Modeling of the Statistical Distribution of Tracheids in Conifer Rings: Finding Universal Criterion for Earlywood–Latewood Distinction
Abstract
:1. Introduction
2. Results
2.1. Statistical Distributions of Tracheids in Terms of Radial Cell Diameter, Cell Wall Thickness, and Derivative Traits
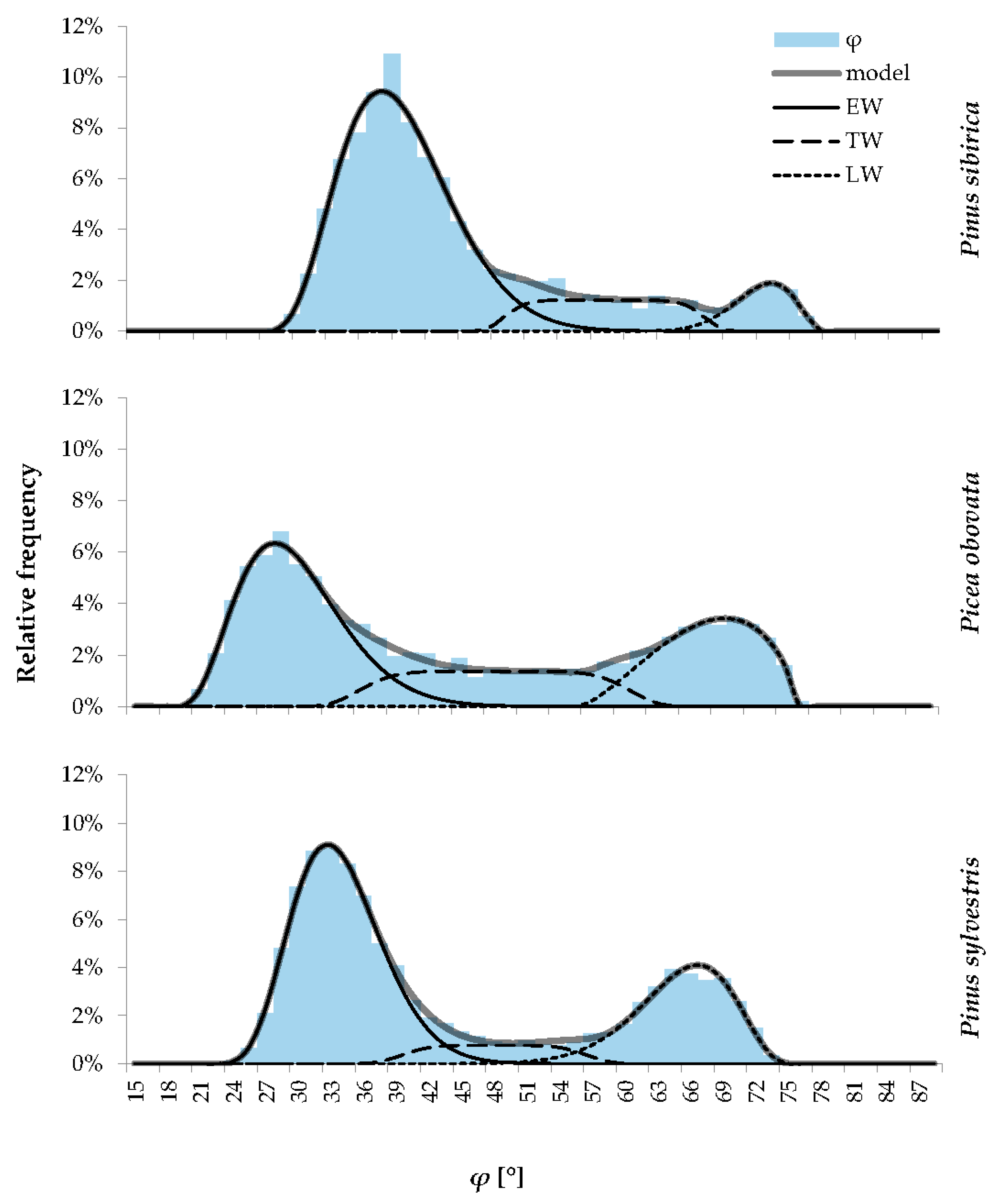
2.2. Functional Modeling of the Statistical Distributions
3. Discussion
- The inclusion of the size–age dynamics of the anatomical structure in juvenile wood.
- The continuation of the analysis of the model stability when generalizing tracheidograms at different spatial scales.
- The analysis of model stability during years of growth depression and/or formation of anomalies in the anatomical structure (light rings, IADFs, etc.).
- The use of the obtained quantitative estimates of earlywood and latewood tracheids in the analysis of the influence of climatic factors on wood structure, etc.
4. Materials and Methods
4.1. Study Area and Sampling Site
4.2. Sampling, Processing and Measurements
4.3. Derivative Criteria for Distinguishing between Tree-Ring Zones
4.4. Modeling of the Cell Statistical Distributions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vaganov, E.A.; Anchukaitis, K.J.; Evans, M.N. How well understood are the processes that create dendroclimatic records? A mechanistic model of the climatic control on conifer tree-ring growth dynamics. In Dendroclimatology: Progress and Prospects; Hughes, M., Swetnam, T., Diaz, H., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 37–75. [Google Scholar]
- Silva, M.D.S.; Funch, L.S.; da Silva, L.B. The growth ring concept: Seeking a broader and unambiguous approach covering tropical species. Biol. Rev. 2019, 94, 1161–1178. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S. Climatic influence on tree wood anatomy: A review. J. Wood Sci. 2021, 67, 1–7. [Google Scholar] [CrossRef]
- Traversari, S.; Giovannelli, A.; Emiliani, G. Wood formation under changing environment: Omics approaches to elucidate the mechanisms driving the early-to-latewood transition in Conifers. Forests 2022, 13, 608. [Google Scholar] [CrossRef]
- Camarero, J.J.; Guerrero-Campo, J.; Gutiérrez, E. Tree-ring growth and structure of Pinus uncinata and Pinus sylvestris in the Central Spanish Pyrenees. Arct. Alp. Res. 1998, 30, 1–10. [Google Scholar] [CrossRef]
- Groombridge, B.; Jenkins, M.D. World Atlas of Biodiversity. Prepared by the UNEP World Conservation Monitoring Centre; University of California Press: Berkeley, CA, USA, 2002. [Google Scholar]
- Vaganov, E.A.; Hughes, M.K.; Shashkin, A.V. Growth Dynamics of Conifer Tree Rings: Images of Past and Future Environments; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Schweingruber, F.H. Wood Structure and Environment; Springer Verlag: Berlin, Germany, 2007. [Google Scholar]
- De Micco, V.; Aronne, G. Seasonal dimorphism in wood anatomy of the Mediterranean Cistus incanus L. subsp. incanus. Trees 2009, 23, 981–989. [Google Scholar] [CrossRef]
- Stoffel, M.; Bollschweiler, M. What tree rings can tell about earth-surface processes: Teaching the principles of dendrogeomorphology. Geogr. Compass 2009, 3, 1013–1037. [Google Scholar] [CrossRef]
- Rahman, M.; Begum, S.; Nakaba, S.; Yamagishi, Y.; Kudo, K.; Nabeshima, E.; Nugroho, W.; Oribe, Y.; Funada, R. Relationship between the earlywood-to-latewood transition and changes in levels of stored starch around the cambium in locally heated stems of the evergreen conifer Chamaecyparis pisifera. Trees 2016, 30, 1619–1631. [Google Scholar] [CrossRef]
- Rosell, J.A.; Olson, M.E.; Anfodillo, T. Scaling of xylem vessel diameter with plant size: Causes, predictions, and outstanding questions. Curr. For. Rep. 2017, 3, 46–59. [Google Scholar] [CrossRef]
- Zhirnova, D.F.; Belokopytova, L.V.; Upadhyay, K.K.; Tripathi, S.K.; Babushkina, E.A.; Vaganov, E.A. 495-year wood anatomical record of Siberian stone pine (Pinus sibirica Du Tour) as climatic proxy on the timberline. Forests 2022, 13, 247. [Google Scholar] [CrossRef]
- Schweingruber, F.H. Tree Rings: Basics and Applications of Dendrochronology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989. [Google Scholar]
- De Micco, V.; Campelo, F.; De Luis, M.; Bräuning, A.; Grabner, M.; Battipaglia, G.; Cherubini, P. Intra-annual density fluctuations in tree rings: How, when, where, and why? IAWA J. 2016, 37, 232–259. [Google Scholar] [CrossRef]
- von Arx, G.; Archer, S.R.; Hughes, M.K. Long-term functional plasticity in plant hydraulic architecture in response to supplemental moisture. Ann. Bot. 2012, 109, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Funada, R.; Kajita, S. Improvement of woody biomass. In Research Approaches to Sustainable Biomass Systems; Tojo, S., Hirasawa, T., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Oxford, UK, 2014; pp. 88–106. [Google Scholar]
- Körner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 2015, 25, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Funada, R.; Kubo, T.; Fushitani, M. Early-and latewood formation in Pinus densiflora trees with different amounts of crown. IAWA J. 1990, 11, 281–288. [Google Scholar] [CrossRef]
- Eckes-Shephard, A.H.; Ljungqvist, F.C.; Drew, D.M.; Rathgeber, C.B.; Friend, A.D. Wood formation modeling—a research review and future perspectives. Front. Plant Sci. 2022, 13, 837648. [Google Scholar] [CrossRef] [PubMed]
- Meko, D.M.; Baisan, C.H. Pilot study of latewood-width of conifers as an indicator of variability of summer rainfall in the North American monsoon region. Int. J. Climatol. 2001, 21, 697–708. [Google Scholar] [CrossRef]
- Creber, G.T.; Chaloner, W.G. Influence of environmental factors on the wood structure of living and fossil trees. Bot. Rev. 1984, 50, 357–448. [Google Scholar] [CrossRef]
- Kaennel, M.; Schweingruber, F.H. (Eds.) Multilingual Glossary of Dendrochronology. Terms and Definitions in English, Spanish, Italian, Portuguese, and Russian; Swiss Federal Research Institute WSL: Birmensdorf, Switzerland, 1995. [Google Scholar]
- Sheppard, P.R.; Graumlich, L.J.; Conkey, L.E. Reflected-light image analysis of conifer tree rings for reconstructing climate. Holocene 1996, 6, 62–68. [Google Scholar] [CrossRef]
- Koubaa, A.; Zhang, S.T.; Makni, S. Defining the transition from earlywood to latewood in black spruce based on intra-ring wood density profiles from X-ray densitometry. Ann. For. Sci. 2002, 59, 511–518. [Google Scholar] [CrossRef]
- Kumar, S. Earlywood–latewood demarcation criteria and their effect on genetic parameters of growth ring density components and efficiency of selection for end-of-rotation density of radiata pine. Silv. Genet. 2002, 51, 241–246. [Google Scholar]
- Clark, A.; Daniels, R.F.; Jordan, L. Juvenile/mature wood transition in loblolly pine as defined by annual ring specific gravity, proportion of latewood, and microfibril angle. Wood Fiber Sci. 2006, 38, 292–299. [Google Scholar]
- Tardif, J.C.; Kames, S.; Nolin, A.F.; Bergeron, Y. Earlywood vessels in black ash (Fraxinus nigra Marsh.) trees show contrasting sensitivity to hydroclimate variables according to flood exposure. Front. Plant Sci. 2021, 12, 754596. [Google Scholar] [CrossRef] [PubMed]
- Antony, F.; Schimleck, L.R.; Daniels, R.F. A comparison of earlywood–latewood demarcation methods—a case study in loblolly pine. IAWA J. 2012, 33, 187–195. [Google Scholar] [CrossRef]
- Babushkina, E.A.; Belokopytova, L.V.; Kostyakova, T.V.; Kokova, V.I. Earlywood and latewood features of Pinus sylvestris in semiarid natural zones of South Siberia. Russ. J. Ecol. 2018, 49, 209–217. [Google Scholar] [CrossRef]
- Zhirnova, D.F.; Belokopytova, L.V.; Babushkina, E.A.; Crivellaro, A.; Vaganov, E.A. Earlywood structure of evergreen conifers near forest line is habitat driven but latewood depends on species and seasons. Trees 2021, 35, 479–492. [Google Scholar] [CrossRef]
- von Arx, G.; Crivellaro, A.; Prendin, A.L.; Čufar, K.; Carrer, M. Quantitative wood anatomy: Practical guidelines. Front. Plant Sci. 2016, 7, 781. [Google Scholar] [CrossRef]
- Denne, M.P. Definition of latewood according to Mork (1928). IAWA J. 1989, 10, 59–62. [Google Scholar] [CrossRef]
- Mork, E. Die Qualitat des Fichtenhozes unter besonder Rucksichtnahme auf Schleif und Papierholz. Der Pap. Fabr. 1928, 26, 741–747. (In German) [Google Scholar]
- Sviderskaya, I.V.; Sukhovol’skii, V.G.; Radosteva, E.Y.; Kirdyanov, A.V. Model estimation of optimal ratio between cell wall thickness and lumen size for tracheids of conifers in temperate zone. J. Sib. Fed. Univ. Biol. 2011, 4, 183–196. (In Russian) [Google Scholar]
- Piermattei, A.; von Arx, G.; Avanzi, C.; Fonti, P.; Gärtner, H.; Piotti, A.; Urbinati, C.; Vendramin, G.G.; Büntgen, U.; Crivellaro, A. Functional relationships of wood anatomical traits in Norway spruce. Front. Plant Sci. 2020, 11, 683. [Google Scholar] [CrossRef]
- Samusevich, A.; Lexa, M.; Vejpustková, M.; Altman, J.; Zeidler, A. Comparison of methods for the demarcation between earlywood and latewood in tree rings of Norway spruce. Dendrochronologia 2020, 60, 125686. [Google Scholar] [CrossRef]
- Gebregeorgis, E.G.; Boniecka, J.; Piatkowski, M.; Robertson, I.; Rathgeber, C.B.K. SabaTracheid 1.0: A novel program for quantitative analysis of conifer wood anatomy—a demonstration on African juniper from the Blue Nile basin. Front. Plant Sci. 2021, 12, 595258. [Google Scholar] [CrossRef]
- Wang, H.; Shao, X.M.; Jiang, Y.; Fang, X.Q.; Wu, S.H. The impacts of climate change on the radial growth of Pinus koraiensis along elevations of Changbai Mountain in northeastern China. For. Ecol. Manag. 2013, 289, 333–340. [Google Scholar] [CrossRef]
- Yu, D.; Liu, J.; Lewis, B.J.; Li, Z.; Zhou, W.; Fang, X.; Wei, Y.; Jiang, S.; Dai, L. Spatial variation and temporal instability in the climate–growth relationship of Korean pine in the Changbai Mountain region of Northeast China. For. Ecol. Manag. 2013, 300, 96–105. [Google Scholar] [CrossRef]
- Cerrato, R.; Salvatore, M.C.; Gunnarson, B.E.; Linderholm, H.W.; Carturan, L.; Brunetti, M.; De Blasi, F.; Baroni, C. A Pinus cembra L. tree-ring record for late spring to late summer temperature in the Rhaetian Alps, Italy. Dendrochronologia 2019, 53, 22–31. [Google Scholar] [CrossRef]
- Belokopytova, L.V.; Fonti, P.; Babushkina, E.A.; Zhirnova, D.F.; Vaganov, E.A. Evidences of different drought sensitivity in xylem cell developmental processes in South Siberia Scots pines. Forests 2020, 11, 294. [Google Scholar] [CrossRef]
- Anfodillo, T.; Petit, G.; Crivellaro, A. Axial conduit widening in woody species: A still neglected anatomical pattern. IAWA J. 2013, 34, 352–364. [Google Scholar] [CrossRef]
- Olson, M.E.; Soriano, D.; Rosell, J.A.; Anfodillo, T.; Donoghue, M.J.; Edwards, E.J.; León-Gómez, C.; Dawson, T.; Camarero Martínez, J.J.; Castorena, M.; et al. Plant height and hydraulic vulnerability to drought and cold. Proc. Natl. Acad. Sci. USA 2018, 115, 7551–7556. [Google Scholar] [CrossRef]
- Sviderskaya, I.V.; Vaganov, E.A.; Fonti, M.V.; Fonti, P. Isometric scaling to model water transport in conifer tree rings across time and environments. J. Exp. Bot. 2021, 72, 2672–2685. [Google Scholar] [CrossRef]
- Koloadin, L.S. Intraspecific and Interspecific Variation in the Xylem Functional Traits of Callitris Species Growing along an Aridity Gradient. Ph.D. Thesis, Western Sydney University, Penrith, NSW, Australia, 2020. [Google Scholar]
- Sasani, N.; Pâques, L.E.; Boulanger, G.; Singh, A.P.; Gierlinger, N.; Rosner, S.; Brendel, O. Physiological and anatomical responses to drought stress differ between two larch species and their hybrid. Trees 2021, 35, 1467–1484. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, Y.; Morris, H.; Vandelook, F.; Jansen, S. Variation in tracheid dimensions of conifer xylem reveals evidence of adaptation to environmental conditions. Front. Plant Sci. 2022, 13, 774241. [Google Scholar] [CrossRef]
- Férriz, M.; Martin-Benito, D.; Fernández-de-Simón, M.B.; Conde, M.; García-Cervigón, A.I.; Aranda, I.; Gea-Izquierdo, G. Functional phenotypic plasticity mediated by water stress and [CO2] explains differences in drought tolerance of two phylogenetically close conifers. Tree Physiol. 2023, 43, 909–924. [Google Scholar] [CrossRef]
- Cartenì, F.; Deslauriers, A.; Rossi, S.; Morin, H.; De Micco, V.; Mazzoleni, S.; Giannino, F. The physiological mechanisms behind the earlywood-to-latewood transition: A process-based modeling approach. Front. Plant Sci. 2018, 9, 1053. [Google Scholar] [CrossRef] [PubMed]
- Piirto, D.D. Wood of Giant Sequoia: Properties and unique characteristics. In Proceedings of the Workshop on Management of Giant Sequoia, Reedley, CA, USA, 24–25 May 1985; 1986; pp. 19–23. [Google Scholar]
- Visscher, G.E. Wood Anatomy of Metasequoia—Separation from Glyptostrobus and Function/Structure Considerations. Master’s Thesis, University of Maine, Orono, ME, USA, 2002. [Google Scholar]
- Zhirnova, D.F.; Belokopytova, L.V.; Krutovsky, K.V.; Kholdaenko, Y.A.; Babushkina, E.A.; Vaganov, E.A. Spatial-coherent dynamics and climatic signals in the radial growth of Siberian stone pine (Pinus sibirica Du Tour) in subalpine stands along the Western Sayan Mountains. Forests 2022, 13, 1994. [Google Scholar] [CrossRef]
- Helama, S.; Melvin, T.M.; Briffa, K.R. Regional curve standardization: State of the art. Holocene 2017, 27, 172–177. [Google Scholar] [CrossRef]
- Babushkina, E.; Belokopytova, L.; Zhirnova, D.; Barabantsova, A.; Vaganov, E. Divergent growth trends and climatic response of Picea obovata along elevational gradient in Western Sayan mountains, Siberia. J. Mt. Sci. 2018, 15, 2378–2397. [Google Scholar] [CrossRef]
- Cook, E.R.; Kairiukstis, L.A. (Eds.) Methods of Dendrochronology. Application in Environmental Sciences; Kluwer Academic Publishers: Dordrecht, Germany, 1990. [Google Scholar]
- Rinn, F. TSAP-Win: Time Series Analysis and Presentation for Dendrochronology and Related Applications: User Reference; RINNTECH: Heidelberg, Germany, 2003. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 68–78. [Google Scholar]
- Gennaretti, F.; Carrer, M.; García-González, I.; Rossi, S.; von Arx, G. Quantitative wood anatomy to explore tree responses to global change. Front. Plant Sci/ 2022, 13, 998895. [Google Scholar] [CrossRef]
- Seo, J.W.; Smiljanić, M.; Wilmking, M. Optimizing cell-anatomical chronologies of Scots pine by stepwise increasing the number of radial tracheid rows included—Case study based on three Scandinavian sites. Dendrochronologia 2014, 32, 205–209. [Google Scholar] [CrossRef]
- Belokopytova, L.V.; Babushkina, E.A.; Zhirnova, D.F.; Panyushkina, I.P.; Vaganov, E.A. Pine and larch tracheids capture seasonal variations of climatic signal at moisture-limited sites. Trees 2019, 33, 227–242. [Google Scholar] [CrossRef]
- Silkin, P.P. Methods of Multiparameter Analysis of Conifers Tree-Rings Structure; Siberian Federal University: Krasnoyarsk, Russia, 2010. (In Russian) [Google Scholar]
- Vaganov, E.A. The tracheidogram method in tree-ring analysis and its application. In Methods of Dendrochronology. Application in Environmental Sciences; Cook, E.R., Kairiukstis, L.A., Eds.; Kluwer Academic Publishers: Dordrecht, Germany, 1990; pp. 63–75. [Google Scholar]
- Johnson, N.L.; Kotz, S.; Balakrishnan, N. Continuous Univariate Distributions, vol. 2; Wiley: New York, NY, USA, 1995. [Google Scholar]
- Nadarajah, S. A generalized normal distribution. J. Appl. Stat. 2005, 32, 685–694. [Google Scholar] [CrossRef]



| Characteristics | Tree Species | |||
|---|---|---|---|---|
| Pinus sibirica | Picea obovata | Pinus sylvestris | ||
| Cover Period | 1968–2017 | 1965–2014 | 1968–2017 | |
| Number of measurements | trees | 5 | 5 | 5 |
| rings | 249 | 245 | 249 | |
| cells before/after averaging over 5 rows | 24,565/4913 | 24,010/4802 | 31,450/6290 | |
| Cell traits: mean (min–max) | D, μm | 35.3 (6.9–55.9) | 28.6 (5.3–52.3) | 34.5 (7.8–52.8) |
| CWT, μm | 2.9 (1.7–5.7) | 2.6 (1.4–6.2) | 3.3 (1.8–7.7) | |
| CWT/D | 0.10 (0.04–0.41) | 0.12 (0.03–0.39) | 0.12 (0.04–0.36) | |
| φ, ° | 5.6 (2.5–22.5) | 6.8 (1.9–21.5) | 6.6 (2.4–20.0) | |
| Di | 1.00 (0.20–1.58) | 1.00 (0.18–1.83) | 1.00 (0.23–1.53) | |
| CWTi | 1.00 (0.60–1.98) | 1.00 (0.55–2.36) | 1.00 (0.55–2.32) | |
| CWTi/Di | 1.20 (0.54–5.07) | 1.33 (0.36–4.33) | 1.21 (0.44–3.77) | |
| φi, ° | 45.8 (28.4–78.8) | 45.6 (20.0–77.0) | 45.0 (23.8–75.2) | |
| Zone | Parameter of Distribution 1 | Tree Species | ||
|---|---|---|---|---|
| Pinus sibirica 2 | Picea obovata | Pinus sylvestris | ||
| EW | A, % | 77.4 (71.7–81.1) | 49.2 | 62.0 |
| μ, ° | 40.52 (39.4–41.18) | 30.57 | 34.60 | |
| median, ° | 40.00 (39.64–41.81) | 29.98 | 34.24 | |
| mode, ° | 38.86 (38.33–39.79) | 28.70 | 33.48 | |
| σ, ° | 4.97 (3.22–5.68) | 4.80 | 4.12 | |
| CWT/D (mean/median/mode) | 0.070 (0.065–0.075) | 0.054 | 0.067 | |
| 0.069 (0.065–0.074) | 0.053 | 0.066 | ||
| 0.066 (0.063–0.070) | 0.050 | 0.064 | ||
| TW | A, % | 14.2 (12.3–19.7) | 22.1 | 8.4 |
| μ, ° | 59.00 (57.51–62.13) | 49.00 | 48.81 | |
| σ, ° | 5.26 (4.78–7.55) | 7.35 | 5.13 | |
| CWT/D (mean = median = mode) | 0.136 (0.123–0.146) | 0.105 | 0.110 | |
| LW | A, % | 8.4 (6.6–8.6) | 28.7 | 29.6 |
| μ, ° | 73.13 (72.02–74.22) | 68.67 | 65.86 | |
| median, ° | 73.42 (72.64–74.45) | 69.01 | 66.37 | |
| mode, ° | 74.11 (73.94–75.10) | 70.29 | 67.48 | |
| σ, ° | 2.61 (1.72–2.92) | 4.38 | 4.44 | |
| CWT/D (mean/median/mode) | 0.270 (0.264–0.293) | 0.233 | 0.216 | |
| 0.275 (0.268–0.298) | 0.237 | 0.221 | ||
| 0.287 (0.278–0.314) | 0.254 | 0.233 | ||
| χ2 | 40.23 (15.47–27.82) | 22.60 | 28.81 | |
| degrees of freedom | 18 (16–17) | 23 | 20 | |
| significance level | 0.002 (0.047–0.49) | 0.48 | 0.09 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belokopytova, L.V.; Zhirnova, D.F.; Yang, B.; Babushkina, E.A.; Vaganov, E.A. Modeling of the Statistical Distribution of Tracheids in Conifer Rings: Finding Universal Criterion for Earlywood–Latewood Distinction. Plants 2023, 12, 3454. https://doi.org/10.3390/plants12193454
Belokopytova LV, Zhirnova DF, Yang B, Babushkina EA, Vaganov EA. Modeling of the Statistical Distribution of Tracheids in Conifer Rings: Finding Universal Criterion for Earlywood–Latewood Distinction. Plants. 2023; 12(19):3454. https://doi.org/10.3390/plants12193454
Chicago/Turabian StyleBelokopytova, Liliana V., Dina F. Zhirnova, Bao Yang, Elena A. Babushkina, and Eugene A. Vaganov. 2023. "Modeling of the Statistical Distribution of Tracheids in Conifer Rings: Finding Universal Criterion for Earlywood–Latewood Distinction" Plants 12, no. 19: 3454. https://doi.org/10.3390/plants12193454
APA StyleBelokopytova, L. V., Zhirnova, D. F., Yang, B., Babushkina, E. A., & Vaganov, E. A. (2023). Modeling of the Statistical Distribution of Tracheids in Conifer Rings: Finding Universal Criterion for Earlywood–Latewood Distinction. Plants, 12(19), 3454. https://doi.org/10.3390/plants12193454







