Elettaria cardamomum (L.) Maton Essential Oil: An Interesting Source of Bioactive Specialized Metabolites as Inhibitors of Acetylcholinesterase and Butyrylcholinesterase
Abstract
:1. Introduction
2. Results and Discussion
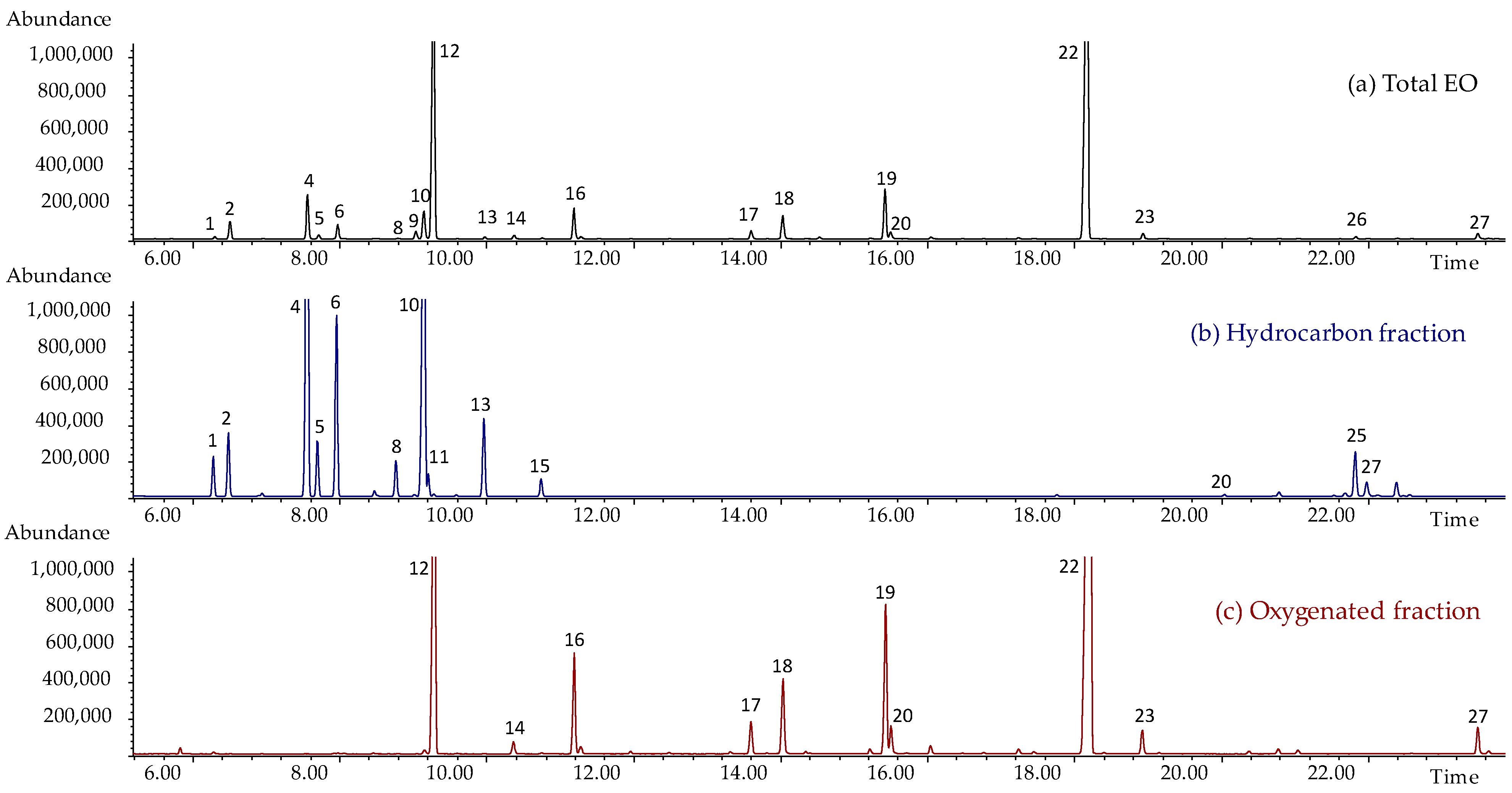
2.1. Chemical Composition of the Investigated Essential Oils
2.2. In Vitro Inhibitory Activity of the Investigated Essential Oil against Acetylcholinesterase and Butyrylcholinesterase
2.3. Identification of Bioactive Components by Bioguided Assay Fractionation
2.4. Effect of Light and Temperature Exposure on EO’s Biological Activity
3. Materials and Methods
3.1. Reagent
3.2. In Vitro Acetylcholinesterase and Butyrylcholinesterase Inhibition Test
3.3. Flash Column Chromatography
3.4. GC-MS Analysis Conditions
3.5. Storage Conditions
3.6. Anova Test and IC50 Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nichols, E.; Szoeke, C.E.I.; Vollset, S.E.; Abbasi, N.; Abd-Allah, F.; Abdela, J.; Aichour, M.T.E.; Akinyemi, R.O.; Alahdab, F.; Asgedom, S.W. Global, Regional, and National Burden of Alzheimer’s Disease and Other Dementias, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Abeysinghe, A.A.D.T.; Deshapriya, R.D.U.S.; Udawatte, C. Alzheimer’s Disease; a Review of the Pathophysiological Basis and Therapeutic Interventions. Life Sci. 2020, 256, 117996. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Webster, C.; Servaes, S.; Morais, J.A.; Rosa-Neto, P. World Alzheimer Report 2022. In Life after Diagnosis: Navigating Treatment, Care and Support, 2022nd ed.; Alzheimer Disease International: London, UK, 2022. [Google Scholar]
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T. Global Estimates on the Number of Persons across the Alzheimer’s Disease Continuum. Alzheimer’s Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Ulep, M.G.; Saraon, S.K.; McLea, S. Alzheimer Disease. J. Nurse Pract. 2018, 14, 129–135. [Google Scholar] [CrossRef]
- Food and Drug Administration Website. FDA Drug Approval Package: Aduhelm (Aducanumab-Avwa). Available online: www.fda.gov (accessed on 15 July 2023).
- Food and Drug Administration Website. FDA Drug Approval Package: LEQEMBI. Available online: www.fda.gov (accessed on 15 July 2023).
- Zhou, S.; Huang, G. The Biological Activities of Butyrylcholinesterase Inhibitors. Biomed. Pharmacother. 2022, 146, 112556. [Google Scholar] [CrossRef]
- de Andrade, P.; Mantoani, S.P.; Gonçalves Nunes, P.S.; Magadán, C.R.; Pérez, C.; Xavier, D.J.; Hojo, E.T.S.; Campillo, N.E.; Martínez, A.; Carvalho, I. Highly Potent and Selective Aryl-1,2,3-Triazolyl Benzylpiperidine Inhibitors toward Butyrylcholinesterase in Alzheimer’s Disease. Bioorg. Med. Chem. 2019, 27, 931–943. [Google Scholar] [CrossRef]
- Essential Oils General Monograph. In European Pharmacopoeia 11th Edition; EDQM Council of Europe: Strasbourg, France, 2023.
- Baser, K.H.C.; Buchbauer, G. (Eds.) Handbook of Essential Oils: Science, Technology and Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Villalta, G.; Salinas, M.; Calva, J.; Bec, N.; Larroque, C.; Vidari, G.; Armijos, C. Selective BuChE Inhibitory Activity, Chemical Composition, and Enantiomeric Content of the Essential Oil from Salvia leucantha Cav. Collected in Ecuador. Plants 2021, 10, 1169. [Google Scholar] [CrossRef]
- Salinas, M.; Bec, N.; Calva, J.; Larroque, C.; Vidari, G.; Armijos, C. Constituents, Enantiomeric Content, and ChE Inhibitory Activity of the Essential Oil from Hypericum laricifolium Juss. Aerial Parts Collected in Ecuador. Plants 2022, 11, 2962. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Warkentin, T.D. Botany, Traditional Uses, Phytochemistry and Biological Activities of Cardamom [Elettaria cardamomum (L.) Maton]—A Critical Review. J. Ethnopharmacol. 2020, 246, 112244. [Google Scholar] [CrossRef]
- Saeed, A.; Sultana, B.; Anwar, F.; Mushtaq, M.; Alkharfy, K.M.; Gilani, A.-H. Antioxidant and Antimutagenic Potential of Seeds and Pods of Green Cardamom (Elettaria cardamomum). Int. J. Pharmacol. 2014, 10, 461–469. [Google Scholar] [CrossRef]
- Hamzaa, R.G.; Osman, N.N. Using of Coffee and Cardamom Mixture to Ameliorate Oxidative Stress Induced in γ-Irradiated Rats. Biochem. Anal. Biochem. 2012, 1, 1000113. [Google Scholar] [CrossRef]
- Yahyazadeh, R.; Ghasemzadeh Rahbardar, M.; Razavi, B.M.; Karimi, G.; Hosseinzadeh, H. The Effect of Elettaria cardamomum (Cardamom) on the Metabolic Syndrome: Narrative Review. Iran. J. Basic Med. Sci. 2021, 24, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Al-Zereini, W.A.; Al-Trawneh, I.N.; Al-Qudah, M.A.; TumAllah, H.M.; Al Rawashdeh, H.A.; Abudayeh, Z.H. Essential Oils from Elettaria cardamomum (L.) Maton Grains and Cinnamomum verum J. Presl Barks: Chemical Examination and Bioactivity Studies. J. Pharm. Pharmacogn. Res. 2022, 10, 173–185. [Google Scholar] [CrossRef]
- Alam, A.; Rehman, N.U.; Ansari, M.N.; Palla, A.H. Effects of Essential Oils of Elettaria cardamomum Grown in India and Guatemala on Gram-Negative Bacteria and Gastrointestinal Disorders. Molecules 2021, 26, 2546. [Google Scholar] [CrossRef]
- Singh, G.; Kiran, S.; Marimuthu, P.; Isidorov, V.; Vinogorova, V. Antioxidant and Antimicrobial Activities of Essential Oil and Various Oleoresins Of Elettaria cardamomum (Seeds and Pods). J. Sci. Food Agric. 2008, 88, 280–289. [Google Scholar] [CrossRef]
- Chen, S.-X.; Xiang, J.-Y.; Han, J.-X.; Yang-Feng; Li, H.Z.; Chen, H.; Xu, M. Essential Oils from Spices Inhibit Cholinesterase Activity and Improve Behavioral Disorder in AlCl3 Induced Dementia. Chem. Biodivers. 2022, 19, e202100443. [Google Scholar] [CrossRef]
- ISO 4733:2004; Oil of Cardamom [Elettaria cardamomum (L.) Maton]. ISO: Geneva, Switzerland, 2004.
- Chowdhury, S.; Kumar, S. Alpha-Terpinyl Acetate: A Natural Monoterpenoid from Elettaria cardamomum as Multi-Target Directed Ligand in Alzheimer’s Disease. J. Funct. Foods 2020, 68, 103892. [Google Scholar] [CrossRef]
- Bicchi, C.; Liberto, E.; Matteodo, M.; Sgorbini, B.; Mondello, L.; Zellner, B.d.; Costa, R.; Rubiolo, P. Quantitative analysis of essential oils: A complex task. Flavour Fragr. J. 2008, 23, 382–391. [Google Scholar] [CrossRef]
- Rubiolo, P.; Sgorbini, B.; Liberto, E.; Cordero, C.; Bicchi, C. Essential oils and volatiles: Sample preparation and analysis. A review. Flavour Fragr. J. 2010, 25, 282–290. [Google Scholar] [CrossRef]
- Sgorbini, B.; Cagliero, C.; Pagani, A.; Sganzerla, M.; Boggia, L.; Bicchi, C.; Rubiolo, P. Determination of free and glucosidically-bound volatiles in plants. Two case studies: L-menthol in peppermint (Mentha × piperita L.) and eugenol in clove (Syzygium aromaticum (L.) Mer. & L.M.Perry). Phytochemistry 2015, 117, 296–305. [Google Scholar] [CrossRef]
- Rhee, I.K.; van de Meent, M.; Ingkaninan, K.; Verpoorte, R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A 2001, 915, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.M.; Peng, J.Q.; Chen, Y.; Tao, L.; Zhang, Y.Y.; Fu, L.Y.; Long, Q.D.; Shen, X.C. 1,8-Cineole: A review of source, biological activities, and application. J. Asian Nat. Prod. Res. 2021, 23, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Al;lured Publishing: Chicago, IL, USA, 2007. [Google Scholar]


| # | ExpIts | LitIts | Compounds | Batches (Average ± σ) | Hydrodist OE | HYDR | OXY | ISO 4733:2004 Min (Central America/ Guatemala) | ISO 4733:2004 Max (Central America/ Guatemala) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 931 | 931 | α-thujene | 0.2 ± 0.03 | 0.2 | 2.3 | |||
| 2 | 939 | 939 | α-pinene | 1.4 ± 0.1 | 1.0 | 3.8 | 1.0 | 2.0 | |
| 3 | 956 | 953 | camphene | tr | 0.2 | ||||
| 4 | 978 | 976 | sabinene | 3.4 ± 0.6 | 1.9 | 36.8 | 3.0 | 5.0 | |
| 5 | 983 | 980 | β-pinene | 0.3 ± 0.05 | 0.3 | 3.3 | |||
| 6 | 992 | 991 | β-myrcene | 1.3 ± 0.4 | 1.3 | 11.1 | tr | 2.5 | |
| 7 | 1010 | 1005 | α-phellandrene | tr | 0.4 | ||||
| 8 | 1022 | 1018 | α-terpinene | 0.1 ± 0.02 | 0.4 | 2.2 | |||
| 9 | 1032 | 1029 | p-cymene | 0.5 ± 0.2 | 0.2 | tr | |||
| 10 | 1036 | 1031 | limonene | 2.2 ± 0.2 | 2.4 | 26.4 | 2.0 | 3.0 | |
| 11 | 1038 | 1031 | β-phellandrene | n.d. | 1.2 | ||||
| 12 | 1041 | 1038 | 1,8-cineole | 30.5 ± 0.7 | 33.6 | 28.5 | 27.0 | 35.0 | |
| 13 | 1065 | 1062 | γ-terpinene | 0.2 ± 0.1 | 0.7 | 5.0 | |||
| 14 | 1078 | 1075 | cis-sabinene hydrate | 0.4 ± 0.1 | tr | 0.5 | |||
| 15 | 1089 | 1088 | α-terpinolene | tr | 0.4 | 1.1 | |||
| 16 | 1104 | 1103 | linalool | 3.4 ± 0.2 | 5.0 | 4.2 | 3.0 | 6.0 | |
| 17 | 1188 | 1184 | 4-terpineol | 0.8 ± 0.04 | 2.5 | 1.4 | 0.8 | 1.5 | |
| 18 | 1202 | 1198 | α-terpineol | 1.9 ± 0.3 | 4.1 | 3.4 | tr | 2.5 | |
| 19 | 1254 | 1251 | linalyl acetate | 6.1 ± 0.7 | 1.7 | 6.6 | 4.0 | 6.0 | |
| 20 | 1257 | 1255 | geraniol | 0.8 ± 0.1 | 1.7 | 1.1 | |||
| 21 | 1276 | 1270 | geranial | 0.3 ± 0.03 | 0.2 | ||||
| 22 | 1355 | 1350 | α-terpinyl acetate | 43.9 ± 0.3 | 40.3 | 52.1 | 35.0 | 45.0 | |
| 23 | 1382 | 1383 | geranyl acetate | 0.7 ± 0.1 | 0.7 | 1.0 | |||
| 24 | 1426 | 1418 | trans-β-caryophyllene | n.d. | n.d. | 0.1 | |||
| 25 | 1486 | 1485 | β-selinene | 0.2 ± 0.1 | n.d. | 3.3 | |||
| 26 | 1490 | 1494 | α-selinene | n.d. | n.d. | 0.3 | |||
| 27 | 1566 | 1565 | (Z)-nerolidol | 0.8 ± 0.1 | 0.5 | 1.2 | 0.5 | 1.0 | |
| Total hydrocarbon compounds | 9.9 ± 1.2 | ||||||||
| Total oxygenated compounds | 89.5 ± 1.4 | ||||||||
| Sample | AChE Inhibition (%) | BChE Inhibition (%) |
|---|---|---|
| Galanthamine | 68.4 ± 2.9 | 24.7 ± 0.6 |
| Batch 1 | 59.2 ± 5.6 | 57.2 ± 0.5 |
| Batch 2 | 60.5 ± 3.1 | 59.4 ± 1.3 |
| Batch 3 | 66.6 ± 1.9 | 54.4 ± 0.9 |
| Batch 4 | 64.0 ± 0.3 | 55.4 ± 1.6 |
| Batch 5 | 62.4 ± 1.1 | 52.4 ± 2.0 |
| Batch 6 | 63.5 ± 1.5 | 61.6 ± 1.2 |
| Hydrodistilled EO | 62.2 ± 2.1 | 57.3 ± 2.0 |
| Inhibitor | IC50 AChE (μg/mL) | IC50 BChE (μg/mL) |
|---|---|---|
| Galanthamine | 0.468 ± 0.002 | 3.40 ± 0.0225 |
| 1,8-Cineole | 14.1 ± 0.556 | not active |
| α-Terpinyl acetate | Activity lower than 50% | 21.9 ± 0.623 |
| Commercial batch | 24.9 ± 0.350 | 25.9 ± 1.71 |
| Hydrodistilled EO | 24.3 ± 0.248 | 37.2 ± 3.24 |
| Sample | AChE Inhibition (%) * | σ | BChE Inhibition (%) * | σ |
|---|---|---|---|---|
| Cardamom EO commercial sample mean | 62.6 | 2.9 | 55.8 | 2.7 |
| Oxygenated fraction | 42.8 | 0.5 | 63.7 | 3.0 |
| Hydrocarbon fraction | n.a. | n.a. | ||
| α-terpinyl acetate | 17.0 | 1.0 | 39.0 | 2.7 |
| 1,8-cineole | 55.4 | 0.7 | n.a. | |
| Mixture of α-terpinyl acetate and 1,8 cineole | 56.7 | 1.2 | 45.8 | 2.0 |
| Linalool | n.a. | 5.0 | 1.4 | |
| α-terpineol | n.a. | n.a. | ||
| Linalyl acetate | n.a. | 10.3 | 3.3 | |
| Mixture of 1,8 cineole, α-terpinyl acetate, linalool, and linalyl acetate | 63.8 | 1.0 | 50.3 | 2.2 |
| Mixture of 1,8 cineole, α-terpinyl acetate, linalool, linalyl acetate, and α-terpineol | 64.1 | 2.1 | 49.4 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavarino, M.; Marengo, A.; Cagliero, C.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Elettaria cardamomum (L.) Maton Essential Oil: An Interesting Source of Bioactive Specialized Metabolites as Inhibitors of Acetylcholinesterase and Butyrylcholinesterase. Plants 2023, 12, 3463. https://doi.org/10.3390/plants12193463
Pavarino M, Marengo A, Cagliero C, Bicchi C, Rubiolo P, Sgorbini B. Elettaria cardamomum (L.) Maton Essential Oil: An Interesting Source of Bioactive Specialized Metabolites as Inhibitors of Acetylcholinesterase and Butyrylcholinesterase. Plants. 2023; 12(19):3463. https://doi.org/10.3390/plants12193463
Chicago/Turabian StylePavarino, Marta, Arianna Marengo, Cecilia Cagliero, Carlo Bicchi, Patrizia Rubiolo, and Barbara Sgorbini. 2023. "Elettaria cardamomum (L.) Maton Essential Oil: An Interesting Source of Bioactive Specialized Metabolites as Inhibitors of Acetylcholinesterase and Butyrylcholinesterase" Plants 12, no. 19: 3463. https://doi.org/10.3390/plants12193463
APA StylePavarino, M., Marengo, A., Cagliero, C., Bicchi, C., Rubiolo, P., & Sgorbini, B. (2023). Elettaria cardamomum (L.) Maton Essential Oil: An Interesting Source of Bioactive Specialized Metabolites as Inhibitors of Acetylcholinesterase and Butyrylcholinesterase. Plants, 12(19), 3463. https://doi.org/10.3390/plants12193463










