Heterologous Expression of OtsB Increases Tuber Yield and Phenotypic Stability in Potato under Both Abiotic and Biotic Stresses
Abstract
1. Introduction
2. Results
2.1. Transgenic Expression of OtsB
2.2. Effect of OtsB on Abiotic Stress Response
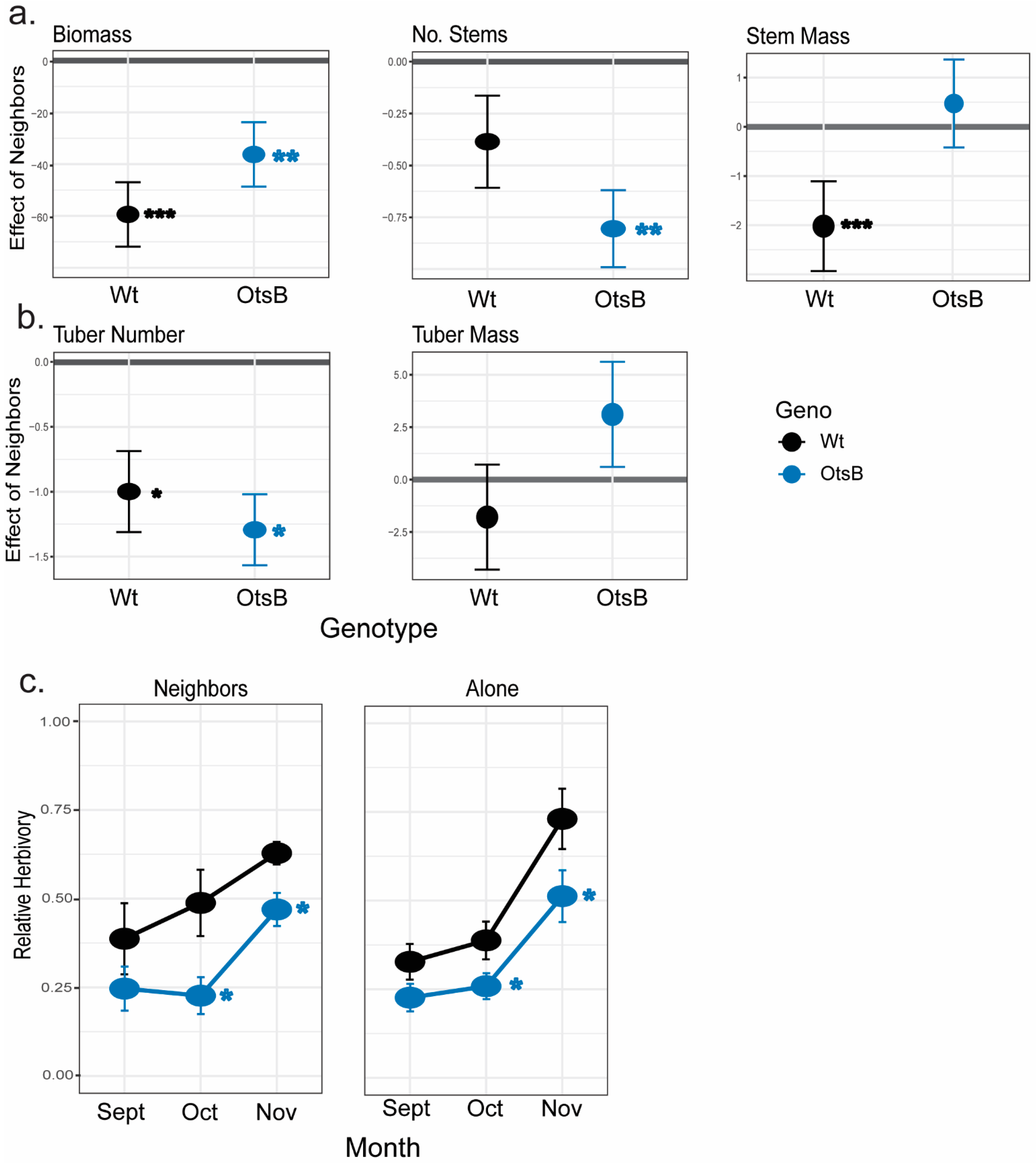
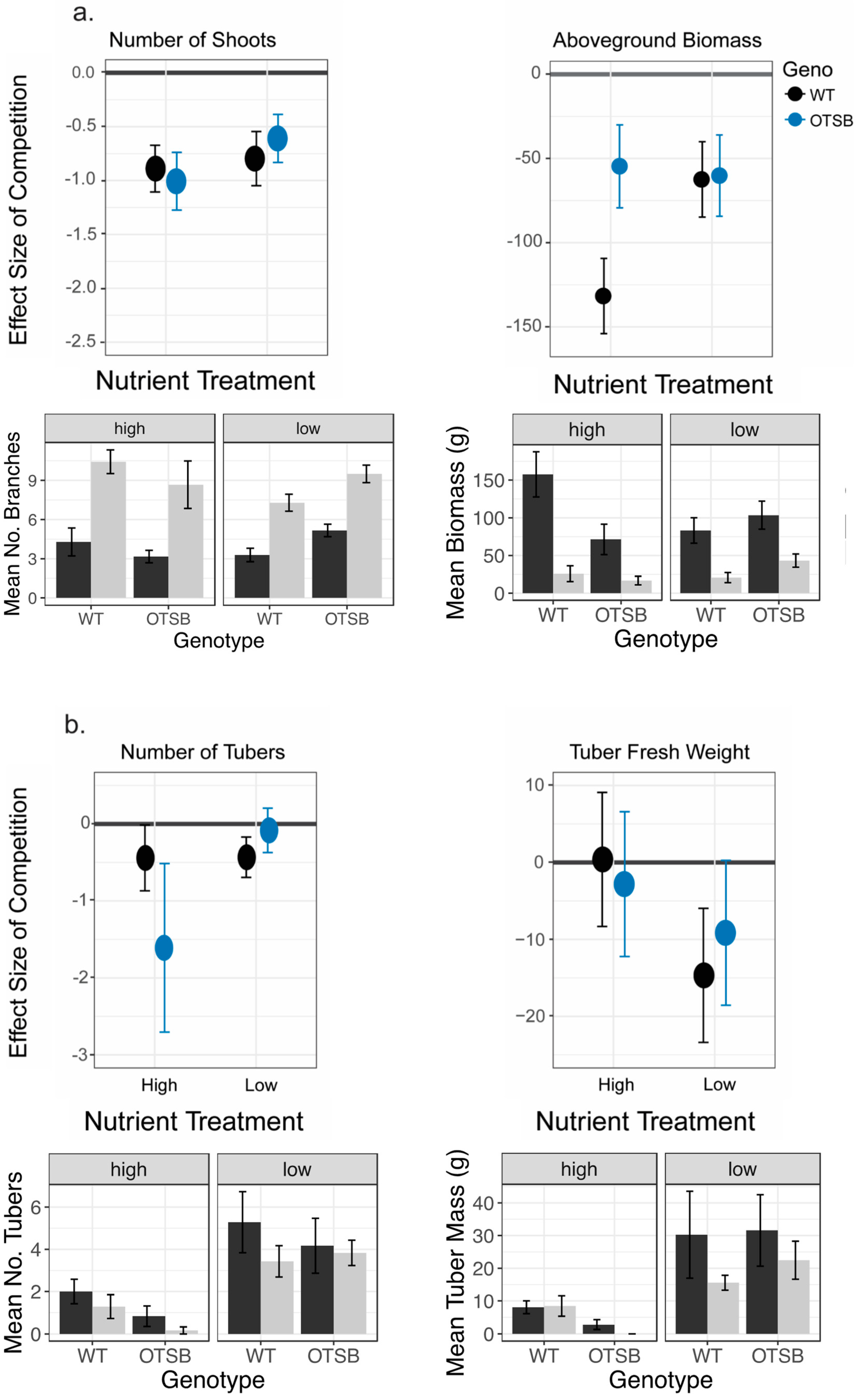
2.3. Effects of OtsB Gene Expression on Biotic Stress
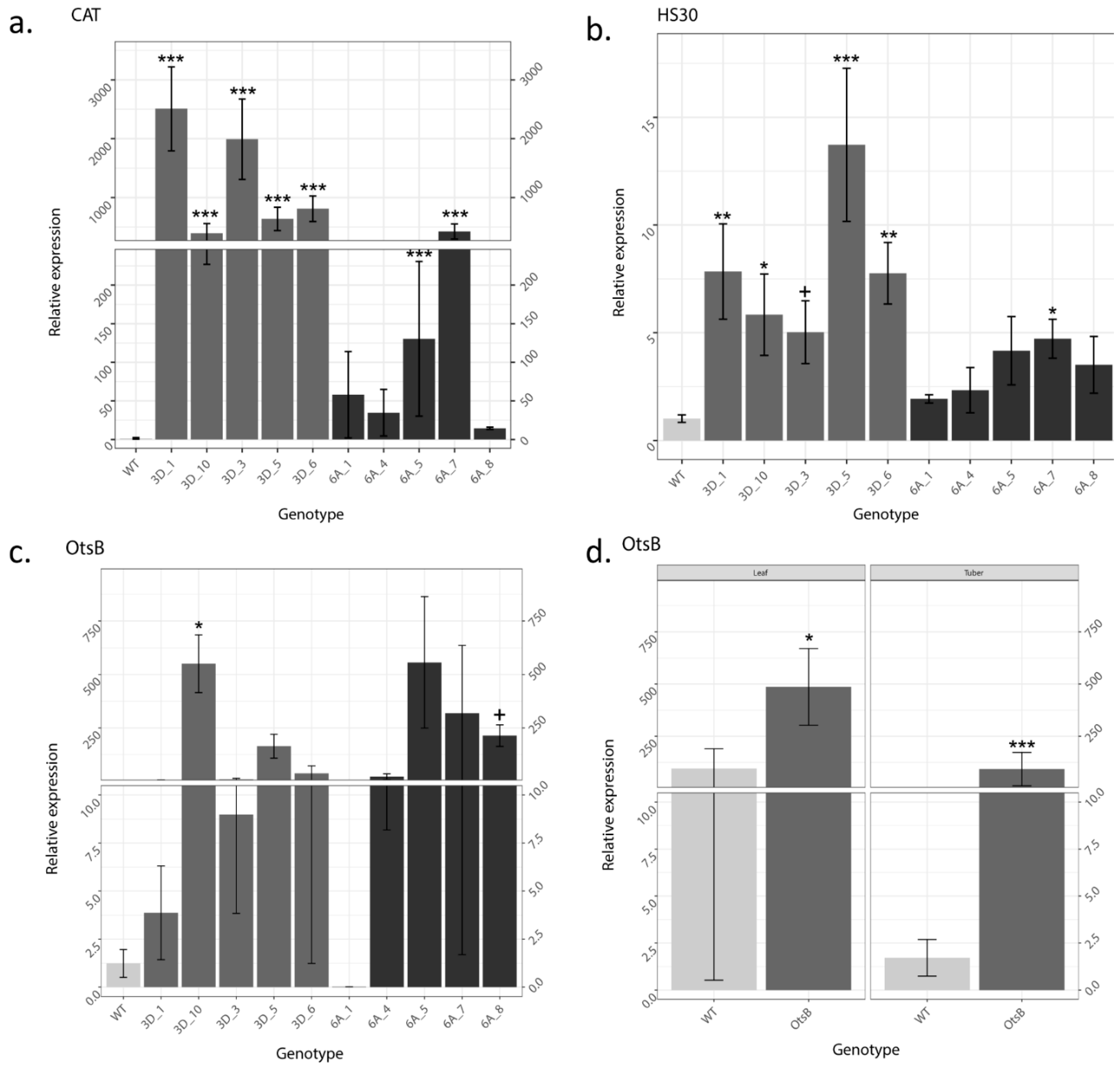
2.4. Increased OtsB Expression Altered Expression of Other Stress Related Genes
3. Discussion
3.1. Exogenous Expression of OtsB Led to Reduced Negative Effects of Stress and Higher Yield
3.2. Increased OtsB Expression Has Potential to Create a Better Climate-Smart Crop
4. Materials and Methods
4.1. Vector Construction
4.2. Plant Material and Transformation
4.3. Plant Propagation
4.4. Abiotic Stress (Heat/Short Photoperiod)
4.5. Trait Measurement
4.6. qRT-PCR
4.7. Biotic Stress (Competition, Herbivory, Nutrient Flux)
4.7.1. Mesocosm 1: Competition and Herbivory under Summer Temperatures
- Plant propagation
- Mesocosm community species composition
- Mesocosm 1 growth conditions
- Mesocosm trait measurements
4.7.2. Mesocosm 2: Competition and Nutrient Availability Manipulation
- Plant propagation
- Mesocosm 2 communities
- Mesocosm 2 growth conditions
4.8. Statistical analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desa, U. World Population Prospects 2019: Highlights; United Nations Department for Economic and Social Affairs: New York, NY, USA, 2019; Volume 11, p. 125. [Google Scholar]
- Singh, A.; Kaushal, N.; Sharma, R.; Bhardwaj, V.; Singh, B.; Singh, R. Effect of elevated temperature on in vitro microtuberization of potato genotypes with different thermotolerance levels. Int. J. Plant Res. 2016, 29, 6. [Google Scholar]
- Herman, D.J.; Knowles, L.O.; Knowles, N.R. Heat stress affects carbohydrate metabolism during cold-induced sweetening of potato (Solanum tuberosum L.). Planta 2017, 245, 563–582. [Google Scholar] [CrossRef] [PubMed]
- Sterrett, S.; Henningre, M.; Lee, G. Relationship of internal heat necrosis of potato to time and temperature after planting. J. Am. Soc. Hortic. Sci. 1991, 116, 697–700. [Google Scholar] [CrossRef]
- Singh, B.; Kukreja, S.; Goutam, U. Impact of heat stress on potato (Solanum tuberosum L.): Present scenario and future opportunities. J. Hortic. Sci. Technol. 2020, 95, 407–424. [Google Scholar]
- Chen, X.; Wang, W.; Cai, P.; Wang, Z.; Li, T.; Du, Y. The role of the MAP kinase-kinase protein StMKK1 in potato immunity to different pathogens. Hortic. Res. 2021, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Van den Ende, W.; Valluru, R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef]
- Poiatti, V.A.D.; Dalmas, F.R.; Astarita, L.V. Defense mechanisms of Solanum tuberosum L. in response to attack by plant-pathogenic bacteria. Biol. Res. 2009, 42, 205–215. [Google Scholar]
- Mukherjee, P.K.; Rahaman, S.; Maity, S.K.; Sinha, B. Weed management practices in potato (Solanum tuberosum L.). J. Crop Weed 2012, 8, 178–180. [Google Scholar]
- Mondani, F.; Golzardi, F.; Ahmadvand, G.; Ghorbani, R.; Moradi, R. Influence of weed competition on potato growth, production and radiation use efficiency. Not. Sci. Biol. 2011, 3, 42–52. [Google Scholar] [CrossRef]
- Arora, A.; Tomar, S.; Gole, M. Yield and quality of potato as influenced by weed management practices and their residual study in soil. Agric. Sci. Dig. 2009, 29, 39–41. [Google Scholar]
- Shah, Z.; Shah, S.H.; Ali, G.S.; Munir, I.; Khan, R.S.; Iqbal, A.; Ahmed, N.; Jan, A. Introduction of Arabidopsis’s heat shock factor HsfA1d mitigates adverse effects of heat stress on potato (Solanum tuberosum L.) plant. Cell Stress Chaperones 2020, 25, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Gangadhar, B.H.; Mishra, R.K.; Kappachery, S.; Baskar, V.; Venkatesh, J.; Nookaraju, A.; Thiruvengadam, M. Enhanced thermo-tolerance in transgenic potato (Solanum tuberosum L.) overexpressing hydrogen peroxide-producing germin-like protein (GLP). Genomics 2021, 113, 3224–3234. [Google Scholar] [PubMed]
- Dou, H.; Xv, K.; Meng, Q.; Li, G.; Yang, X. Potato plants ectopically expressing Arabidopsis thaliana CBF 3 exhibit enhanced tolerance to high-temperature stress. Plant Cell Environ. 2015, 38, 61–72. [Google Scholar] [CrossRef]
- Tang, L.; Kwon, S.-Y.; Kim, S.-H.; Kim, J.-S.; Choi, J.S.; Cho, K.Y.; Sung, C.K.; Kwak, S.-S.; Lee, H.-S. Enhanced tolerance of transgenic potato plants expressing both superoxide dismutase and ascorbate peroxidase in chloroplasts against oxidative stress and high temperature. Plant Cell Rep. 2006, 25, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A.; Park, S.W. Engineered resistance against filamentous pathogens in Solanum tuberosum. J. Gen. Plant Pathol. 2012, 78, 377–388. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Luo, Y.; Li, W.-M.; Wang, W. Trehalose: Protector of antioxidant enzymes or reactive oxygen species scavenger under heat stress? Environ. Exp. Bot. 2008, 63, 378–384. [Google Scholar] [CrossRef]
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément, C. Trehalose and plant stress responses: Friend or foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef]
- Lyu, J.I.; Park, J.H.; Kim, J.-K.; Bae, C.-H.; Jeong, W.-J.; Min, S.R.; Liu, J.R. Enhanced tolerance to heat stress in transgenic tomato seeds and seedlings overexpressing a trehalose-6-phosphate synthase/phosphatase fusion gene. Plant Biotechnol. Rep. 2018, 12, 399–408. [Google Scholar] [CrossRef]
- Nuccio, M.L.; Wu, J.; Mowers, R.; Zhou, H.-P.; Meghji, M.; Primavesi, L.F.; Paul, M.J.; Chen, X.; Gao, Y.; Haque, E.; et al. Expression of trehalose-6-phosphate phosphatase in maize ears improves yield in well-watered and drought conditions. Nat. Biotechnol. 2015, 33, 862–869. [Google Scholar] [CrossRef]
- Thiel, J.; Rolletschek, H.; Friedel, S.; E Lunn, J.; Nguyen, T.H.; Feil, R.; Tschiersch, H.; Müller, M.; Borisjuk, L. Seed-specific elevation of non-symbiotic hemoglobin AtHb1: Beneficial effects and underlying molecular networks in Arabidopsis thaliana. BMC Plant Biol. 2011, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.B.; Engler, J.; Iyer, S.; Gerats, T.; Van Montagu, M.; Caplan, A.B. Effects of osmoprotectants upon NaCl stress in rice. Plant Physiol. 1997, 115, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, R. Amelioration of postharvest chilling stress by trehalose in pepper. Sci. Hortic. 2018, 232, 52–56. [Google Scholar] [CrossRef]
- Jang, I.-C.; Oh, S.-J.; Seo, J.-S.; Choi, W.-B.; Song, S.I.; Kim, C.H.; Kim, Y.S.; Seo, H.-S.; Choi, Y.D.; Nahm, B.H.; et al. Expression of a bifunctional fusion of the Escherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth. Plant Physiol. 2003, 131, 516–524. [Google Scholar] [CrossRef]
- Pin, P.A.; Nilsson, O. The multifaceted roles of FLOWERING LOCUS T in plant development. Plant Cell Environ. 2012, 35, 1742–1755. [Google Scholar] [CrossRef]
- Navarro, C.; Abelenda, J.A.; Cruz-Oró, E.; Cuéllar, C.A.; Tamaki, S.; Silva, J.; Shimamoto, K.; Prat, S. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 2011, 478, 119–122. [Google Scholar] [CrossRef]
- Lehretz, G.G.; Sonnewald, S.; Lugassi, N.; Granot, D.; Sonnewald, U. Future-proofing potato for drought and heat tolerance by overexpression of hexokinase and SP6A. Front. Plant Sci. 2020, 11, 614534. [Google Scholar] [CrossRef]
- Hastilestari, B.R.; Lorenz, J.; Reid, S.; Hofmann, J.; Pscheidt, D.; Sonnewald, U.; Sonnewald, S. Deciphering source and sink responses of potato plants (Solanum tuberosum L.) to elevated temperatures. Plant Cell Environ. 2018, 41, 2600–2616. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.; Terry, N.; Sears, T.; Kim, H.; Zayed, A.; Hwang, S.; van Dun, K.; Voogd, E.; Verwoerd, T.C.; Krutwagen, R.W.; et al. Trehalose-producing transgenic tobacco plants show improved growth performance under drought stress. J. Plant Physiol. 1998, 152, 525–532. [Google Scholar] [CrossRef]
- Bianchi, G.; Gamba, A.; Limiroli, R.; Pozzi, N.; Elster, R.; Salamini, F.; Bartels, D. The unusual sugar composition in leaves of the resurrection plant Myrothamnus flabellifolia. Physiol. Plant. 1993, 87, 223–226. [Google Scholar] [CrossRef]
- Goddijn, O.J.M.; van Dun, K. Trehalose metabolism in plants. Trends Plant Sci. 1999, 4, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Iordachescu, M.; Imai, R. Trehalose biosynthesis in response to abiotic stresses. J. Integr. Plant Biol. 2008, 50, 1223–1229. [Google Scholar] [CrossRef]
- Zeidler, S.; Hubloher, J.; Schabacker, K.; Lamosa, P.; Santos, H.; Müller, V. Trehalose, a temperature- and salt-induced solute with implications in pathobiology of Acinetobacter baumannii. Environ. Microbiol. 2017, 19, 5088–5099. [Google Scholar] [CrossRef] [PubMed]
- Giaever, H.M.; Styrvold, O.B.; Kaasen, I.; Strøm, A.R. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J. Bacteriol. 1988, 170, 2841–2849. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, A.M.; Barth, J.X.; Hahn, M.C.P.; Scarlett, C.O.; Genin, S.; Allen, C. Trehalose synthesis contributes to osmotic stress tolerance and virulence of the bacterial wilt pathogen Ralstonia solanacearum. Mol. Plant-Microbe Interact. 2019, 33, 462–473. [Google Scholar] [CrossRef]
- Woodcock, S.D.; Syson, K.; Little, R.H.; Ward, D.; Sifouna, D.; Brown, J.K.M.; Bornemann, S.; Malone, J.G. Trehalose and α-glucan mediate distinct abiotic stress responses in Pseudomonas aeruginosa. PLoS Genet. 2021, 17, e1009524. [Google Scholar] [CrossRef]
- Al-Naama, M.; Ewaze, J.O.; Green, B.J.; Scott, J.A. Trehalose accumulation in Baudoinia compniacensis following abiotic stress. Int. Biodeterior. Biodegradation 2009, 63, 765–768. [Google Scholar] [CrossRef]
- Liu, X.-M.; Wu, X.-L.; Gao, W.; Qu, J.-B.; Chen, Q.; Huang, C.-Y.; Zhang, J.-X. Protective roles of trehalose in Pleurotus pulmonarius during heat stress response. J. Integr. Agric. 2019, 18, 428–437. [Google Scholar] [CrossRef]
- Ocón, A.; Hampp, R.; Requena, N. Trehalose turnover during abiotic stress in arbuscular mycorrhizal fungi. New Phytol. 2007, 174, 879–891. [Google Scholar] [CrossRef]
- Penna, S. Building stress tolerance through over-producing trehalose in transgenic plants. Trends Plant Sci. 2003, 8, 355–357. [Google Scholar] [CrossRef]
- Bae, H.; Herman, E.; Bailey, B.; Bae, H.J.; Sicher, R. Exogenous trehalose alters Arabidopsis transcripts involved in cell wall modification, abiotic stress, nitrogen metabolism, and plant defense. Physiol. Plant. 2005, 125, 114–126. [Google Scholar] [CrossRef]
- Vanaporn, M.; Titball, R.W. Trehalose and bacterial virulence. Virulence 2020, 11, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Petzold, E.W.; Himmelreich, U.; Mylonakis, E.; Rude, T.; Toffaletti, D.; Cox, G.M.; Miller, J.L.; Perfect, J.R. Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infect. Immun. 2006, 74, 5877–5887. [Google Scholar] [CrossRef]
- Puttikamonkul, S.; Willger, S.D.; Grahl, N.; Perfect, J.R.; Movahed, N.; Bothner, B.; Park, S.; Paderu, P.; Perlin, D.S.; Cramer, R.A., Jr. Trehalose 6-phosphate phosphatase is required for cell wall integrity and fungal virulence but not trehalose biosynthesis in the human fungal pathogen Aspergillus fumigatus. Mol. Microbiol. 2010, 77, 891–911. [Google Scholar] [CrossRef]
- Djonović, S.; Urbach, J.M.; Drenkard, E.; Bush, J.; Feinbaum, R.; Ausubel, J.L.; Traficante, D.; Risech, M.; Kocks, C.; Fischbach, M.A.; et al. Trehalose biosynthesis promotes Pseudomonas aeruginosa pathogenicity in plants. PLoS Pathog. 2013, 9, e1003217. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, Y.; Yu, D. Trehalose phosphate synthase 5-dependent trehalose metabolism modulates basal defense responses in Arabidopsis thaliana. J. Integr. Plant Biol. 2019, 61, 509–527. [Google Scholar] [CrossRef]
- Tayeh, C.; Randoux, B.; Vincent, D.; Bourdon, N.; Reignault, P. Exogenous trehalose induces defenses in wheat before and during a biotic stress caused by powdery mildew. Phytopathology 2013, 104, 293–305. [Google Scholar] [CrossRef]
- Louis, J.; Shah, J. Arabidopsis thaliana—Myzus persicae interaction: Shaping the understanding of plant defense against phloem-feeding aphids. Front. Plant Sci. 2013, 4, 00213. [Google Scholar] [CrossRef]
- Singh, V.; Louis, J.; Ayre, B.G.; Reese, J.C.; Shah, J. TREHALOSE PHOSPHATE SYNTHASE11-dependent trehalose metabolism promotes Arabidopsis thaliana defense against the phloem-feeding insect Myzus persicae. Plant J. 2011, 67, 94–104. [Google Scholar] [CrossRef]
- Singh, V.; Shah, J. Tomato responds to green peach aphid infestation with the activation of trehalose metabolism and starch accumulation. Plant Signal. Behav. 2012, 7, 605–607. [Google Scholar] [CrossRef][Green Version]
- Gibson, S.I. Control of plant development and gene expression by sugar signaling. Curr. Opin. Plant Biol. 2005, 8, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Veyres, N.; Danon, A.; Aono, M.; Galliot, S.; Karibasappa, Y.B.; Diet, A.; Grandmottet, F.; Tamaoki, M.; Lesur, D.; Pilard, S.; et al. The Arabidopsis sweetie mutant is affected in carbohydrate metabolism and defective in the control of growth, development and senescence. Plant J. 2008, 55, 665–686. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, T.; Yu, X.; Yang, Y.; Wang, C.; Yang, Q.; Wang, X. Enhanced sugar accumulation and regulated plant hormone signalling genes contribute to cold tolerance in hypoploid Saccharum spontaneum. BMC Genom. 2020, 21, 507. [Google Scholar] [CrossRef] [PubMed]
- Cortina, C.; Culiáñez-Macià, F.A. Tomato abiotic stress enhanced tolerance by trehalose biosynthesis. Plant Sci. 2005, 169, 75–82. [Google Scholar] [CrossRef]
- Romero, C.; Bellés, J.M.; Vayá, J.L.; Serrano, R.; Culiáñez-Macià, F.A. Expression of the yeast trehalose-6-phosphate synthase gene in transgenic tobacco plants: Pleiotropic phenotypes include drought tolerance. Planta 1997, 201, 293–297. [Google Scholar] [CrossRef]
- Gabriel, C.; Fernhout, J.; Fichtner, F.; Feil, R.; Lunn, J.E.; Kossmann, J.; Lloyd, J.R.; van der Vyver, C. Genetic manipulation of trehalose-6-phosphate synthase results in changes in the soluble sugar profile in transgenic sugarcane stems. Plant Direct 2021, 5, e358. [Google Scholar] [CrossRef]
- Meitzel, T.; Radchuk, R.; McAdam, E.L.; Thormählen, I.; Feil, R.; Munz, E.; Hilo, A.; Geigenberger, P.; Ross, J.J.; Lunn, J.E.; et al. Trehalose 6-phosphate promotes seed filling by activating auxin biosynthesis. New Phytol. 2021, 229, 1553–1565. [Google Scholar] [CrossRef]
- Logan, B.A.; Monson, R.K.; Potosnak, M.J. Biochemistry and physiology of foliar isoprene production. Trends Plant Sci. 2000, 5, 477–481. [Google Scholar] [CrossRef]
- Kerstiens, G.; Possell, M. Is competence for isoprene emission related to the mode of phloem loading? New Phytol. 2001, 152, 368–372. [Google Scholar] [CrossRef]
- Balderrama-Carmona, A.P.; Silva-Beltrán, N.P.; Alvarez, L.A.Z.; Bante, N.P.A.; Palacio, E.F.M. Consequences of herbicide use in rural environments and their effect on agricultural workers. In Sustainability Concept in Developing Countries; Kulsreshta, S.N., Ed.; IntechOpen: London, UK, 2020; pp. 53–66. [Google Scholar]
- Sullivan, T.P.; Sullivan, D.S. Vegetation management and ecosystem disturbance: Impact of glyphosate herbicide on plant and animal diversity in terrestrial systems. Environ. Rev. 2003, 11, 37–59. [Google Scholar] [CrossRef]
- Choung, C.B.; Hyne, R.V.; Stevens, M.M.; Hose, G.C. The ecological effects of a herbicide–insecticide mixture on an experimental freshwater ecosystem. Environ. Pollut. 2013, 172, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Morelli, G.; Ruberti, I. Light and shade in the photocontrol of Arabidopsis growth. Trends Plant Sci. 2002, 7, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Whitelam, G.C. The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant Cell Environ. 1997, 20, 840–844. [Google Scholar] [CrossRef]
- Griffith, T.M.; Sultan, S.E. Plastic and constant developmental traits contribute to adaptive differences in co-cccurring Polygonum species. Oikos 2006, 114, 5–14. [Google Scholar] [CrossRef]
- Franklin, K.A.; Quail, P.H. Phytochrome functions in Arabidopsis development. J. Exp. Bot. 2010, 61, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.C.; Strasser, B.; Cerdán, P.D.; Amasino, R.M. Acceleration of flowering during shade avoidance in Arabidopsis alters the balance between Flowering Locus C-mediated repression and photoperiodic induction of flowering. Plant Physiol. 2008, 148, 1681–1694. [Google Scholar] [CrossRef]
- Lymperopoulos, P.; Msanne, J.; Rabara, R. Phytochrome and phytohormones: Working in tandem for plant growth and development. Front. Plant Sci. 2018, 9, 1037. [Google Scholar] [CrossRef]
- Spies, J.M.; Warkentin, T.D.; Shirtliffe, S.J. Variation in field pea (Pisum sativum) cultivars for basal branching and weed competition. Weed Sci. 2011, 59, 218–223. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Fischer, M. Adaptive evolution of plastic foraging responses in a clonal plant. Ecology 2001, 82, 3309–3319. [Google Scholar] [CrossRef]
- Donohue, K.; Pyle, E.H.; Messiqua, D.; Heschel, M.S.; Schmitt, J. Density dependence and population differentiation of genetic architecture in Impatiens capensis in natural environments. Evolution 2000, 54, 1969–1981. [Google Scholar]
- Crawford, S.; Shinohara, N.; Sieberer, T.; Williamson, L.; George, G.; Hepworth, J.; Müller, D.; Domagalska, M.A.; Leyser, O. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 2010, 137, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Flynn, H.C.; Smith, J.; Smith, K.A.; Wright, J.; Smith, P.; Massheder, J. Climate- and crop-responsive emission factors significantly alter estimates of current and future nitrous oxide emissions from fertilizer use. Glob. Chang. Biol. 2005, 11, 1522–1536. [Google Scholar] [CrossRef]
- Byrnes, B.H. Environmental effects of N fertilizer use—An overview. Fertil. Res. 1990, 26, 209–215. [Google Scholar] [CrossRef]
- Khan, M.N.; Mohammad, F. Eutrophication: Challenges and solutions. In Eutrophication: Causes, Consequences and Control; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–15. [Google Scholar]
- Withers, P.J.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and eutrophication: Where do we go from here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef]
- Snyder, C.; Bruulsema, T.; Jensen, T.; Fixen, P. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Gleń-Karolczyk, K.; Boligłowa, E.; Antonkiewicz, J. Organic fertilization shapes the biodiversity of fungal communities associated with potato dry rot. Appl. Soil Ecol. 2018, 129, 43–51. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Optimizing nitrogen fertilization to improve qualitative performances and physiological and yield responses of potato (Solanum tuberosum L.). Agronomy 2020, 10, 352–365. [Google Scholar] [CrossRef]
- Zebarth, B.J.; Bélanger, G.; Cambouris, A.N.; Ziadi, N. Nitrogen Fertilization Strategies in Relation to Potato Tuber Yield Quality, and Crop N Recovery. In Sustainable Potato Production: Global Case Studies; dans He, Z., Larkin, R., Honeycutt, W., Eds.; Springer: New York, NY, USA, 2012; pp. 165–186. [Google Scholar]
- Setayesh, R.; Kafi, M.; Mehrjerdi, M.Z. Low sensitivity to photoperiod may increase potato yield in short day through the maintenance of sink and source balance. Pak. J. Bot. 2017, 49, 929–933. [Google Scholar]
- Engler, C.; Youles, M.; Gruetzner, R.; Ehnert, T.-M.; Werner, S.; Jones, J.D.G.; Patron, N.J.; Marillonnet, S. A Golden Gate modular cloning toolbox for plants. ACS Synth. Biol. 2014, 3, 839–843. [Google Scholar] [CrossRef]
- Chronis, D.; Chen, S.; Lang, P.; Tran, T.; Thurston, D.; Wang, X. Potato transformation. Bio-Protocol 2014, 4, e1017. [Google Scholar] [CrossRef]
- OPS-Diagnostics. CTAB Protocol for Isolating DNA from Plant Tissues; OPS-Diagnostics: Lebanon, NJ, USA, 2019. [Google Scholar]
- Mellars, G.; Gomez, K. Mutation detection by Southern blotting. Methods Mol. Biol. 2011, 688, 281–291. [Google Scholar] [PubMed]
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive responses of potato (Solanum tuberosum L.) plants to heat stress and infection with potato virus Y. Front. Microbiol. 2018, 9, 2582. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Package “Emmeans”. R Package Version 4.0-3. 2020. [Google Scholar]



| (a) | ||||
| Fixed Effect | Df | Number of Nodes | Height | Shoot Fresh Weight |
| LR ChiSq | LR ChiSq | LR ChiSq | ||
| Genotype | 10 | 11.626 | 96.44 *** | 50.773 *** |
| Treatment | 2 | 102.861 *** | 598.55 *** | 69.38 *** |
| Genotype × Treatment | 20 | 13.428 *** | 61.95 *** | 40.574 * |
| (b) | ||||
| Fixed Effect | Df | Number of Tubers | Tuber Fresh Weight | |
| LR ChiSq | LR ChiSq | |||
| Genotype | 10 | 5.5162 | 67.035 *** | |
| Treatment | 2 | 0.7014 | 225.176 *** | |
| Genotype × Treatment | 20 | 19.3275 | 64.806 *** | |
| Stem Number | Stem Mass | Biomass | Tuber Number | Tuber Mass | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed effect | LR Chisq | LR Chisq | LR Chisq | LR Chisq | LR Chisq | |||||
| Geno | 12.41 | *** | 2.72 | 0.01 | 5.61 | * | 0.22 | |||
| Competition | 21.37 | *** | 0.98 | 29.63 | *** | 38.24 | *** | 3.95 | * | |
| Geno x Competition | 2.1 | 4.31 | * | 1.74 | 0.5 | 0.91 | ||||
| Number of Shoots | Aboveground Biomass | Number of Tubers | Tuber Mass | |
|---|---|---|---|---|
| Fixed effect | LR Chisq | LR Chisq | LR Chisq | LR Chisq |
| Geno | 0.18 | 1.32 | 2.77 | 0.09 |
| Competition | 51.63 *** | 47.86 *** | 4.20 * | 2.14 |
| Nutrient | 0.43 | 0.48 | 49.40 *** | 19.02 *** |
| Geno × competition | 0.03 | 3.02 | 0.2 | 0.02 |
| Geno × nutrient | 6.10 * | 9.13 ** | 5.45 * | 1.46 |
| Competition × nutrient | 1 | 2.33 | 0.55 | 1.49 |
| Geno x competition × nutrient | 0.4 | 2.68 | 1.77 | 0.23 |
| Trait | Geno | Community | Estimate (SE) | ||
|---|---|---|---|---|---|
| Number of shoots | OtsB | Alone | −0.09 | (0.19) | |
| Number of shoots | OtsB | Neighbors | −0.49 | (0.29) | |
| Number of shoots | WT | Alone | 0.36 | (0.18) | + |
| Number of shoots | WT | Neighbors | 0.27 | (0.28) | |
| Biomass | OtsB | Alone | −32.05 | (23.70) | |
| Biomass | OtsB | Neighbors | −26.48 | (23.70) | |
| Biomass | WT | Alone | 74.36 | (21.94) | * |
| Biomass | WT | Neighbors | 5.10 | (21.94) | |
| Number of tubers | OtsB | Alone | −1.61 | (0.49) | * |
| Number of tubers | OtsB | Neighbors | −3.14 | (1.02) | * |
| Number of tubers | WT | Alone | −0.97 | (0.31) | * |
| Number of tubers | WT | Neighbors | −0.98 | (0.39) | * |
| Tuber mass | OtsB | Alone | −28.78 | (9.41) | * |
| Tuber mass | OtsB | Neighbors | −22.45 | (9.41) | * |
| Tuber mass | WT | Alone | −22.19 | (8.71) | * |
| Tuber mass | WT | Neighbors | −7.13 | (8.71) | |
| Gene Name | Abbreviation |
|---|---|
| Catalase | CAT |
| Elongation factor 1 alpha | EFI |
| Heat shock protein 30 | HSP30 |
| OtsB/trehalose-6-phosphate phosphatase | OtsB |
| StSP3D | 3D |
| StSP6A | 6A |
| 60S ribosomal protein L8 | L8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, B.L.; Kakeshpour, T.; Occhialini, A.; King, G.; Sichterman, M.; Harbison, S.A.; Rigoulot, S.B.; Brabazon, H.; Stewart, C.N., Jr.; Lenaghan, S.C. Heterologous Expression of OtsB Increases Tuber Yield and Phenotypic Stability in Potato under Both Abiotic and Biotic Stresses. Plants 2023, 12, 3394. https://doi.org/10.3390/plants12193394
Morgan BL, Kakeshpour T, Occhialini A, King G, Sichterman M, Harbison SA, Rigoulot SB, Brabazon H, Stewart CN Jr., Lenaghan SC. Heterologous Expression of OtsB Increases Tuber Yield and Phenotypic Stability in Potato under Both Abiotic and Biotic Stresses. Plants. 2023; 12(19):3394. https://doi.org/10.3390/plants12193394
Chicago/Turabian StyleMorgan, Britany Lauren, Tayebeh Kakeshpour, Alessandro Occhialini, Gabriella King, Megan Sichterman, Stacee A. Harbison, Stephen B. Rigoulot, Holly Brabazon, Charles Neal Stewart, Jr., and Scott C. Lenaghan. 2023. "Heterologous Expression of OtsB Increases Tuber Yield and Phenotypic Stability in Potato under Both Abiotic and Biotic Stresses" Plants 12, no. 19: 3394. https://doi.org/10.3390/plants12193394
APA StyleMorgan, B. L., Kakeshpour, T., Occhialini, A., King, G., Sichterman, M., Harbison, S. A., Rigoulot, S. B., Brabazon, H., Stewart, C. N., Jr., & Lenaghan, S. C. (2023). Heterologous Expression of OtsB Increases Tuber Yield and Phenotypic Stability in Potato under Both Abiotic and Biotic Stresses. Plants, 12(19), 3394. https://doi.org/10.3390/plants12193394







