Botanical Collection Patterns and Conservation Categories of the Most Traded Timber Species from the Ecuadorian Amazon: The Role of Protected Areas
Abstract
:1. Introduction
2. Results
2.1. Collection Patterns of Harvested Timber Species from the Ecuadorian Amazon
2.2. Protection Coverage of Timber Species within Current Conservation Initiatives
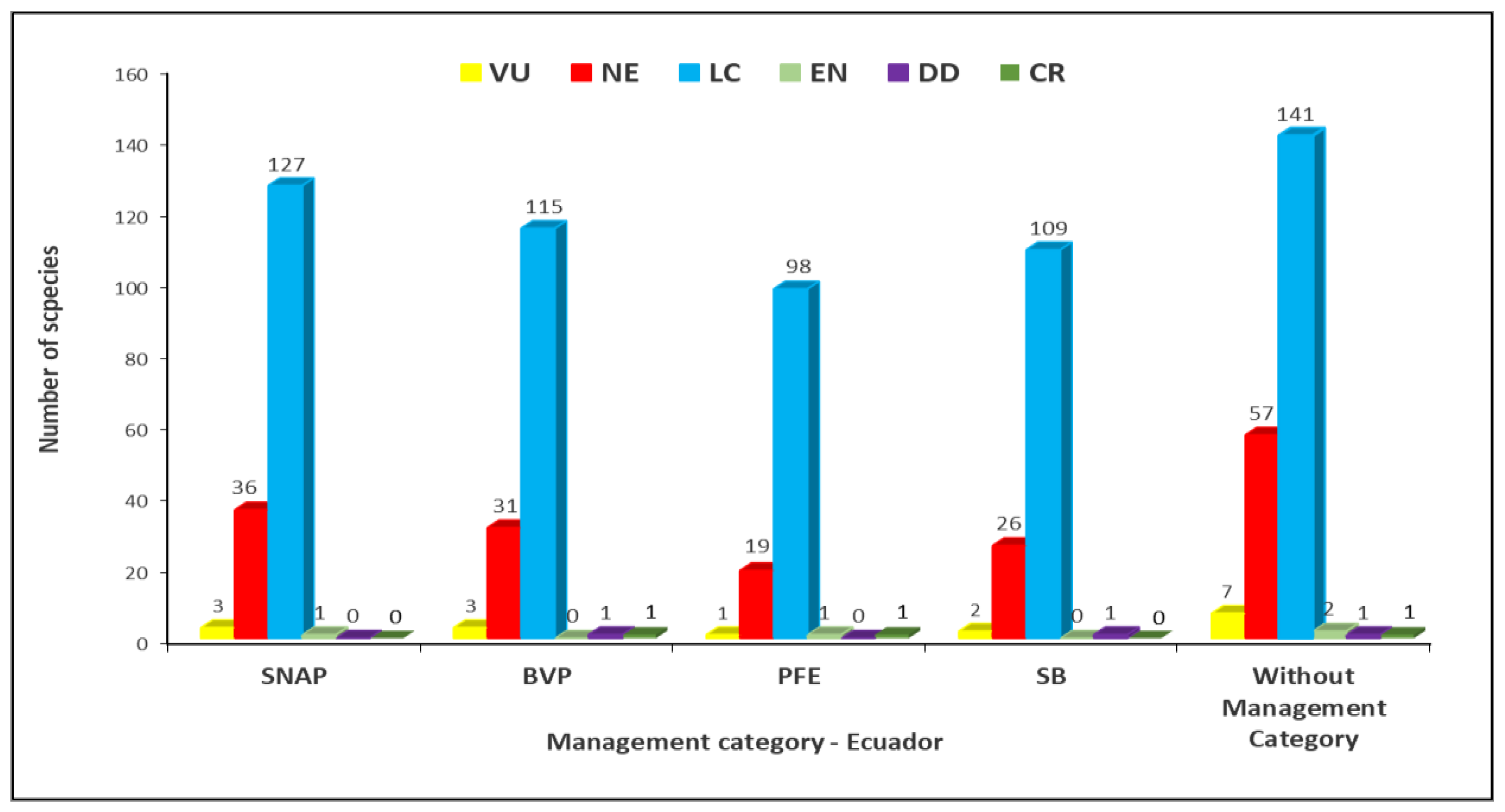
2.3. IUCN Conservation Categories for the Analyzed Timber Species
3. Discussion
3.1. Collection Patterns across Provinces
3.2. Collection Patterns and Coverage Extent: The Role of Protected Areas
3.3. IUCN Conservation Categories
3.4. Considerations for Current and Future Conservation of the Most Traded Timber Species in the EAR
3.4.1. Promotion of Botanical Sampling and Its Digitization in an Open Access Database
3.4.2. Current Tools to Assess the Preliminary Conservation Status of Species
4. Materials and Methods
4.1. Study Area
4.2. Selection of Forest Species with Harvesting Records in the EAR
4.3. Patterns of Collection of Timber Species Used in the EAR
4.4. Protection Coverage within the Current Conservation Initiatives in Ecuador
4.5. IUCN Conservation Categories for the Analyzed Timber Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cazzolla Gatti, R.; Reich, P.B.; Gamarra, J.G.P.; Crowther, T.; Hui, C.; Morera, A.; Bastin, J.-F.; De-Miguel, S.; Nabuurs, G.-J.; Svenning, J.-C. The Number of Tree Species on Earth. Proc. Natl. Acad. Sci. USA 2022, 119, e2115329119. [Google Scholar] [CrossRef]
- Raven, P.H.; Gereau, R.E.; Phillipson, P.B.; Chatelain, C.; Jenkins, C.N.; Ulloa Ulloa, C. The Distribution of Biodiversity Richness in the Tropics. Sci. Adv. 2020, 6, eabc6228. [Google Scholar] [CrossRef]
- Comer, P.J.; Valdez, J.; Pereira, H.M.; Acosta-Muñoz, C.; Campos, F.; Bonet García, F.J.; Claros, X.; Castro, L.; Dallmeier, F.; Domic Rivadeneira, E.Y. Conserving Ecosystem Diversity in the Tropical Andes. Remote Sens. 2022, 14, 2847. [Google Scholar] [CrossRef]
- Hu, X.; Huang, B.; Verones, F.; Cavalett, O.; Cherubini, F. Overview of Recent Land-cover Changes in Biodiversity Hotspots. Front. Ecol. Environ. 2021, 19, 91–97. [Google Scholar] [CrossRef]
- Ulloa Ulloa, C.; Acevedo-Rodríguez, P.; Beck, S.; Belgrano, M.J.; Bernal, R.; Berry, P.E.; Brako, L.; Celis, M.; Davidse, G.; Forzza, R.C. An Integrated Assessment of the Vascular Plant Species of the Americas. Science 2017, 358, 1614–1617. [Google Scholar] [CrossRef]
- Tirira, D.G. La Conservación de Mamíferos En El Ecuador: The Mammals Conservation in Ecuador. Mamm. Aequat. 2021, 3, 7–8. [Google Scholar] [CrossRef]
- Ortega-Andrade, H.M.; Rodes Blanco, M.; Cisneros-Heredia, D.F.; Guerra Arévalo, N.; López de Vargas-Machuca, K.G.; Sánchez-Nivicela, J.C.; Armijos-Ojeda, D.; Cáceres Andrade, J.F.; Reyes-Puig, C.; Quezada Riera, A.B. Red List Assessment of Amphibian Species of Ecuador: A Multidimensional Approach for Their Conservation. PLoS ONE 2021, 16, e0251027. [Google Scholar] [CrossRef]
- Reyes-Puig, C.; Almendáriz, A.; Torres-Carvajal, O. Diversity, Threat, and Conservation of Reptiles from Continental Ecuador. Amphib. Reptile Conserv. 2017, 11, 51–58. [Google Scholar]
- Freile, J.F.; Santander, T.; Carrasco, L.; Cisneros-Heredia, D.F.; Guevara, E.A.; Sánchez-Nivicela, M.; Tinoco, B.A. Lista Roja de Las Aves Del Ecuador Continental; Ministerio del Ambiente, Aves y Conservación, Comité Ecuatoriano de Registros Ornitológicos, Universidad del Azuay, Red Aves Ecuador y Universidad San Francisco de Quito: Quito, Ecuador, 2019. [Google Scholar]
- MAATE Sistema Nacional de Indicadores Ambientales y Sostenibilidad (SINIAS). Available online: http://sinias.ambiente.gob.ec:8099/proyecto-sinias-web/estadisticasAmbientales.jsf?menu=01 (accessed on 5 July 2023).
- Bass, M.S.; Finer, M.; Jenkins, C.N.; Kreft, H.; Cisneros-Heredia, D.F.; McCracken, S.F.; Pitman, N.C.A.; English, P.H.; Swing, K.; Villa, G. Global Conservation Significance of Ecuador’s Yasuní National Park. PLoS ONE 2010, 5, e8767. [Google Scholar] [CrossRef]
- Cardoso, D.; Sarkinen, T.; Alexander, S.; Amorim, A.M.; Bittrich, V.; Celis, M.; Daly, D.C.; Fiaschi, P.; Funk, V.A.; Giacomin, L.L.; et al. Amazon Plant Diversity Revealed by a Taxonomically Verified Species List. Proc. Natl. Acad. Sci. USA 2017, 114, 10695–10700. [Google Scholar] [CrossRef]
- Lozano, P.; Cabrera, O.; Peyre, G.; Cleef, A.; Toulkeridis, T. Plant Diversity and Composition Changes along an Altitudinal Gradient in the Isolated Volcano Sumaco in the Ecuadorian Amazon. Diversity 2020, 12, 229. [Google Scholar] [CrossRef]
- Valencia, R.; Balslev, H. High Tree Alpha-Diversity in Amazonian Ecuador. Biodivers. Conserv. 1994, 3, 21–28. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Pitman, N.C.A.; Terborgh, J.W.; Silman, M.R.; Núñez, V.P.; Neill, D.A.; Cerón, C.E.; Palacios, W.A.; Aulestia, M. A Comparison of Tree Species Diversity in Two Upper Amazonian Forests. Ecology 2002, 83, 3210–3224. [Google Scholar] [CrossRef]
- Kreft, H.; Köster, N.; Küper, W.; Nieder, J.; Barthlott, W. Diversity and Biogeography of Vascular Epiphytes in Western Amazonia, Yasuní, Ecuador. J. Biogeogr. 2004, 31, 1463–1476. [Google Scholar] [CrossRef]
- Ter Steege, H.; Pitman, N.C.A.; Sabatier, D.; Baraloto, C.; Salomão, R.P.; Guevara, J.E.; Phillips, O.L.; Castilho, C.V.; Magnusson, W.E.; Molino, J.-F. Hyperdominance in the Amazonian Tree Flora. Science 2013, 342, 1243092. [Google Scholar] [CrossRef]
- Guevara, J.E.; Mogollón, H.; Pitman, N.C.A.; Cerón, C.; Palacios, W.A.; Neill, D.A. A Floristic Assessment of Ecuador’s Amazon Tree Flora. For. Struct. Funct. Dyn. West. Amazon. 2017, 27–52. [Google Scholar]
- Torres, B.; Günter, S.; Acevedo-Cabra, R.; Knoke, T. Livelihood Strategies, Ethnicity and Rural Income: The Case of Migrant Settlers and Indigenous Populations in the Ecuadorian Amazon. For. Policy Econ. 2018, 86, 22–34. [Google Scholar] [CrossRef]
- Vasco, C.; Torres, B.; Pacheco, P.; Griess, V. The Socioeconomic Determinants of Legal and Illegal Smallholder Logging: Evidence from the Ecuadorian Amazon. For. Policy Econ. 2017, 78, 133–140. [Google Scholar] [CrossRef]
- Torres, B.; Cayambe, J.; Paz, S.; Ayerve, K.; Heredia-R, M.; Torres, E.; Luna, M.; Toulkeridis, T.; García, A. Livelihood Capitals, Income Inequality, and the Perception of Climate Change: A Case Study of Small-Scale Cattle Farmers in the Ecuadorian Andes. Sustainability 2022, 14, 5028. [Google Scholar] [CrossRef]
- Torres, B.; Eche, D.; Torres, Y.; Bravo, C.; Velasco, C.; García, A. Identification and Assessment of Livestock Best Management Practices (BMPs) Using the REDD+ Approach in the Ecuadorian Amazon. Agronomy 2021, 11, 1336. [Google Scholar] [CrossRef]
- Torres, B.; Espinoza, Í.; Torres, A.; Herrera-Feijoo, R.; Luna, M.; García, A. Livelihood Capitals and Opportunity Cost for Grazing Areas’ Restoration: A Sustainable Intensification Strategy in the Ecuadorian Amazon. Animals 2023, 13, 714. [Google Scholar] [CrossRef]
- Gordillo, F.; Torres, B.; Tamayo, F. Sobre La Disposición Al Pago de Hogares Ecuatorianos Para La Conservación Forestal En Ecuador. In Deforestación en Paisajes Tropicales del Ecuador: Bases Científicas Para Perspectivas Políticas; INABIO: Quito, Ecuador, 2020; pp. 144–150. ISBN 978-9942-932-334. [Google Scholar]
- Krause, T.; Loft, L. Benefit Distribution and Equity in Ecuador’s Socio Bosque Program. Soc. Nat. Resour. 2013, 26, 1170–1184. [Google Scholar] [CrossRef]
- Sierra, R.; Calva, O.; Guevara, A. La Deforestación En El Ecuador, 1990–2018. In Factores Promotores y Tendencias Recientes; ProAmazonía: Quito, Ecuador, 2021. [Google Scholar]
- Sierra, R. Patrones y Factores de Deforestación En El Ecuador Continental, 1990–2010. Y Un Acercamiento a Los Próximos 10 Años. Conserv. Int. Ecuad. For. Trends Quito 2013, 10, 57. [Google Scholar]
- Bilsborrow, R.E.; Barbieri, A.F.; Pan, W. Changes in Population and Land Use over Time in the Ecuadorian Amazon. Acta Amaz. 2004, 34, 635–647. [Google Scholar] [CrossRef]
- Kleemann, J.; Zamora, C.; Villacis-Chiluisa, A.B.; Cuenca, P.; Koo, H.; Noh, J.K.; Fürst, C.; Thiel, M. Deforestation in Continental Ecuador with a Focus on Protected Areas. Land 2022, 11, 268. [Google Scholar] [CrossRef]
- Noh, J.K.; Echeverria, C.; Gaona, G.; Kleemann, J.; Koo, H.; Fürst, C.; Cuenca, P. Forest Ecosystem Fragmentation in Ecuador: Challenges for Sustainable Land Use in the Tropical Andean. Land 2022, 11, 287. [Google Scholar] [CrossRef]
- Fischer, R.; Tamayo Cordero, F.; Ojeda Luna, T.; Ferrer Velasco, R.; DeDecker, M.; Torres, B.; Giessen, L.; Günter, S. Interplay of Governance Elements and Their Effects on Deforestation in Tropical Landscapes: Quantitative Insights from Ecuador. World Dev. 2021, 148, 105665. [Google Scholar] [CrossRef]
- Tapia-Armijos, M.F.; Homeier, J.; Espinosa, C.I.; Leuschner, C.; de la Cruz, M. Deforestation and Forest Fragmentation in South Ecuador since the 1970s–Losing a Hotspot of Biodiversity. PLoS ONE 2015, 10, e0133701. [Google Scholar] [CrossRef]
- Tapia-Armijos, M.F.; Homeier, J.; Munt, D.D. Spatio-Temporal Analysis of the Human Footprint in South Ecuador: Influence of Human Pressure on Ecosystems and Effectiveness of Protected Areas. Appl. Geogr. 2017, 78, 22–32. [Google Scholar] [CrossRef]
- Castillo Vizuete, D.D.; Gavilanes Montoya, A.V.; Chávez Velásquez, C.R.; Borz, S.A. A Critical Review on the Perspectives of the Forestry Sector in Ecuador. Land 2023, 12, 258. [Google Scholar] [CrossRef]
- Wasserstrom, R.; Southgate, D. Deforestación, Reforma Agraria y Desarrollo Petrolero En Ecuador, 1964–1994. Nat. Resour. 2013, 4, 34–44. [Google Scholar]
- Mejía, E.; Pacheco, P. Forest Use and Timber Markets in the Ecuadorian Amazon; CIFOR: Bogor, Indonesia, 2014; Volume 111, ISBN 6021504143. [Google Scholar]
- Guevara Andino, J.E.; Pitman, N.C.A.; Ulloa Ulloa, C.; Romoleroux, K.; Fernández-Fernández, D.; Ceron, C.; Palacios, W.; Neill, D.A.; Oleas, N.; Altamirano, P. Trees of Amazonian Ecuador: A Taxonomically Verified Species List with Data on Abundance and Distribution. Ecology 2019, 100, e02894. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Pearse, W.D.; Dalrymple, R.L.; Zanne, A.E. What We (Don’t) Know about Global Plant Diversity. Ecography 2019, 42, 1819–1831. [Google Scholar] [CrossRef]
- Nic Lughadha, E.; Walker, B.E.; Canteiro, C.; Chadburn, H.; Davis, A.P.; Hargreaves, S.; Lucas, E.J.; Schuiteman, A.; Williams, E.; Bachman, S.P. The Use and Misuse of Herbarium Specimens in Evaluating Plant Extinction Risks. Philos. Trans. R. Soc. B 2019, 374, 20170402. [Google Scholar] [CrossRef]
- Lang, P.L.M.; Willems, F.M.; Scheepens, J.F.; Burbano, H.A.; Bossdorf, O. Using Herbaria to Study Global Environmental Change. New Phytol. 2019, 221, 110–122. [Google Scholar] [CrossRef]
- Meineke, E.K.; Davis, C.C.; Davies, T.J. The Unrealized Potential of Herbaria for Global Change Biology. Ecol. Monogr. 2018, 88, 505–525. [Google Scholar] [CrossRef]
- Cuesta, F.; Peralvo, M.; Merino-Viteri, A.; Bustamante, M.; Baquero, F.; Freile, J.F.; Muriel, P.; Torres-Carvajal, O. Priority Areas for Biodiversity Conservation in Mainland Ecuador. Neotrop. Biodivers. 2017, 3, 93–106. [Google Scholar] [CrossRef]
- Mestanza-Ramón, C.; Henkanaththegedara, S.M.; Vásconez Duchicela, P.; Vargas Tierras, Y.; Sánchez Capa, M.; Constante Mejía, D.; Jimenez Gutierrez, M.; Charco Guamán, M.; Mestanza Ramón, P. In-Situ and Ex-Situ Biodiversity Conservation in Ecuador: A Review of Policies, Actions and Challenges. Diversity 2020, 12, 315. [Google Scholar] [CrossRef]
- Mestanza-Ramón, C.; Monar-Nuñez, J.; Guala-Alulema, P.; Montenegro-Zambrano, Y.; Herrera-Chávez, R.; Milanes, C.B.; Arguello-Guadalupe, C.; Buñay-Guisñan, P.; Toledo-Villacís, M. A Review to Update the Protected Areas in Ecuador and an Analysis of Their Main Impacts and Conservation Strategies. Environments 2023, 10, 79. [Google Scholar] [CrossRef]
- Nic Lughadha, E.; Bachman, S.P.; Leão, T.C.C.; Forest, F.; Halley, J.M.; Moat, J.; Acedo, C.; Bacon, K.L.; Brewer, R.F.A.; Gâteblé, G. Extinction Risk and Threats to Plants and Fungi. Plants People Planet 2020, 2, 389–408. [Google Scholar] [CrossRef]
- IUCN IUCN Red List. Available online: https://www.iucnredlist.org/ (accessed on 10 July 2023).
- Neill, D.A. ¿ Cuantas Especies Nativas de Plantas Vasculares Hay En Ecuador? Rev. Amaz. Cienc. Tecnol. 2012, 1, 70–83. [Google Scholar] [CrossRef]
- Torres, B.; Vasseur, L.; López, R.; Lozano, P.; García, Y.; Arteaga, Y.; Bravo, C.; Barba, C.; García, A. Structure and above Ground Biomass along an Elevation Small-Scale Gradient: Case Study in an Evergreen Andean Amazon Forest, Ecuador. Agrofor. Syst. 2020, 94, 1235–1245. [Google Scholar] [CrossRef]
- García-Cox, W.; López-Tobar, R.; Herrera-Feijoo, R.J.; Tapia, A.; Heredia-R, M.; Toulkeridis, T.; Torres, B. Floristic Composition, Structure, and Aboveground Biomass of the Moraceae Family in an Evergreen Andean Amazon Forest, Ecuador. Forests 2023, 14, 1406. [Google Scholar] [CrossRef]
- Zizka, A.; Antonelli, A.; Silvestro, D. Sampbias, a Method for Quantifying Geographic Sampling Biases in Species Distribution Data. Ecography 2021, 44, 25–32. [Google Scholar] [CrossRef]
- Stropp, J.; Umbelino, B.; Correia, R.A.; Campos-Silva, J.V.; Ladle, R.J.; Malhado, A.C.M. The Ghosts of Forests Past and Future: Deforestation and Botanical Sampling in the Brazilian Amazon. Ecography 2020, 43, 979–989. [Google Scholar] [CrossRef]
- Herrera-Feijoo, R.J.; Torres, B.; López-Tobar, R.; Tipán-Torres, C.; Toulkeridis, T.; Heredia-R, M.; Mateo, R.G. Modelling Climatically Suitable Areas for Mahogany (Swietenia Macrophylla King) and Their Shifts across Neotropics: The Role of Protected Areas. Forests 2023, 14, 385. [Google Scholar] [CrossRef]
- Jumbo, E.; Avila, A.; Herrera Feijoo, R.J.; Chicaiza Ortiz, Á.F.; Morocho Cuenca, M.; Chicaiza Ortiz, C.D. Evaluación de La Biodiversidad, Amenazas y Estatus de Conservación de La Flora y Fauna Del Bosque Petrificado Puyango. Green World J. 2021, 4, 18. [Google Scholar] [CrossRef]
- de Araujo, M.L.; Ramos, F.N. Targeting the Survey Efforts: Gaps and Biases in Epiphyte Sampling at a Biodiversity Hotspot. For. Ecol. Manag. 2021, 498, 119544. [Google Scholar] [CrossRef]
- Neill, D.A.; Palacios, W.A. Árboles de La Amazonía Ecuatoriana; Ministerio de Agricultura y Ganadería: Quito, Ecuador, 1989. [Google Scholar]
- Enquist, B.J.; Feng, X.; Boyle, B.; Maitner, B.; Newman, E.A.; Jørgensen, P.M.; Roehrdanz, P.R.; Thiers, B.M.; Burger, J.R.; Corlett, R.T. The Commonness of Rarity: Global and Future Distribution of Rarity across Land Plants. Sci. Adv. 2019, 5, eaaz0414. [Google Scholar] [CrossRef]
- Engemann, K.; Enquist, B.J.; Sandel, B.; Boyle, B.; Jørgensen, P.M.; Morueta-Holme, N.; Peet, R.K.; Violle, C.; Svenning, J. Limited Sampling Hampers “Big Data” Estimation of Species Richness in a Tropical Biodiversity Hotspot. Ecol. Evol. 2015, 5, 807–820. [Google Scholar] [CrossRef]
- Hughes, A.C.; Orr, M.C.; Ma, K.; Costello, M.J.; Waller, J.; Provoost, P.; Yang, Q.; Zhu, C.; Qiao, H. Sampling Biases Shape Our View of the Natural World. Ecography 2021, 44, 1259–1269. [Google Scholar] [CrossRef]
- García-Roselló, E.; González-Dacosta, J.; Lobo, J.M. The Biased Distribution of Existing Information on Biodiversity Hinders Its Use in Conservation, and We Need an Integrative Approach to Act Urgently. Biol. Conserv. 2023, 283, 110118. [Google Scholar] [CrossRef]
- Nabe-Nielsen, J. Diversity and Distribution of Lianas in a Neotropical Rain Forest, Yasuní National Park, Ecuador. J. Trop. Ecol. 2001, 17, 1–19. [Google Scholar] [CrossRef]
- Valencia, R.; Condit, R.; Foster, R.B.; Romoleroux, K.; Villa Munoz, G.; Svenning, J.-C.; Magard, E.; Bass, M.; Losos, E.C.; Balslev, H. Yasuni Forest Dynamics Plot, Ecuador. Trop. For. Divers. Dynamism Find. Large-Scale Plot Netw. 2004, 609, 620. [Google Scholar]
- Villarroel, C.; Alexander, F. Spatial Relations between Lianas and Trees in Yasuni National Park. Bachelor’s Thesis, Universidad de Investigación de Tecnología Experimental Yachay, Quito, Ecuador, 2022. [Google Scholar]
- Homeier, J.; Breckle, S.; Günter, S.; Rollenbeck, R.T.; Leuschner, C. Tree Diversity, Forest Structure and Productivity along Altitudinal and Topographical Gradients in a Species-rich Ecuadorian Montane Rain Forest. Biotropica 2010, 42, 140–148. [Google Scholar] [CrossRef]
- Ballesteros, J.L.; Bracco, F.; Cerna, M.; Vita Finzi, P.; Vidari, G. Ethnobotanical Research at the Kutukú Scientific Station, Morona-Santiago, Ecuador. BioMed Res. Int. 2016, 2016, 9105746. [Google Scholar] [CrossRef] [PubMed]
- Benítez, S. The Condor Bioreserve in Ecuador. Mt. Res. Dev. 2003, 23, 212–214. [Google Scholar] [CrossRef]
- Churchill, S.P.; Neill, D.; Jaramillo, E.; Quizhpe, W. Bryophytes from the Cordillera Del Cóndor, Ecuador. Trop. Bryol. 2009, 30, 92–101. [Google Scholar]
- Espinosa, F.R. Economic Valuation of Environmental Goods and Services of the Protector Forest Kutukú–Shaimi, SE Ecuador. Int. J. Energy Environ. Econ. 2019, 27, 117–132. [Google Scholar]
- de Jorgenson, A.B. El Petróleo:¿ Una Amenaza o Una Oportunidad Para La Conservación y El Desarrollo Sostenible En Ecuador? In Petróleo y Desarrollo Sostenible en Ecuador 1. Las Reglas de Juego; Flacso-Sede Ecuador: Quito, Ecuador, 2003; p. 181. [Google Scholar]
- Bio-Parques, F.; Valarezo, V.; Gómez, J.; Mejía, L.; Célleri, Y. Plan de Manejo de La Reserva de Biosfera Sumaco; Ministerio del Ambiente Proyecto Gran Sumaco: Tena, Ecuador, 2001. [Google Scholar]
- López, Á.C.; Rivadeneira, T.; Andrade, J.; Aguirre, P. Las Áreas Protegidas Como Una Contribución Al Desarrollo Sustentable: Caso Del Bosque Protector Sumaco, Ecuador. Sustentabilidad 2012, 8, 8. [Google Scholar]
- Acevedo, V.L.; Ulloa, J.; Osejo, J.A. Cartografía Histórica de Áreas Naturales Protegidas y Territorios Indígenas de La Amazonía Ecuatoriana; FLACSO, Guatemala: Quito, Ecuador, 2016; ISBN 9942217592. [Google Scholar]
- Zambrano, M.; Robles, M.; Izurieta, S.; Torres, B.; Bravo, C.; Martínez, C. Atlas Geográfico de La Provincia de Pastaza; Gobierno Provincial de Pastaza, The Nature Conservancy, Universidad Estatal Amazónica y Conservación Internacional Ecuador: Puyo, Ecuador, 2019; p. 53. [Google Scholar]
- Rivadeneira Torres, N.H. Plan de Ordenamiento Forestal Del Bosque Protector Kutukú–Shaimi. Bachelor’s Thesis, Escuela Superior Politécnica de Chimborazo, Riobamba, Ecuador, 2012. [Google Scholar]
- Jumbo Salazar, C.A.; Arévalo Delgado, C.D.; Ramirez-Cando, L.J. Medición de Carbono Del Estrato Arbóreo Del Bosque Natural Tinajillas-Limón Indanza, Ecuador. LA GRANJA. Rev. Cienc. La Vida 2018, 27, 51–63. [Google Scholar] [CrossRef]
- Jadán, O.; Aguirre, Z. Flora de Los Tepuyes de La Cuenca Alta Del Río Nangaritza, Cordillera Del Cóndor. In Evaluación Ecológica Rápida la Biodiversidad los Tepuyes la Cuenca Alta del Río Nangaritza, Cordillera del Cóndor, Ecuador; Conservación Internacional: Quito, Ecuador, 2011; pp. 41–48. [Google Scholar]
- GADPAZ. Plan Provincial de Educación Ambiental Para El Desarrollo Sostenible 2021–2025 Para La Provincia de Pastaza; Gobierno Provincial de Pastaza: Pastaza, Ecuador, 2021. [Google Scholar]
- Stickler, C.M.; Duchelle, A.E.; Ardila, J.P.; Nepstad, D.C.; David, O.R.; Chan, C.; Rojas, J.G.; Vargas, R.; Bezerra, T.P.; Pritchard, L. The State of Jurisdictional Sustainability; Earth Innovation Institute: San Francisco, CA, USA; Center for International Forestry Research: Bogor, Indonesia; Governors’ Climate & Forests Task Force Secretariat: Boulder, CO, USA, 2018; Available online: https//earthinnovation.org/stateof-jurisdictional-sustaina (accessed on 10 July 2023).
- Bonilla-Bedoya, S.; Estrella-Bastidas, A.; Ordoñez, M.; Sánchez, A.; Herrera, M.A. Patterns of Timber Harvesting and Its Relationship with Sustainable Forest Management in the Western Amazon, Ecuador Case. J. Sustain. For. 2017, 36, 433–453. [Google Scholar] [CrossRef]
- Reed, P. REDD+ and the Indigenous Question: A Case Study from Ecuador. Forests 2011, 2, 525–549. [Google Scholar] [CrossRef]
- Fa, J.E.; Watson, J.E.M.; Leiper, I.; Potapov, P.; Evans, T.D.; Burgess, N.D.; Molnár, Z.; Fernández-Llamazares, Á.; Duncan, T.; Wang, S. Importance of Indigenous Peoples’ Lands for the Conservation of Intact Forest Landscapes. Front. Ecol. Environ. 2020, 18, 135–140. [Google Scholar] [CrossRef]
- Thomas, N.; Baltezar, P.; Lagomasino, D.; Stovall, A.; Iqbal, Z.; Fatoyinbo, L. Trees Outside Forests Are an Underestimated Resource in a Country with Low Forest Cover. Sci. Rep. 2021, 11, 7919. [Google Scholar] [CrossRef]
- Peros, C.S.; Dasgupta, R.; Estoque, R.C.; Basu, M. Ecosystem Services of ‘Trees Outside Forests (TOF)’and Their Contribution to the Contemporary Sustainability Agenda: A Systematic Review. Environ. Res. Commun. 2022, 4, 112002. [Google Scholar] [CrossRef]
- Di Cristofaro, M.; Sallustio, L.; Sitzia, T.; Marchetti, M.; Lasserre, B. Landscape Preference for Trees Outside Forests along an Urban–Rural–Natural Gradient. Forests 2020, 11, 728. [Google Scholar] [CrossRef]
- Yadav, Y.; Chhetri, B.B.K.; Raymajhi, S.; Tiwari, K.R.; Sitaula, B.K. Evaluating Contribution of Trees Outside Forests for Income of Rural Livelihoods of Terai Region of Nepal. Open J. For. 2020, 10, 388–400. [Google Scholar] [CrossRef]
- Ghosh, M.; Sinha, B. Policy Analysis for Realizing the Potential of Timber Production from Trees Outside Forests (TOF) in India. Int. For. Rev. 2018, 20, 89–103. [Google Scholar] [CrossRef]
- Skole, D.L.; Mbow, C.; Mugabowindekwe, M.; Brandt, M.S.; Samek, J.H. Trees Outside of Forests as Natural Climate Solutions. Nat. Clim. Chang. 2021, 11, 1013–1016. [Google Scholar] [CrossRef]
- Ter Steege, H.; Pitman, N.C.A.; Killeen, T.J.; Laurance, W.F.; Peres, C.A.; Guevara, J.E.; Salomão, R.P.; Castilho, C.V.; Amaral, I.L.; de Almeida Matos, F.D. Estimating the Global Conservation Status of More than 15,000 Amazonian Tree Species. Sci. Adv. 2015, 1, e1500936. [Google Scholar] [CrossRef] [PubMed]
- Brummitt, N.A.; Bachman, S.P.; Griffiths-Lee, J.; Lutz, M.; Moat, J.F.; Farjon, A.; Donaldson, J.S.; Hilton-Taylor, C.; Meagher, T.R.; Albuquerque, S. Green Plants in the Red: A Baseline Global Assessment for the IUCN Sampled Red List Index for Plants. PLoS ONE 2015, 10, e0135152. [Google Scholar] [CrossRef] [PubMed]
- Bachman, S.P.; Field, R.; Reader, T.; Raimondo, D.; Donaldson, J.; Schatz, G.E.; Lughadha, E.N. Progress, Challenges and Opportunities for Red Listing. Biol. Conserv. 2019, 234, 45–55. [Google Scholar] [CrossRef]
- Heberling, J.M.; Miller, J.T.; Noesgaard, D.; Weingart, S.B.; Schigel, D. Data Integration Enables Global Biodiversity Synthesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2018093118. [Google Scholar] [CrossRef]
- Monsarrat, S.; Boshoff, A.F.; Kerley, G.I.H. Accessibility Maps as a Tool to Predict Sampling Bias in Historical Biodiversity Occurrence Records. Ecography 2019, 42, 125–136. [Google Scholar] [CrossRef]
- Park, D.S.; Feng, X.; Akiyama, S.; Ardiyani, M.; Avendaño, N.; Barina, Z.; Bärtschi, B.; Belgrano, M.; Betancur, J.; Bijmoer, R. The Colonial Legacy of Herbaria. Nat. Hum. Behav. 2023, 7, 1059–1068. [Google Scholar] [CrossRef]
- Nelson, G.; Ellis, S. The History and Impact of Digitization and Digital Data Mobilization on Biodiversity Research. Philos. Trans. R. Soc. B 2019, 374, 20170391. [Google Scholar] [CrossRef]
- Davis, C.C. The Herbarium of the Future. Trends Ecol. Evol. 2023, 38, 412–423. [Google Scholar] [CrossRef]
- Jackowiak, B.; Lawenda, M.; Nowak, M.M.; Wolniewicz, P.; Błoszyk, J.; Urbaniak, M.; Szkudlarz, P.; Jędrasiak, D.; Wiland-Szymańska, J.; Bajaczyk, R. Open Access to the Digital Biodiversity Database: A Comprehensive Functional Model of the Natural History Collections. Diversity 2022, 14, 596. [Google Scholar] [CrossRef]
- Albani Rocchetti, G.; Armstrong, C.G.; Abeli, T.; Orsenigo, S.; Jasper, C.; Joly, S.; Bruneau, A.; Zytaruk, M.; Vamosi, J.C. Reversing Extinction Trends: New Uses of (Old) Herbarium Specimens to Accelerate Conservation Action on Threatened Species. New Phytol. 2021, 230, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Cobos, M.E.; Jiménez, L.; Nuñez-Penichet, C.; Romero-Alvarez, D.; Simões, M. Sample Data and Training Modules for Cleaning Biodiversity Information. Biodivers. Inform. 2018, 13, 49–50. [Google Scholar] [CrossRef]
- ENF. Inventario Nacional Forestal. Available online: http://enf.ambiente.gob.ec/web_enf/ (accessed on 10 July 2023).
- Nuñez-Penichet, C.; Cobos, M.E.; Soberón, J.; Gueta, T.; Barve, N.; Barve, V.; Navarro-Sigüenza, A.G.; Peterson, A.T. Selection of Sampling Sites for Biodiversity Inventory: Effects of Environmental and Geographical Considerations. Methods Ecol. Evol. 2022, 13, 1595–1607. [Google Scholar] [CrossRef]
- SoberónM, J.; LlorenteB, J. The Use of Species Accumulation Functions for the Prediction of Species Richness. Conserv. Biol. 1993, 7, 480–488. [Google Scholar] [CrossRef]
- Velásquez-Tibatá, J. Package WhereNext. Available online: https://github.com/jivelasquezt/WhereNext-Pkg (accessed on 10 July 2023).
- Merow, C.; Galante, P.J.; Kass, J.M.; Aiello-Lammens, M.E.; Babich Morrow, C.; Gerstner, B.E.; Grisales Betancur, V.; Moore, A.C.; Noguera-Urbano, E.A.; Pinilla-Buitrago, G.E. Operationalizing Expert Knowledge in Species’ Range Estimates Using Diverse Data Types. Front. Biogeogr. 2022, 14, e53589. [Google Scholar] [CrossRef]
- Agnello, G.; Vercammen, A.; Knight, A.T. Understanding Citizen Scientists’ Willingness to Invest in, and Advocate for, Conservation. Biol. Conserv. 2022, 265, 109422. [Google Scholar] [CrossRef]
- Soteropoulos, D.L.; De Bellis, C.R.; Witsell, T. Citizen Science Contributions to Address Biodiversity Loss and Conservation Planning in a Rapidly Developing Region. Diversity 2021, 13, 255. [Google Scholar] [CrossRef]
- Huntley, B.J. Building Biodiversity Knowledge: Mobilising Citizen Science. In Strategic Opportunism: What Works in Africa: Twelve Fundamentals for Conservation Success; Springer: Berlin/Heidelberg, Germany, 2023; pp. 71–91. [Google Scholar]
- Paton, A.; Antonelli, A.; Carine, M.; Forzza, R.C.; Davies, N.; Demissew, S.; Dröge, G.; Fulcher, T.; Grall, A.; Holstein, N. Plant and Fungal Collections: Current Status, Future Perspectives. Plants People Planet 2020, 2, 499–514. [Google Scholar] [CrossRef]
- Hedrick, B.P.; Heberling, J.M.; Meineke, E.K.; Turner, K.G.; Grassa, C.J.; Park, D.S.; Kennedy, J.; Clarke, J.A.; Cook, J.A.; Blackburn, D.C. Digitization and the Future of Natural History Collections. Bioscience 2020, 70, 243–251. [Google Scholar] [CrossRef]
- Walker, B.E.; Tucker, A.; Nicolson, N. Harnessing Large-Scale Herbarium Image Datasets through Representation Learning. Front. Plant Sci. 2022, 12, 806407. [Google Scholar] [CrossRef]
- Chulif, S.; Lee, S.H.; Chang, Y.L.; Chai, K.C. A Machine Learning Approach for Cross-Domain Plant Identification Using Herbarium Specimens. Neural Comput. Appl. 2023, 35, 5963–5985. [Google Scholar] [CrossRef]
- Hussein, B.R.; Malik, O.A.; Ong, W.-H.; Slik, J.W.F. Applications of Computer Vision and Machine Learning Techniques for Digitized Herbarium Specimens: A Systematic Literature Review. Ecol. Inform. 2022, 69, 101641. [Google Scholar] [CrossRef]
- Goëau, H.; Lorieul, T.; Heuret, P.; Joly, A.; Bonnet, P. Can Artificial Intelligence Help in the Study of Vegetative Growth Patterns from Herbarium Collections? An Evaluation of the Tropical Flora of the French Guiana Forest. Plants 2022, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.V.; Andermann, T.; Zizka, A.; Kozlowski, G.; Silvestro, D. Global Estimation and Mapping of the Conservation Status of Tree Species Using Artificial Intelligence. Front. Plant Sci. 2022, 13, 839792. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.; Young, R.P.; Hilton-Taylor, C.; Hoffmann, M.; Rodríguez, J.P.; Stuart, S.N.; Milner-Gulland, E.J. A Framework for Evaluating the Impact of the IUCN Red List of Threatened Species. Conserv. Biol. 2020, 34, 632–643. [Google Scholar] [CrossRef]
- Cazalis, V.; Santini, L.; Lucas, P.M.; González-Suárez, M.; Hoffmann, M.; Benítez-López, A.; Pacifici, M.; Schipper, A.M.; Böhm, M.; Zizka, A. Prioritizing the Reassessment of Data Deficient Species on the IUCN Red List. Conserv. Biol. 2023. [Google Scholar] [CrossRef]
- Zizka, A.; Silvestro, D.; Vitt, P.; Knight, T.M. Automated Conservation Assessment of the Orchid Family with Deep Learning. Conserv. Biol. 2021, 35, 897–908. [Google Scholar] [CrossRef]
- Dauby, G.; Stévart, T.; Droissart, V.; Cosiaux, A.; Deblauwe, V.; Simo-Droissart, M.; Sosef, M.S.M.; Lowry, P.P.; Schatz, G.E.; Gereau, R.E. ConR: An R Package to Assist Large-scale Multispecies Preliminary Conservation Assessments Using Distribution Data. Ecol. Evol. 2017, 7, 11292–11303. [Google Scholar] [CrossRef]
- Zizka, A.; Andermann, T.; Silvestro, D. IUCNN–Deep Learning Approaches to Approximate Species’ Extinction Risk. Divers. Distrib. 2022, 28, 227–241. [Google Scholar] [CrossRef]
- Mace, G.M.; Lande, R. Assessing Extinction Threats: Toward a Reevaluation of IUCN Threatened Species Categories. Conserv. Biol. 1991, 5, 148–157. [Google Scholar] [CrossRef]
- Ballantyne, M.; Pickering, C.M. Tourism and Recreation: A Common Threat to IUCN Red-Listed Vascular Plants in Europe. Biodivers. Conserv. 2013, 22, 3027–3044. [Google Scholar] [CrossRef]
- Duenas, M.-A.; Hemming, D.J.; Roberts, A.; Diaz-Soltero, H. The Threat of Invasive Species to IUCN-Listed Critically Endangered Species: A Systematic Review. Glob. Ecol. Conserv. 2021, 26, e01476. [Google Scholar] [CrossRef]
- Walker, B.E.; Leão, T.C.C.; Bachman, S.P.; Lucas, E.; Nic Lughadha, E. Evidence-based Guidelines for Automated Conservation Assessments of Plant Species. Conserv. Biol. 2023, 37, e13992. [Google Scholar] [CrossRef] [PubMed]
- Herkt, K.M.B.; Skidmore, A.K.; Fahr, J. Macroecological Conclusions Based on IUCN Expert Maps: A Call for Caution. Glob. Ecol. Biogeogr. 2017, 26, 930–941. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- MAATE. Sistema de Admistración Forestal (SAF). Available online: https://saf.ambiente.gob.ec/saf2/ (accessed on 28 June 2023).
- Carmona-Higuita, M.J.; Mendieta-Leiva, G.; Gómez-Díaz, J.A.; Villalobos, F.; Ramos, F.N.; Elias, J.P.C.; Jiménez-López, D.A.; Zuluaga, A.; Holst, B.; Kessler, M. Conservation Status of Vascular Epiphytes in the Neotropics. 2023. Available online: https://assets.researchsquare.com/files/rs-2773328/v1/a709b427-f900-4639-a064-ca91582051b3.pdf?c=1680715479 (accessed on 10 July 2023).
- Hijmans, R.J.; Guarino, L.; Bussink, C.; Mathur, P.; Cruz, M.; Barrentes, I.; Rojas, E. Diva-Gis. 2004. Available online: https://www.diva-gis.org/ (accessed on 10 July 2023).
- MAATE. Interactive Map of MAATE. Available online: http://ide.ambiente.gob.ec/mapainteractivo/ (accessed on 23 February 2023).
- Chamberlain, S.A.; Szöcs, E. Taxize: Taxonomic Search and Retrieval in R. F1000Research 2013, 2, 191. Available online: https://f1000research.com/articles/2-191 (accessed on 10 July 2023). [CrossRef]
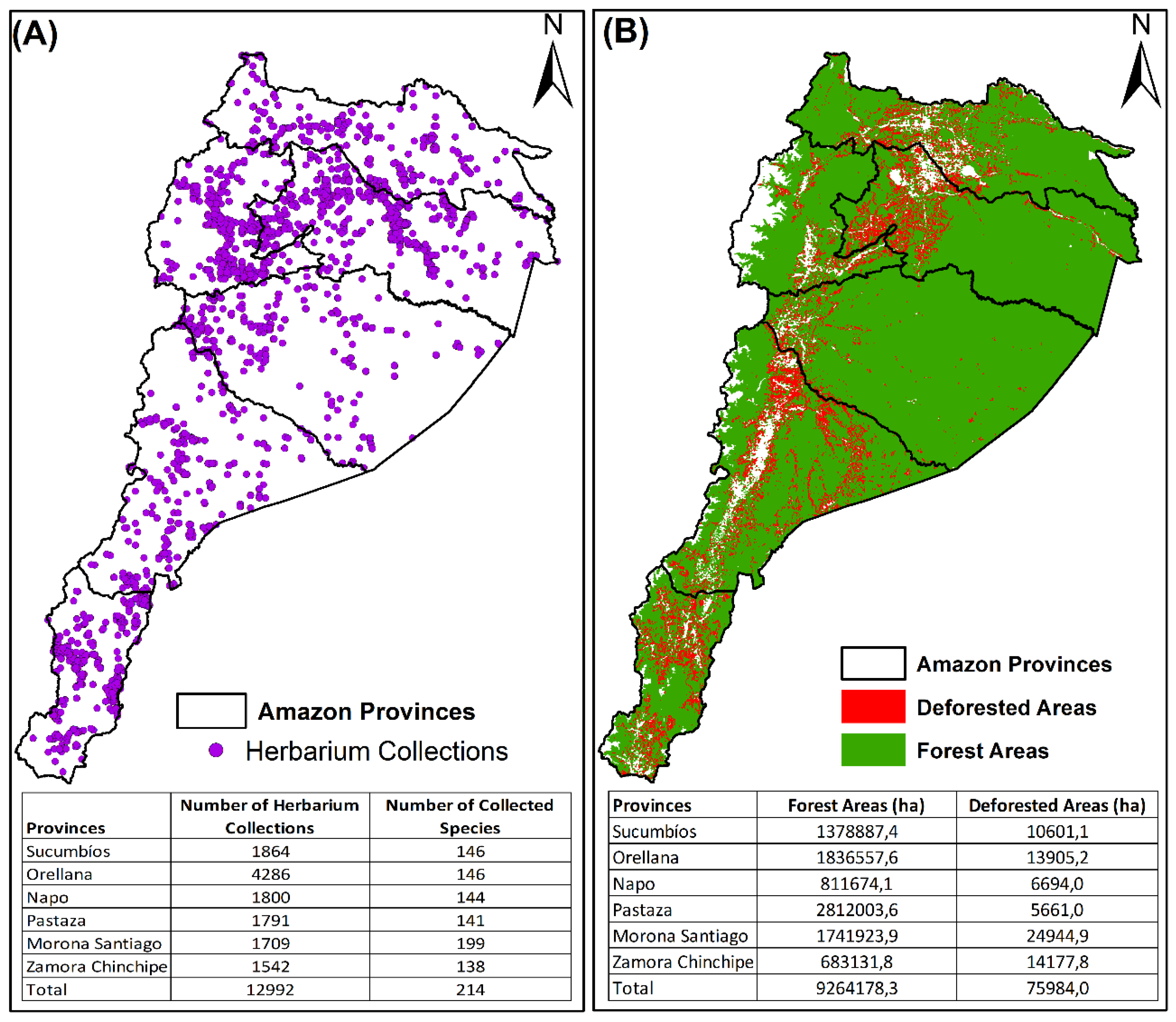
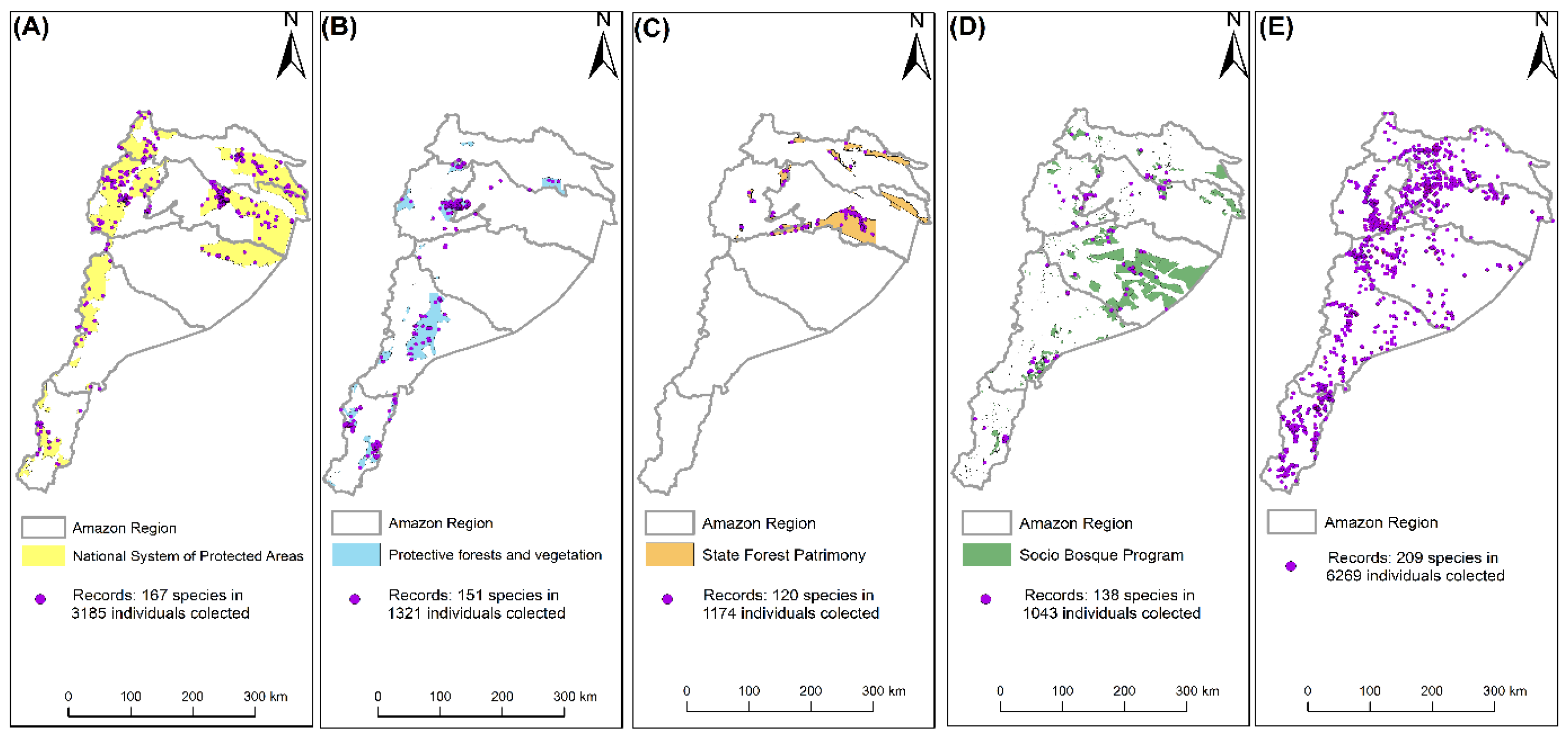

| Province | Individuals | % | Species |
|---|---|---|---|
| Sucumbíos | 1864 | 14.3 | 146 |
| Orellana | 4286 | 33.0 | 146 |
| Napo | 1800 | 13.9 | 144 |
| Pastaza | 1791 | 13.8 | 141 |
| Morona Santiago | 1709 | 13.2 | 199 |
| Zamora Chinchipe | 1542 | 11.9 | 138 |
| Total | 12,992 | 100 | 214 |
| Data | Format |
|---|---|
| Protected areas | Vector (Polygons) |
| Forests and protective vegetation | Vector (Polygons) |
| State Forest Heritage | Vector (Polygons) |
| Socio Bosque Program | Vector (Polygons) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Tobar, R.; Herrera-Feijoo, R.J.; Mateo, R.G.; García-Robredo, F.; Torres, B. Botanical Collection Patterns and Conservation Categories of the Most Traded Timber Species from the Ecuadorian Amazon: The Role of Protected Areas. Plants 2023, 12, 3327. https://doi.org/10.3390/plants12183327
López-Tobar R, Herrera-Feijoo RJ, Mateo RG, García-Robredo F, Torres B. Botanical Collection Patterns and Conservation Categories of the Most Traded Timber Species from the Ecuadorian Amazon: The Role of Protected Areas. Plants. 2023; 12(18):3327. https://doi.org/10.3390/plants12183327
Chicago/Turabian StyleLópez-Tobar, Rolando, Robinson J. Herrera-Feijoo, Rubén G. Mateo, Fernando García-Robredo, and Bolier Torres. 2023. "Botanical Collection Patterns and Conservation Categories of the Most Traded Timber Species from the Ecuadorian Amazon: The Role of Protected Areas" Plants 12, no. 18: 3327. https://doi.org/10.3390/plants12183327
APA StyleLópez-Tobar, R., Herrera-Feijoo, R. J., Mateo, R. G., García-Robredo, F., & Torres, B. (2023). Botanical Collection Patterns and Conservation Categories of the Most Traded Timber Species from the Ecuadorian Amazon: The Role of Protected Areas. Plants, 12(18), 3327. https://doi.org/10.3390/plants12183327








