Rhizobiome Transplantation: A Novel Strategy beyond Single-Strain/Consortium Inoculation for Crop Improvement
Abstract
:1. Introduction
2. Rhizospheric Soil: A Rich Source of PGPMs
3. Single versus Group Inoculation for Crop Improvement
4. Plant Microbiome Engineering Strategies
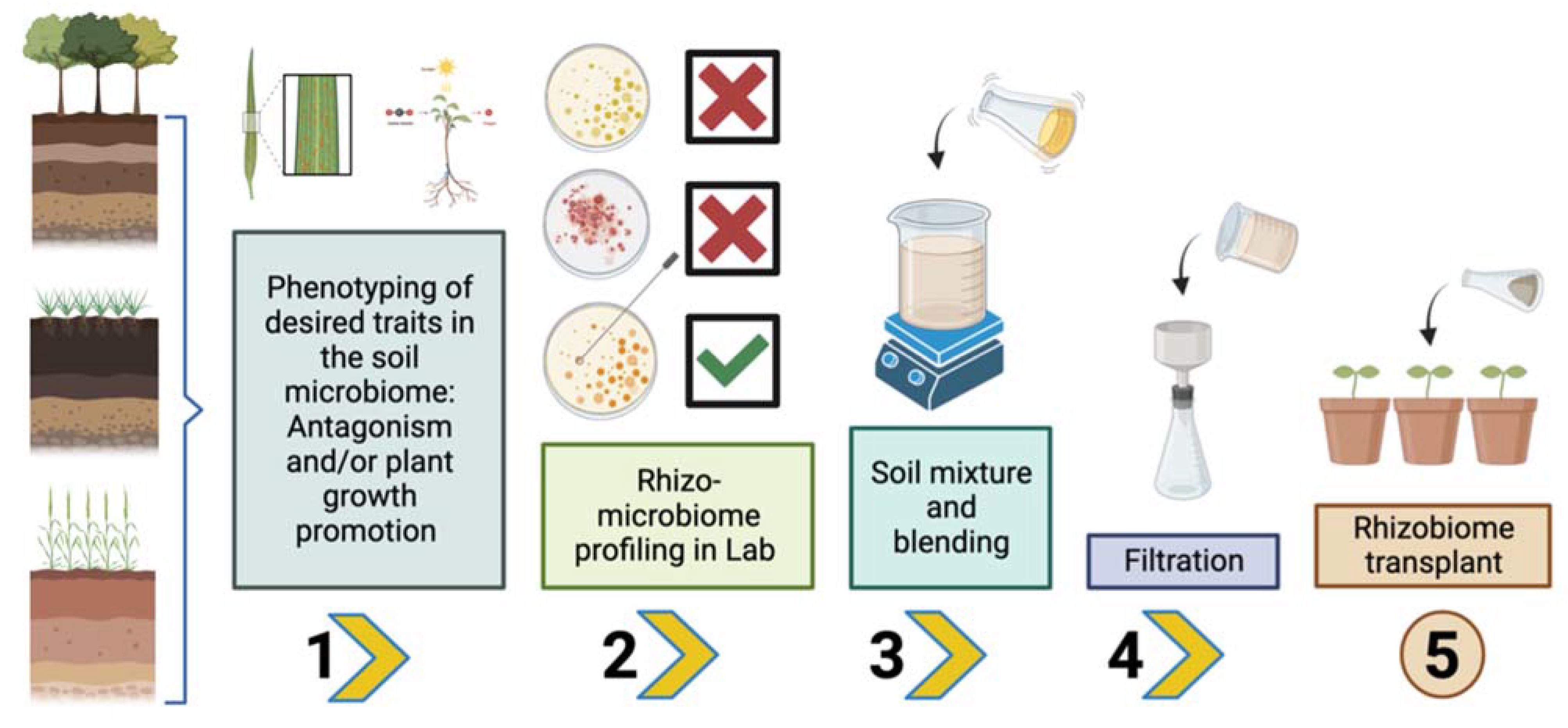
5. Rhizosphere Microbiome Transplantation
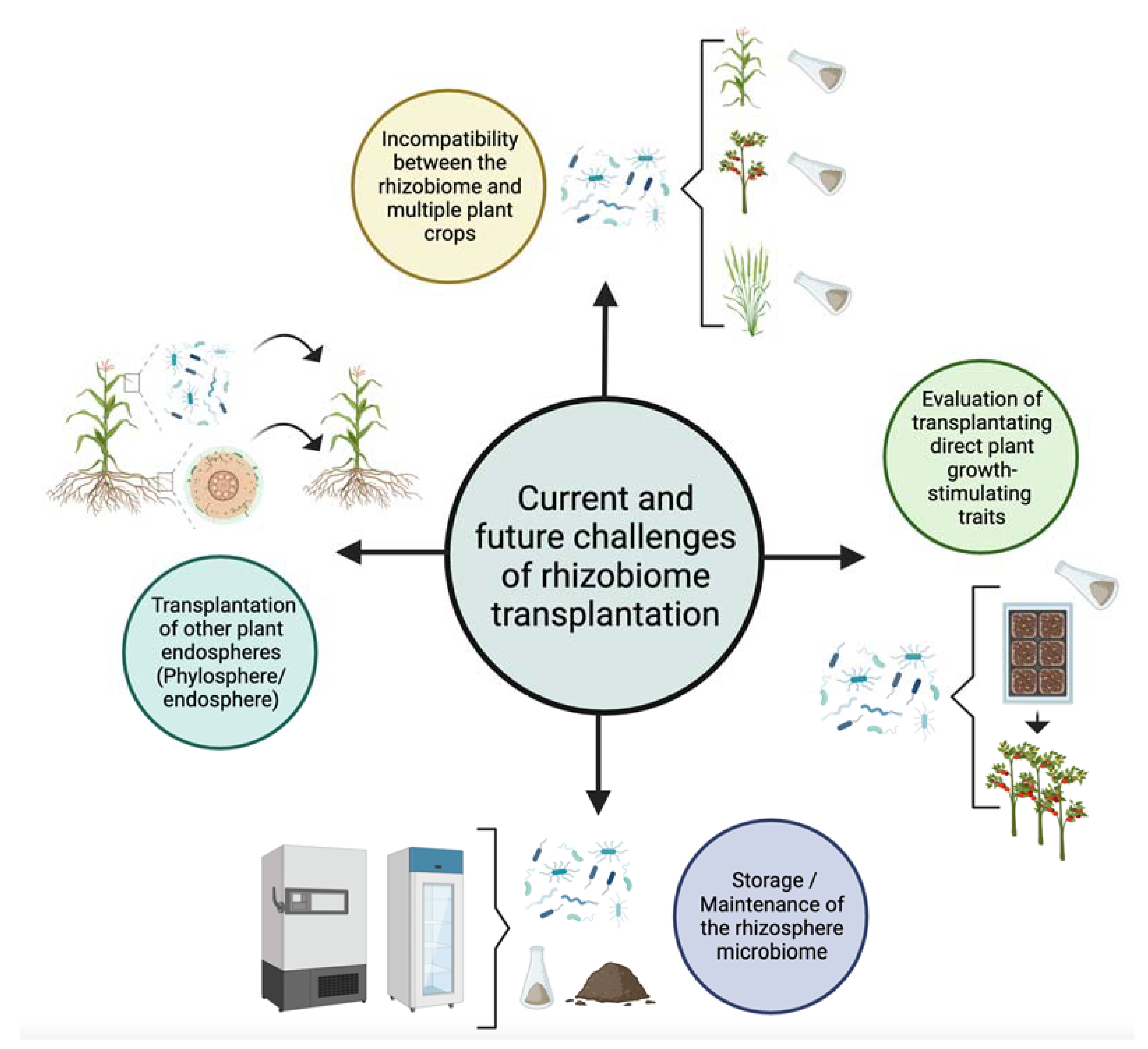
6. Current and Future Challenges
6.1. Phyllosphere or Endosphere Microbiome Transplantation?
6.2. Incompatibility between the Rhizobiome and the Plant
6.3. How to Preserve a Rhizobiome for Future Applications?
6.4. Transplantation of Plant Growth-Stimulating Traits
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- D’Hondt, K.; Kostic, T.; McDowell, R.; Eudes, F.; Singh, B.K.; Sarkar, S.; Markakis, M.; Schelkle, B.; Maguin, E.; Sessitsch, A. Microbiome innovations for a sustainable future. Nat. Microbiol. 2021, 6, 138–142. [Google Scholar] [CrossRef]
- Parizadeh, M.; Arrieta, M.-C. The global human gut microbiome: Genes, lifestyles, and diet. Trends Mol. Med. 2023. In press. [Google Scholar] [CrossRef] [PubMed]
- Shoubridge, A.P.; Choo, J.M.; Martin, A.M.; Keating, D.J.; Wong, M.-L.; Licinio, J.; Rogers, G.B. The gut microbiome and mental health: Advances in research and emerging priorities. Mol. Psychiatry 2022, 27, 1908–1919. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Baedke, J.; Fábregas-Tejeda, A.; Nieves Delgado, A. The holobiont concept before Margulis. J. Exp. Zool. Part B Mol. Dev. Evol. 2020, 334, 149–155. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, P.C.; Dubey, R.K.; Tripathi, V.; Gupta, V.K.; Singh, H.B. Plant Growth-Promoting Microorganisms for Environmental Sustainability. Trends Biotechnol. 2016, 34, 847–850. [Google Scholar] [CrossRef]
- Shah, A.; Nazari, M.; Antar, M.; Msimbira, L.A.; Naamala, J.; Lyu, D.; Rabileh, M.; Zajonc, J.; Smith, D.L. PGPR in Agriculture: A Sustainable Approach to Increasing Climate Change Resilience. Front. Sustain. Food Syst. 2021, 5, 667546. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Vanderleyden, J.; Okon, Y. Plant Growth-Promoting Effects of Diazotrophs in the Rhizosphere. CRC. Crit. Rev. Plant Sci. 2003, 22, 107–149. [Google Scholar] [CrossRef]
- Glick, B.R. Beneficial Plant-Bacterial Interactions; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9783030443689. [Google Scholar]
- Vocciante, M.; Grifoni, M.; Fusini, D.; Petruzzelli, G.; Franchi, E. The Role of Plant Growth-Promoting Rhizobacteria (PGPR) in Mitigating Plant’s Environmental Stresses. Appl. Sci. 2022, 12, 1231. [Google Scholar] [CrossRef]
- Singh, D.P.; Singh, H.B.; Prabha, R. Microbial inoculants in sustainable agricultural productivity: Volume 1: Research perspectives. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–343. [Google Scholar] [CrossRef]
- Bradáčová, K.; Florea, A.S.; Bar-Tal, A.; Minz, D.; Yermiyahu, U.; Shawahna, R.; Kraut-Cohen, J.; Zolti, A.; Erel, R.; Dietel, K.; et al. Microbial Consortia versus Single-Strain Inoculants: An advantage in PGPM-assisted tomato production? Agronomy 2019, 9, 105. [Google Scholar] [CrossRef]
- Kost, T.; Stopnisek, N.; Agnoli, K.; Eberl, L.; Weisskopf, L.; Basu, A.; Prasad, P.; Das, S.S.N.; Kalam, S.; Sayyed, R.Z.; et al. Trichoderma koningiopsis controls Fusarium oxysporum causing damping-off in Pinus massoniana seedlings by regulating active oxygen metabolism, osmotic potential, and the rhizosphere microbiome. Front. Microbiol. 2020, 5, 104352. [Google Scholar] [CrossRef]
- Kumar, P.; Thakur, S.; Dhingra, G.K.; Singh, A.; Pal, M.K.; Harshvardhan, K.; Dubey, R.C.; Maheshwari, D.K. Inoculation of siderophore producing rhizobacteria and their consortium for growth enhancement of wheat plant. Biocatal. Agric. Biotechnol. 2018, 15, 264–269. [Google Scholar] [CrossRef]
- Lin, L. Bottom-up synthetic ecology study of microbial consortia to enhance lignocellulose bioconversion. Biotechnol. Biofuels Bioprod. 2022, 15, 14. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Porras-Troncoso, M.D.; Olmedo-Monfil, V.; Herrera-Estrella, A. Trichoderma species: Versatile plant symbionts. Phytopathology 2019, 109, 6–16. [Google Scholar] [CrossRef]
- Santoyo, G.; Gamalero, E.; Glick, B.R. Mycorrhizal-Bacterial Amelioration of Plant Abiotic and Biotic Stress. Front. Sustain. Food Syst. 2021, 5, 672881. [Google Scholar] [CrossRef]
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; Santos-Villalobos, S.d.L.; Orozco-Mosqueda, M.d.C.; Glick, B.R. Plant Growth Stimulation by Microbial Consortia. Agronomy 2021, 11, 219. [Google Scholar] [CrossRef]
- Choi, K.; Choi, J.; Lee, P.A.; Roy, N.; Khan, R.; Lee, H.J.; Weon, H.Y.; Kong, H.G.; Lee, S.W.; Manichanh, C.; et al. Alteration of Bacterial Wilt Resistance in Tomato Plant by Microbiota Transplant. ISME Commun. 2022, 11, 1186. [Google Scholar] [CrossRef]
- Jiang, G.; Zhang, Y.; Gan, G.; Li, W.; Wan, W.; Jiang, Y.; Yang, T.; Zhang, Y.; Xu, Y.; Wang, Y.; et al. Exploring rhizo-microbiome transplants as a tool for protective plant-microbiome manipulation. ISME Commun. 2022, 2, 10. [Google Scholar] [CrossRef]
- Nouri, E.; Breuillin-Sessoms, F.; Feller, U.; Reinhardt, D. Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in petunia hybrida. PLoS ONE 2014, 9, e90841. [Google Scholar] [CrossRef] [PubMed]
- Schoenborn, L.; Yates, P.S.; Grinton, B.E.; Hugenholtz, P.; Janssen, P.H. Liquid serial dilution is inferior to solid media for isolation of cultures representative of the phylum-level diversity of soil bacteria. Appl. Environ. Microbiol. 2004, 70, 4363–4366. [Google Scholar] [CrossRef] [PubMed]
- Sessitsch, A.; Wakelin, S.; Schloter, M.; Maguin, E.; Cernava, T.; Champomier-Verges, M.-C.; Charles, T.C.; Cotter, P.D.; Ferrocino, I.; Kriaa, A.; et al. Microbiome Interconnectedness throughout Environments with Major Consequences for Healthy People and a Healthy Planet. Microbiol. Mol. Biol. Rev. 2023, e0021222. [Google Scholar] [CrossRef]
- Nadarajah, K. Soil Health: The Contribution of Microflora and Microfauna BT. In Mycorrhizosphere and Pedogenesis; Varma, A., Choudhary, D.K., Eds.; Springer: Singapore, 2019; pp. 383–400. ISBN 978-981-13-6480-8. [Google Scholar]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Nazir, N.; Kamili, A.N.; Zargar, M.Y.; Khan, I.; Shah, D.; Parray, J.A.; Tyub, S. Effect of Root Exudates on Rhizosphere Soil Microbial Communities. J. Res. Dev. 2016, 16, 88–95. [Google Scholar]
- Avis, T.J.; Gravel, V.; Antoun, H.; Tweddell, R.J. Multifaceted beneficial effects of rhizosphere microorganisms on plant health and productivity. Soil Biol. Biochem. 2008, 40, 1733–1740. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, D.; Liu, Y.; Li, S.; Shen, Q.; Zhang, R. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 2014, 374, 689–700. [Google Scholar] [CrossRef]
- Pande, A.; Mun, B.G.; Lee, D.S.; Khan, M.; Lee, G.M.; Hussain, A.; Yun, B.W. NO Network for Plant–Microbe Communication Underground: A Review. Front. Plant Sci. 2021, 12, 658679. [Google Scholar] [CrossRef] [PubMed]
- Omirou, M.; Fasoula, D.A.; Ioannides, I.M. Bradyrhizobium inoculation alters indigenous AMF community assemblages and interacts positively with AMF inoculum to improve cowpea performance. Appl. Soil Ecol. 2016, 108, 381–389. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Yadav, A.N.; Santoyo, G.; Babalola, O.O. Understanding the plant-microbe interactions in environments exposed to abiotic stresses: An overview. Microbiol. Res. 2023, 271, 127368. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Esquivel, A.A.; Castro-Mercado, E.; García-Pineda, E. Comparative Effects of Azospirillum brasilense Sp245 and Pseudomonas aeruginosa PAO1 Lipopolysaccharides on Wheat Seedling Growth and Peroxidase Activity. J. Plant Growth Regul. 2021, 40, 1903–1911. [Google Scholar] [CrossRef]
- Cassán, F.D.; Lucangeli, C.D.; Bottini, R.; Piccoli, P.N. Azospirillum spp. metabolize [17,17-2H2] gibberellin A20 to [17,17-2H2] gibberellin A1 in vivo in dy rice mutant seedlings. Plant Cell Physiol. 2001, 42, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Scudeletti, D.; Crusciol, C.A.C.; Momesso, L.; Bossolani, J.W.; Moretti, L.G.; De Oliveira, E.F.; Tubaña, B.S.; Silva, M.d.A.; de Castro, S.G.Q.; Hungria, M. Inoculation with Azospirillum brasilense as a strategy to enhance sugarcane biomass production and bioenergy potential. Eur. J. Agron. 2023, 144, 126749. [Google Scholar] [CrossRef]
- Arora, P.K.; Srivastava, A.; Garg, S.K.; Singh, V.P. Recent advances in degradation of chloronitrophenols. Bioresour. Technol. 2018, 250, 902–909. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and plants—With special reference to induced systemic resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manag. 2020, 273, 111118. [Google Scholar] [CrossRef]
- Khatoon, Z.; del Carmen Orozco-Mosqueda, M.; Huang, S.; Nascimento, F.X.; Santoyo, G. Peptide Antibiotics Produced by Bacillus Species: First Line of Attack in the Biocontrol of Plant Diseases. In Bacilli in Agrobiotechnology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 31–46. [Google Scholar] [CrossRef]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of Two Plant-Growth Promoting Bacillus velezensis Isolates Against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef]
- de los Santos Villalobos, S.; Robles, R.I.; Parra Cota, F.I.; Larsen, J.; Lozano, P.; Tiedje, J.M. Bacillus cabrialesii sp. Nov., an endophytic plant growth promoting bacterium isolated from wheat (triticum turgidum subsp. durum) in the yaqui valley, Mexico. Int. J. Syst. Evol. Microbiol. 2019, 69, 3939–3945. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Nazir, R.; Sessitsch, A.; Elhottová, D.; Korenblum, E.; Van Overbeek, L.S.; Van Elsas, J.D. The new species Enterobacter oryziphilus sp. nov. and Enterobacter oryzendophyticus sp. nov. are key inhabitants of the endosphere of rice. BMC Microbiol. 2013, 13, 164. [Google Scholar] [CrossRef]
- Singh, R.P.; Mishra, S.; Jha, P.; Raghuvanshi, S.; Jha, P.N. Effect of inoculation of zinc-resistant bacterium Enterobacter ludwigii CDP-14 on growth, biochemical parameters and zinc uptake in wheat (Triticum aestivum L.) plant. Ecol. Eng. 2018, 116, 163–173. [Google Scholar] [CrossRef]
- Hoque, M.N.; Hannan, A.; Imran, S.; Paul, N.C.; Mondal, M.F.; Sadhin, M.M.R.; Bristi, J.M.; Dola, F.S.; Hanif, M.A.; Ye, W.; et al. Plant Growth-Promoting Rhizobacteria-Mediated Adaptive Responses of Plants Under Salinity Stress. J. Plant Growth Regul. 2023, 42, 1307–1326. [Google Scholar] [CrossRef]
- Kang, S.M.; Radhakrishnan, R.; You, Y.H.; Khan, A.L.; Lee, K.E.; Lee, J.D.; Lee, I.J. Enterobacter asburiae KE17 association regulates physiological changes and mitigates the toxic effects of heavy metals in soybean. Plant Biol. 2015, 17, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Kamran, S.; Shahid, I.; Baig, D.N.; Rizwan, M.; Malik, K.A.; Mehnaz, S. Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Front. Microbiol. 2017, 8, 2593. [Google Scholar] [CrossRef]
- Ortiz, M.; Hernández, J..; Valenzuela, B.; De Los Santo, S.; Del Carmen Rocha, M.; Santoyo, G. Diversity of cultivable endophytic bacteria associated with blueberry plants (Vaccinium corymbosum L.) cv. Biloxi with plant growth-promoting traits. Chil. J. Agric. Anim. Sci. 2018, 34, 140–151. [Google Scholar] [CrossRef]
- Chen, C.; Xin, K.; Liu, H.; Cheng, J.; Shen, X.; Wang, Y.; Zhang, L. Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci. Rep. 2017, 7, 41564. [Google Scholar] [CrossRef]
- Shariati, V.J.; Malboobi, M.A.; Tabrizi, Z.; Tavakol, E.; Owilia, P.; Safari, M. Comprehensive genomic analysis of a plant growth-promoting rhizobacterium Pantoea agglomerans strain P5 /631/208/212/748 /631/208/464 /38/43/45 article. Sci. Rep. 2017, 7, 15610. [Google Scholar] [CrossRef]
- Ramette, A.; Frapolli, M.; Saux, M.F.L.; Gruffaz, C.; Meyer, J.M.; Défago, G.; Sutra, L.; Moënne-Loccoz, Y. Pseudomonas protegens sp. nov., widespread plant-protecting bacteria producing the biocontrol compounds 2,4-diacetylphloroglucinol and pyoluteorin. Syst. Appl. Microbiol. 2011, 34, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Couillerot, O.; Ramírez-Trujillo, A.; Walker, V.; Von Felten, A.; Jansa, J.; Maurhofer, M.; Défago, G.; Prigent-Combaret, C.; Comte, G.; Caballero-Mellado, J.; et al. Comparison of prominent Azospirillum strains in Azospirillum-Pseudomonas-Glomus consortia for promotion of maize growth. Appl. Microbiol. Biotechnol. 2013, 97, 4639–4649. [Google Scholar] [CrossRef]
- Pereira, S.I.A.; Abreu, D.; Moreira, H.; Vega, A.; Castro, P.M.L. Plant growth-promoting rhizobacteria (PGPR) improve the growth and nutrient use efficiency in maize (Zea mays L.) under water deficit conditions. Heliyon 2020, 6, e05106. [Google Scholar] [CrossRef]
- Kang, S.M.; Adhikari, A.; Lee, K.E.; Park, Y.G.; Shahzad, R.; Lee, I.J. Gibberellin producing rhizobacteria pseudomonas koreensis MU2 enhance growth of lettuce (lactuca sativa) and Chinese cabbage (Brassica rapa, chinensis). J. Microbiol. Biotechnol. Food Sci. 2019, 9, 166–170. [Google Scholar] [CrossRef]
- Kaur, G.; Reddy, M.S. Effects of Phosphate-Solubilizing Bacteria, Rock Phosphate and Chemical Fertilizers on Maize-Wheat Cropping Cycle and Economics. Pedosphere 2015, 25, 428–437. [Google Scholar] [CrossRef]
- Hernández-León, R.; Rojas-Solís, D.; Contreras-Pérez, M.; Orozco-Mosqueda, M.d.C.; Macías-Rodríguez, L.I.; la Cruz, H.R.-D.; Valencia-Cantero, E.; Santoyo, G. Characterization of the antifungal and plant growth-promoting effects of diffusible and volatile organic compounds produced by Pseudomonas fluorescens strains. Biol. Control 2015, 81, 83–92. [Google Scholar] [CrossRef]
- Pueppke, S.G.; Broughton, W.J. Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Mol. Plant-Microbe Interact. 1999, 12, 293–318. [Google Scholar] [CrossRef]
- Suárez, R.; Wong, A.; Ramírez, M.; Barraza, A.; Orozco, M.D.C.; Cevallos, M.A.; Lara, M.; Hernández, G.; Iturriaga, G. Improvement of drought tolerance and grain yield in common bean by overexpressing trehalose-6-phosphate synthase in rhizobia. Mol. Plant-Microbe Interact. 2008, 21, 958–966. [Google Scholar] [CrossRef]
- Peralta, H.; Mora, Y.; Salazar, E.; Encarnación, S.; Palacios, R.; Mora, J. Engineering the nifH promoter region and abolishing poly-β -hydroxybutyrate accumulation in Rhizobium etli enhance nitrogen fixation in symbiosis with Phaseolus vulgaris. Appl. Environ. Microbiol. 2004, 70, 3272–3281. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, B.; Kaur, S.; Prasanna, R.; Ranjan, K.; Kanchan, A.; Hossain, F.; Shivay, Y.S.; Nain, L. Microbial inoculation of seeds characteristically shapes the rhizosphere microbiome in desi and kabuli chickpea types. J. Soils Sediments 2017, 17, 2040–2053. [Google Scholar] [CrossRef]
- Pandey, R.P.; Srivastava, A.K.; Gupta, V.K.; O’Donovan, A.; Ramteke, P.W. Enhanced yield of diverse varieties of chickpea (Cicer arietinum L.) by different isolates of Mesorhizobium ciceri. Environ. Sustain. 2018, 1, 425–435. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The PGPR stenotrophomonas maltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front. Microbiol. 2017, 8, 1945. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Nafady, N.A.; Bashandy, S.R.; Hassan, A.A. Mitigation of effect of salt stress on the nodulation, nitrogen fixation and growth of chickpea (Cicer arietinum L.) by triple microbial inoculation. Rhizosphere 2019, 10, 100148. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Berg, G. Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth, and nodulation of soybean under salt stress. Plant Soil 2016, 405, 35–45. [Google Scholar] [CrossRef]
- Morales-Cedeño, L.R.; de los Santos-Villalobos, S.; Santoyo, G. Functional and Genomic Analysis of Rouxiella badensis SER3 as a Novel Biocontrol Agent of Fungal Pathogens. Front. Microbiol. 2021, 12, 709855. [Google Scholar] [CrossRef]
- Queiroz, P.S.; Barboza, N.R.; Cordeiro, M.M.; Leão, V.A.; Guerra-Sá, R. Rich growth medium promotes an increased on Mn(II) removal and manganese oxide production by Serratia marcescens strains isolates from wastewater. Biochem. Eng. J. 2018, 140, 148–156. [Google Scholar] [CrossRef]
- Rathore, A.S.; Gupta, R.D. Chitinases from Bacteria to Human: Properties, Applications, and Future Perspectives. Enzyme Res. 2015, 2015, 791907. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guzmán, P.; Santoyo, G. Action mechanisms, biodiversity, and omics approaches in biocontrol and plant growth-promoting Pseudomonas: An updated review. Biocontrol Sci. Technol. 2022, 32, 527–550. [Google Scholar] [CrossRef]
- Luziatelli, F.; Gatti, L.; Ficca, A.G.; Medori, G.; Silvestri, C.; Melini, F.; Muleo, R.; Ruzzi, M. Metabolites Secreted by a Plant-Growth-Promoting Pantoea agglomerans Strain Improved Rooting of Pyrus communis L. cv Dar Gazi Cuttings. Front. Microbiol. 2020, 11, 539359. [Google Scholar] [CrossRef]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]
- Chávez-moctezuma, M.P.; Martínez-cámara, R.; Hernández-salmerón, J.; Valencia-cantero, E. Comparative genomic and functional analysis of Arthrobacter sp. UMCV2 reveals the presence of luxR-related genes inducible by the biocompound N,N-dimethylhexadecilamine. Front. Microbiol. 2022, 13, 1040932. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.H.; Hwang, B.S.; Choi, A.; Chung, E.J. Isolation and characterization of strain Rouxiella sp. S1S-2 producing antibacterial compound. Korean J. Microbiol. 2020, 56, 152–159. [Google Scholar] [CrossRef]
- Singh, R.P.; Jha, P.N. The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLoS ONE 2016, 11, e0155026. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, L.J.; Pan, S.Y.; Li, X.W.; Xu, M.J.; Zhang, C.M.; Xing, K.; Qin, S. Antifungal potential evaluation and alleviation of salt stress in tomato seedlings by a halotolerant plant growth-promoting actinomycete Streptomyces sp. KLBMP5084. Rhizosphere 2020, 16, 100262. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Kumar, A.; Santos-villalobos, S.D.L.; Parra-cota, F.I.; Orozco-mosqueda, M.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma Species: Our Best Fungal Allies in the Biocontrol of Plant Diseases—A Review. Plants 2023, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Imperiali, N.; Chiriboga, X.; Schlaeppi, K.; Fesselet, M.; Villacrés, D.; Jaffuel, G.; Bender, S.F.; Dennert, F.; Blanco-Pérez, R.; van der Heijden, M.G.A.; et al. Combined field inoculations of Pseudomonas bacteria, arbuscular mycorrhizal fungi, and entomopathogenic nematodes and their effects on wheat performance. Front. Plant Sci. 2017, 8, 1809. [Google Scholar] [CrossRef]
- Moreira, H.; Pereira, S.I.A.; Vega, A.; Castro, P.M.L.; Marques, A.P.G.C. Synergistic effects of arbuscular mycorrhizal fungi and plant growth-promoting bacteria benefit maize growth under increasing soil salinity. J. Environ. Manage. 2020, 257, 109982. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhou, Y.; Yue, T.; Huang, Y.; He, C.; Jiang, W.; Liu, H.; Zeng, H.; Wang, J. Plant growth-promoting rhizobacteria Bacillus velezensis JB0319 promotes lettuce growth under salt stress by modulating plant physiology and changing the rhizosphere bacterial community. Environ. Exp. Bot. 2023, 213, 105451. [Google Scholar] [CrossRef]
- Balderas-Ruíz, K.A.; Bustos, P.; Santamaria, R.I.; González, V.; Cristiano-Fajardo, S.A.; Barrera-Ortíz, S.; Mezo-Villalobos, M.; Aranda-Ocampo, S.; Guevara-García, A.; Galindo, E.; et al. Bacillus velezensis 83 a bacterial strain from mango phyllosphere, useful for biological control and plant growth promotion. AMB Express 2020, 10, 163. [Google Scholar] [CrossRef]
- Saubidet, M.I.; Fatta, N.; Barneix, A.J. The effect of inoculation with Azospirillum brasilense on growth and nitrogen utilization by wheat plants. Plant Soil 2002, 245, 215–222. [Google Scholar] [CrossRef]
- SkZ, A.; Vardharajula, S.; Vurukonda, S.S.K.P. Transcriptomic profiling of maize (Zea mays L.) seedlings in response to Pseudomonas putida stain FBKV2 inoculation under drought stress. Ann. Microbiol. 2018, 68, 331–349. [Google Scholar] [CrossRef]
- Alori, E.T.; Babalola, O.O. Microbial inoculants for improving crop quality and human health in Africa. Front. Microbiol. 2018, 9, 2213. [Google Scholar] [CrossRef]
- Compant, S.; Reiter, B.; Sessitsch, A.; Nowak, J.; Clément, C.; Barka, E.A. Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl. Environ. Microbiol. 2005, 71, 1685–1693. [Google Scholar] [CrossRef]
- Goswami, M.; Deka, S. Plant growth-promoting rhizobacteria—Alleviators of abiotic stresses in soil: A review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Saravanakumar, D.; Thomas, A.; Banwarie, N. Antagonistic potential of lipopeptide producing Bacillus amyloliquefaciens against major vegetable pathogens. Eur. J. Plant Pathol. 2019, 154, 319–335. [Google Scholar] [CrossRef]
- Mendis, H.C.; Thomas, V.P.; Schwientek, P.; Salamzade, R.; Chien, J.T.; Waidyarathne, P.; Kloepper, J.; De La Fuente, L. Strain-specific quantification of root colonization by plant growth promoting rhizobacteria Bacillus firmus I-1582 and Bacillus amyloliquefaciens QST713 in non-sterile soil and field conditions. PLoS ONE 2018, 13, e0193119. [Google Scholar] [CrossRef]
- Hernández-Soberano, C.; Ruíz-Herrera, L.F.; Valencia-Cantero, E. Endophytic bacteria Arthrobacter agilis UMCV2 and Bacillus methylotrophicus M4-96 stimulate achene germination, in vitro growth, and greenhouse yield of strawberry (Fragaria × ananassa). Sci. Hortic. 2020, 261, 109005. [Google Scholar] [CrossRef]
- Huang, Y. hong Comparison of rhizosphere and endophytic microbial communities of Chinese leek through high-throughput 16S rRNA gene Illumina sequencing. J. Integr. Agric. 2018, 17, 359–367. [Google Scholar] [CrossRef]
- Szymańska, S.; Płociniczak, T.; Piotrowska-Seget, Z.; Hrynkiewicz, K. Endophytic and rhizosphere bacteria associated with the roots of the halophyte Salicornia europaea L.—Community structure and metabolic potential. Microbiol. Res. 2016, 192, 37–51. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Zetter-Salmón, E.; Contreras-Pérez, M.; del Carmen Rocha-Granados, M.; Macías-Rodríguez, L.; Santoyo, G. Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal. Agric. Biotechnol. 2018, 13, 46–52. [Google Scholar] [CrossRef]
- Hafiz, F.B.; Moradtalab, N.; Goertz, S.; Rietz, S.; Dietel, K.; Rozhon, W.; Humbeck, K.; Geistlinger, J.; Neumann, G.; Schellenberg, I. Synergistic Effects of a Root-Endophytic Trichoderma Fungus and Bacillus on Early Root Colonization and Defense Activation against Verticillium longisporum in Rapeseed. Mol. Plant-Microbe Interact. 2022, 35, 380–392. [Google Scholar] [CrossRef]
- Ni, H.; Wu, Y.; Zong, R.; Ren, S.; Pan, D.; Yu, L.; Li, J.; Qu, Z.; Wang, Q.; Zhao, G.; et al. Combination of Aspergillus niger MJ1 with Pseudomonas stutzeri DSM4166 or mutant Pseudomonas fluorescens CHA0-nif improved crop quality, soil properties, and microbial communities in barrier soil. Front. Microbiol. 2023, 14, 1064358. [Google Scholar] [CrossRef]
- Chen, D.; Hou, Q.; Jia, L.; Sun, K. Combined use of two trichoderma strains to promote growth of pakchoi (Brassica chinensis L.). Agronomy 2021, 11, 726. [Google Scholar] [CrossRef]
- Sellappan, R.; Thangavel, K.; Uthandi, S. Bioprotective potential of maize apoplastic fluid bacterium (Bacillus amyloliquefaciens) and arbuscular mycorrhizal fungi (Glomus intraradices) against Spodoptera frugiperda infestation in maize. Physiol. Mol. Plant Pathol. 2023, 127, 102050. [Google Scholar] [CrossRef]
- Faizal Azizi, M.M.; Lau, H.Y. Advanced diagnostic approaches developed for the global menace of rice diseases: A review. Can. J. Plant Pathol. 2022, 44, 627–651. [Google Scholar] [CrossRef]
- Joshi, B.; Chaudhary, A.; Singh, H.; Kumar, P.A. Prospective evaluation of individual and consortia plant growth promoting rhizobacteria for drought stress amelioration in rice (Oryza sativa L.). Plant Soil 2020, 457, 225–240. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, S.; Dixit, V.; Kumar, M.; Agarwal, L.; Chauhan, P.S.; Nautiyal, C.S. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant Signal. Behav. 2016, 11, e1071004. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Fadiji, A.E.; Babalola, O.O.; Glick, B.R.; Santoyo, G. Rhizobiome engineering: Unveiling complex rhizosphere interactions to enhance plant growth and health. Microbiol. Res. 2022, 263, 127137. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; del Carmen Rocha-Granados, M.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ferrarezi, J.A.; Carvalho-Estrada, P.d.A.; Batista, B.D.; Aniceto, R.M.; Tschoeke, B.A.P.; de Maia Andrade, P.A.; de Moura Lopes, B.; Bonatelli, M.L.; Odisi, E.J.; Azevedo, J.L.; et al. Effects of inoculation with plant growth-promoting rhizobacteria from the Brazilian Amazon on the bacterial community associated with maize in field. Appl. Soil Ecol. 2022, 170, 104297. [Google Scholar] [CrossRef]
- Coniglio, A.; Larama, G.; Molina, R.; Mora, V.; Torres, D.; Marin, A.; Avila, A.I.; Lede NoirCarlan, C.; Erijman, L.; Figuerola, E.L.; et al. Modulation of Maize Rhizosphere Microbiota Composition by Inoculation with Azospirillum argentinense Az39 (Formerly A. brasilense Az39). J. Soil Sci. Plant Nutr. 2022, 22, 3553–3567. [Google Scholar] [CrossRef]
- De Zutter, N.; Ameye, M.; Debode, J.; De Tender, C.; Ommeslag, S.; Verwaeren, J.; Vermeir, P.; Audenaert, K.; De Gelder, L. Shifts in the rhizobiome during consecutive in planta enrichment for phosphate-solubilizing bacteria differentially affect maize P status. Microb. Biotechnol. 2021, 14, 1594–1612. [Google Scholar] [CrossRef]
- Matsumura, E.E.; Secco, V.A.; Moreira, R.S.; dos Santos, O.J.A.P.; Hungria, M.; de Oliveira, A.L.M. Composition and activity of endophytic bacterial communities in field-grown maize plants inoculated with Azospirillum brasilense. Ann. Microbiol. 2015, 65, 2187–2200. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; De Bruijn, I.; Dekkers, E.; Van Der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Bziuk, N.; Maccario, L.; Sørensen, S.J.; Schikora, A.; Smalla, K. Barley Rhizosphere Microbiome Transplantation—A Strategy to Decrease Susceptibility of Barley Grown in Soils with Low Microbial Diversity to Powdery Mildew. Front. Microbiol. 2022, 13, 830905. [Google Scholar] [CrossRef] [PubMed]
- Morales Moreira, Z.P.; Chen, M.Y.; Yanez Ortuno, D.L.; Haney, C.H. Engineering plant microbiomes by integrating eco-evolutionary principles into current strategies. Curr. Opin. Plant Biol. 2023, 71, 102316. [Google Scholar] [CrossRef]
- Berg, G.; Grube, M.; Schloter, M.; Smalla, K. The plant microbiome and its importance for plant and human health. Front. Microbiol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, M.; Sharma, S.; Prasad, R. Probiotics and Plant Health; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9789811034732. [Google Scholar]
- Schlechter, R.O.; Miebach, M.; Remus-Emsermann, M.N.P. Driving factors of epiphytic bacterial communities: A review. J. Adv. Res. 2019, 19, 57–65. [Google Scholar] [CrossRef]
- van Horn, C.; Somera, T.S.; Mazzola, M. Comparative analysis of the rhizosphere and endophytic microbiomes across apple rootstock genotypes in replant orchard soils. Phytobiomes J. 2021, 5, 231–243. [Google Scholar] [CrossRef]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef]
- Jousset, A.; Lee, S.W. Coming of age for the rhizosphere microbiome transplantation. Soil Ecol. Lett. 2023, 5, 4–5. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef]
- Raklami, A.; Bechtaoui, N.; Tahiri, A.I.; Anli, M.; Meddich, A.; Oufdou, K. Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front. Microbiol. 2019, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, N.; Huang, Q.; Raza, W.; Li, R.; Vivanco, J.M.; Shen, Q. Organic acids from root exudates of banana help root colonization of PGPR strain Bacillus amyloliquefaciens NJN-6. Sci. Rep. 2015, 5, 13438. [Google Scholar] [CrossRef]
- Kost, T.; Stopnisek, N.; Agnoli, K.; Eberl, L.; Weisskopf, L. Oxalotrophy, a widespread trait of plant-associated Burkholderia species, is involved in successful root colonization of lupin and maize by Burkholderia phytofirmans. Front. Microbiol. 2013, 4, 421. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Lonhienne, T.G.A.; Yeoh, Y.K.; Donose, B.C.; Webb, R.I.; Parsons, J.; Liao, W.; Sagulenko, E.; Lakshmanan, P.; Hugenholtz, P.; et al. Crosstalk between sugarcane and a plant-growth promoting Burkholderia species. Sci. Rep. 2016, 6, 37389. [Google Scholar] [CrossRef]
- Khadeejath Rajeela, T.H.; Gopal, M.; Gupta, A.; Bhat, R.; Thomas, G. V Cross-compatibility evaluation of plant growth promoting rhizobacteria of coconut and cocoa on yield and rhizosphere properties of vegetable crops. Biocatal. Agric. Biotechnol. 2017, 9, 67–73. [Google Scholar] [CrossRef]
- De los Santos Villalobos, S.; Parra Cota, F.I.; Herrera Sepúlveda, A.; Valenzuela Aragón, B.; Estrada Mora, J.C. Colmena: Colección de microorganismos edáficos y endófitos nativos, para contribuir a la seguridad alimentaria nacional. Rev. Mex. Cienc. Agrícolas 2018, 9, 191–202. [Google Scholar] [CrossRef]
- Rojas-sánchez, B.; Guzmán-guzmán, P.; Orozco-mosqueda, M.D.C.; Rojas-s, B.; Guzm, P.; Morales-cedeño, L.R.; Orozco-mosqueda, M.C.; Saucedo-mart, B.C.; Juan, M.S.; Fadiji, A.E.; et al. Bioencapsulation of Microbial Inoculants: Mechanisms, Formulation Types and Application Techniques Bioencapsulation of Microbial Inoculants: Mechanisms, Formulation Types and Application Techniques. Appl. Biosci. 2022, 1, 198–220. [Google Scholar] [CrossRef]
- Talib, K.M.; Luhuai, J.; Chen, X.; Akbar, A.; Tahir, A.; Iqbal, I.; Ali, I. Isolation, Culture, and Maintenance of Extremophilic Fungi BT. In Extremophilic Fungi: Ecology, Physiology and Applications; Sahay, S., Ed.; Springer: Singapore, 2022; pp. 3–32. ISBN 978-981-16-4907-3. [Google Scholar]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Group/Species | Role | Beneficiated Plant Crop | Reference |
|---|---|---|---|
| Azospirillum brasilense, A. lipoferum, Gluconacetobacter diazotrophicus, A. brasilense | Biofertilization and biostimulation | Sugarcane (Saccharum officinarum), maize (Zea mays), wheat (Triticum aestivum L.), rice (Oryza sativa) | [35,36,37] |
| Bacillus amyloliquefaciens, B. aryabhattai, B. cereus, B. endophyticus, B. megaterium, B. mojavensis, B. cabrialesii, B. subtilis, B. pumilus | Biofertilization, bioprotection and biostimulation | Sugarcane (Saccharum officinarum), maize (Zea mays), wheat (Triticum aestivum L.), rice (Oryza sativa), stone pine (Pinus pinea L.), cucumber (Cucumis sativus), chickpea (Cicer arietinum), tomato (Solanum lycopersicum L.), sweet and chili peppers (Capsicum annuum L.), tea plants (Camellia sinensis), mung bean (Vigna radiata) | [38,39,40,41,42,43] |
| Enterobacter oryzae, E. asburiae, E. ludwigii, E. cloacae, E. oryziphilus, E. oryzendophyticus | Biofertilization, bioprotection and biostimulation, bioremediation | Mangart and jam (Acacia acuminate), wheat (Triticum aestivum L.), alfalfa (Medicago sativa L.) | [40,44,45,46,47] |
| Pantoea agglomerans, Pantoea dispersa, P. allii, P. alaghi | Biostimulation and bioremediation | Maize (Zea mays L.), wheat (Triticum aestivum L.) | [48,49,50,51] |
| Pseudomonas plecoglossicida, P. azotoformans, P. fluorescens, P. koreensis, P. protegens | Biofertilization, bioprotection and biostimulation, bioremediation | Pearl millet (Pennisetumglaucum), maize (Zea mays), wheat (Triticum aestivum L.), rice (Oryza sativa), tomato (Solanum lycopersicum L.), Medicago truncatula | [52,53,54,55,56,57] |
| Rhizobium meliloti, Rhizobium leguminosarum, Rhizobium phaseoli, R. etli, Rhizobium sp., Mesorhizobium loti, Mesorhizobium cicero | Biofertilization and biostimulation | Diverse legume plants, soybean (Glycine max L.), alfalfa (Medicago sativa L.), common bean (Phaseolus vulgaris), chickpea (Cicer arietinum L.) | [58,59,60,61,62] |
| Stenotrophomonas maltophilia, S. rhizophila | Biofertilization and bioprotection | Maize (Zea mays), canola (Brassica napus), chickpea (Cicer arietinum L.), wheat (Triticum aestivum L.) | [63,64,65] |
| Serratia plymuthica, S. marcescens, Rouxiella badensis | Biofertilization, bioremediation, post-harvest bioprotection | Field pumpkin (Poa pratensis), summer squash (Cucurbita pepo), berries | [66,67,68] |
| Strategy | Advantages | Disadvantages |
|---|---|---|
| Single-strain or -species inoculation |
|
|
| Dual or consortium inoculation |
|
|
| Rhizobiome transplantation |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orozco-Mosqueda, M.d.C.; Kumar, A.; Babalola, O.O.; Santoyo, G. Rhizobiome Transplantation: A Novel Strategy beyond Single-Strain/Consortium Inoculation for Crop Improvement. Plants 2023, 12, 3226. https://doi.org/10.3390/plants12183226
Orozco-Mosqueda MdC, Kumar A, Babalola OO, Santoyo G. Rhizobiome Transplantation: A Novel Strategy beyond Single-Strain/Consortium Inoculation for Crop Improvement. Plants. 2023; 12(18):3226. https://doi.org/10.3390/plants12183226
Chicago/Turabian StyleOrozco-Mosqueda, Ma. del Carmen, Ajay Kumar, Olubukola Oluranti Babalola, and Gustavo Santoyo. 2023. "Rhizobiome Transplantation: A Novel Strategy beyond Single-Strain/Consortium Inoculation for Crop Improvement" Plants 12, no. 18: 3226. https://doi.org/10.3390/plants12183226
APA StyleOrozco-Mosqueda, M. d. C., Kumar, A., Babalola, O. O., & Santoyo, G. (2023). Rhizobiome Transplantation: A Novel Strategy beyond Single-Strain/Consortium Inoculation for Crop Improvement. Plants, 12(18), 3226. https://doi.org/10.3390/plants12183226









