Syzygium cumini (L.) Extract-Derived Green Titanium Dioxide Nanoparticles Induce Caspase-Dependent Apoptosis in Hepatic Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
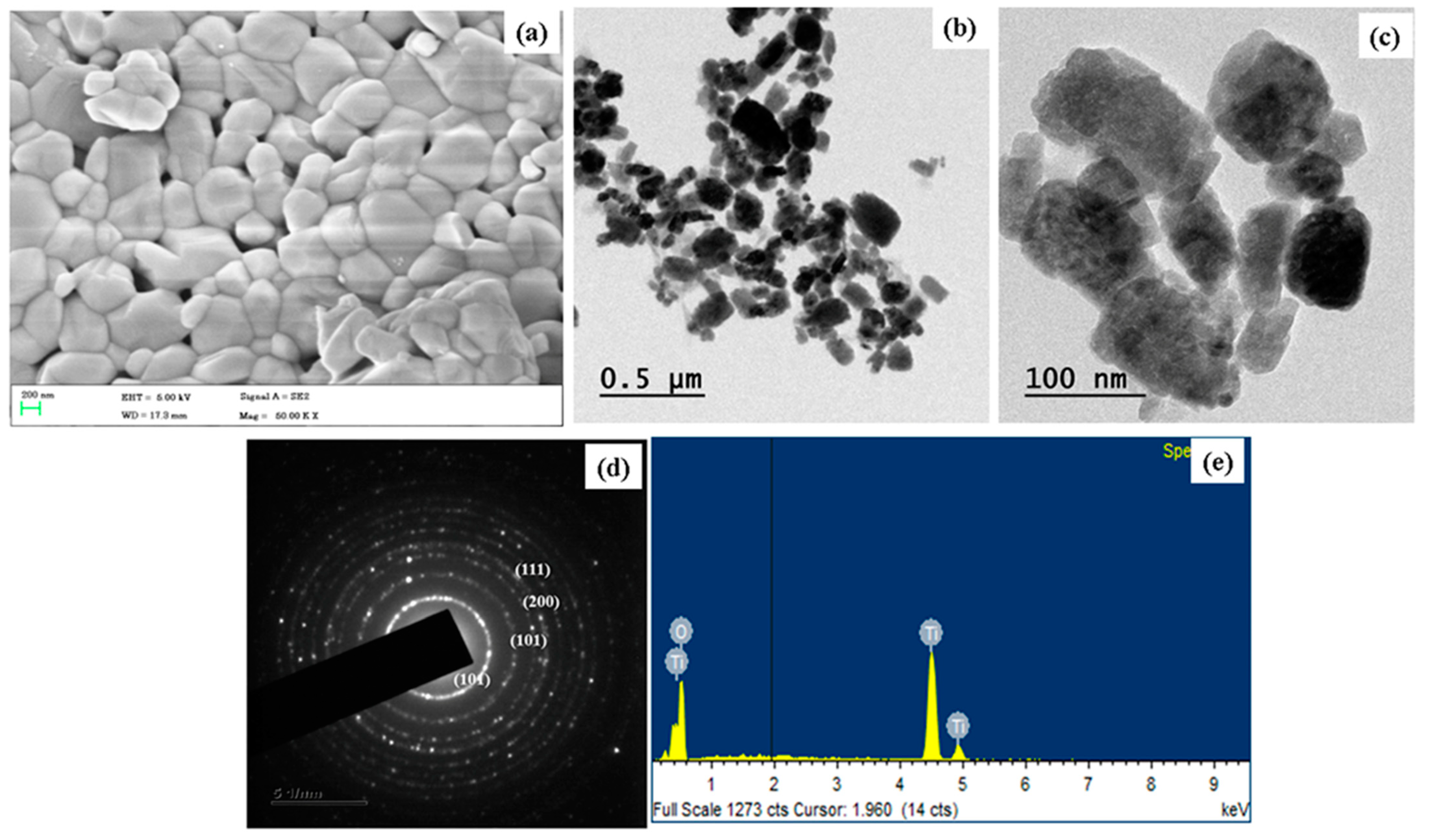
2.1. Characterization of TiO2 NPs
2.2. Morphology Analysis and Chemical Composition
2.3. Cell Viability Assay
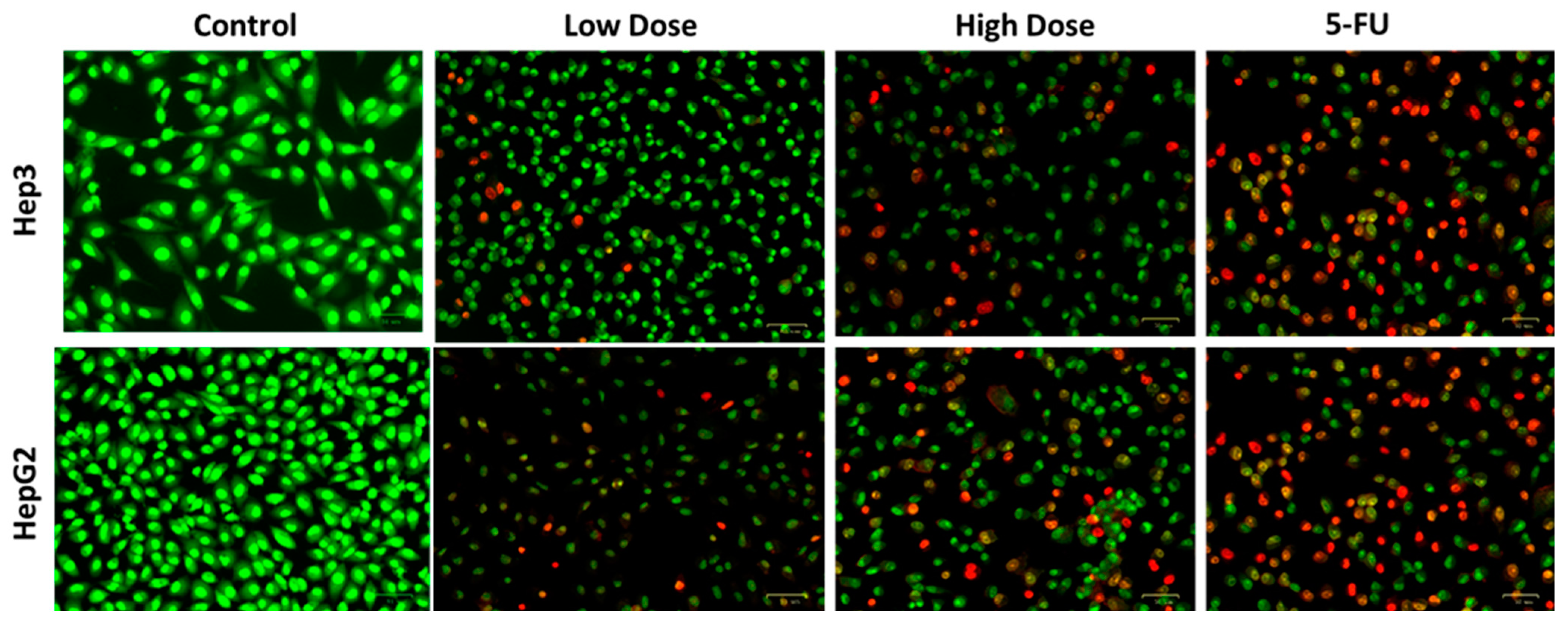
2.4. Apoptosis Analysis by Dual Staining
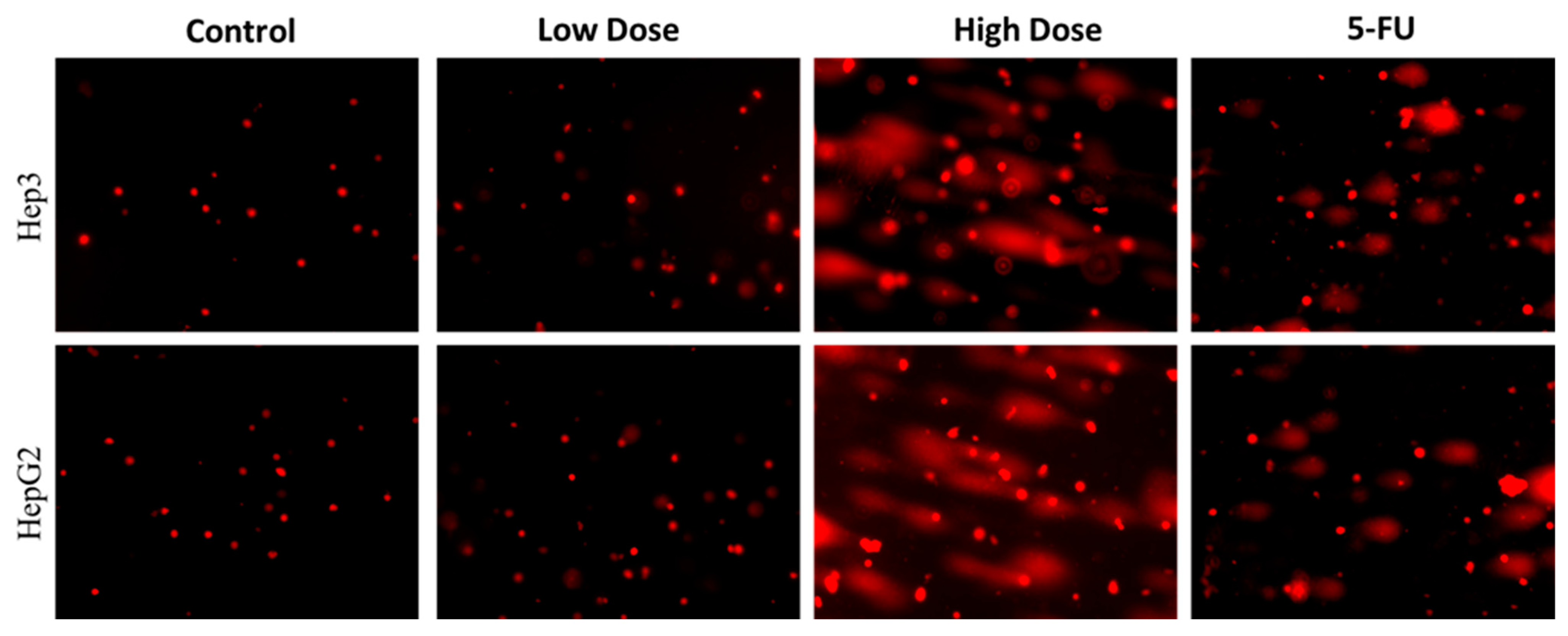
2.5. Analysis of Nuclear Damage by Comet Assay
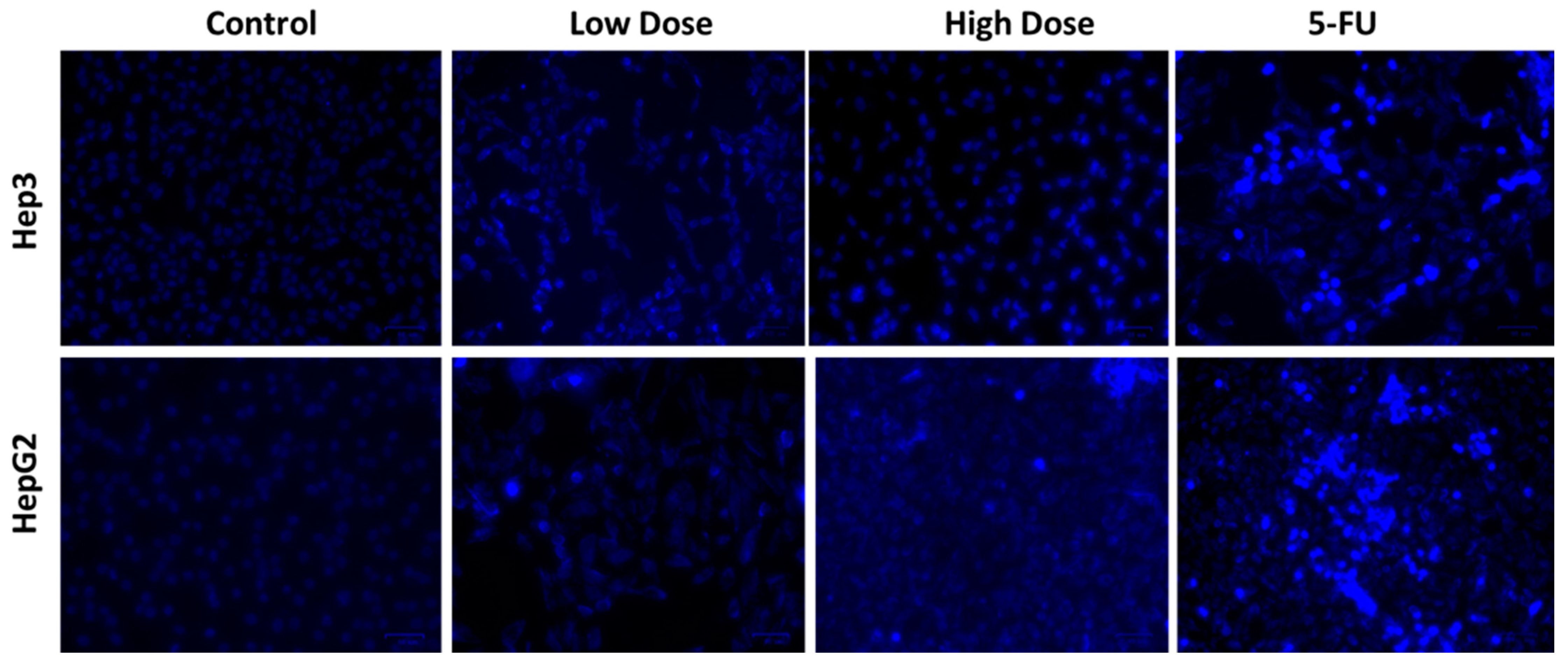
2.6. Apoptosis Analysis by DAPI Staining
2.7. MMP Analysis by Rh-123 Staining
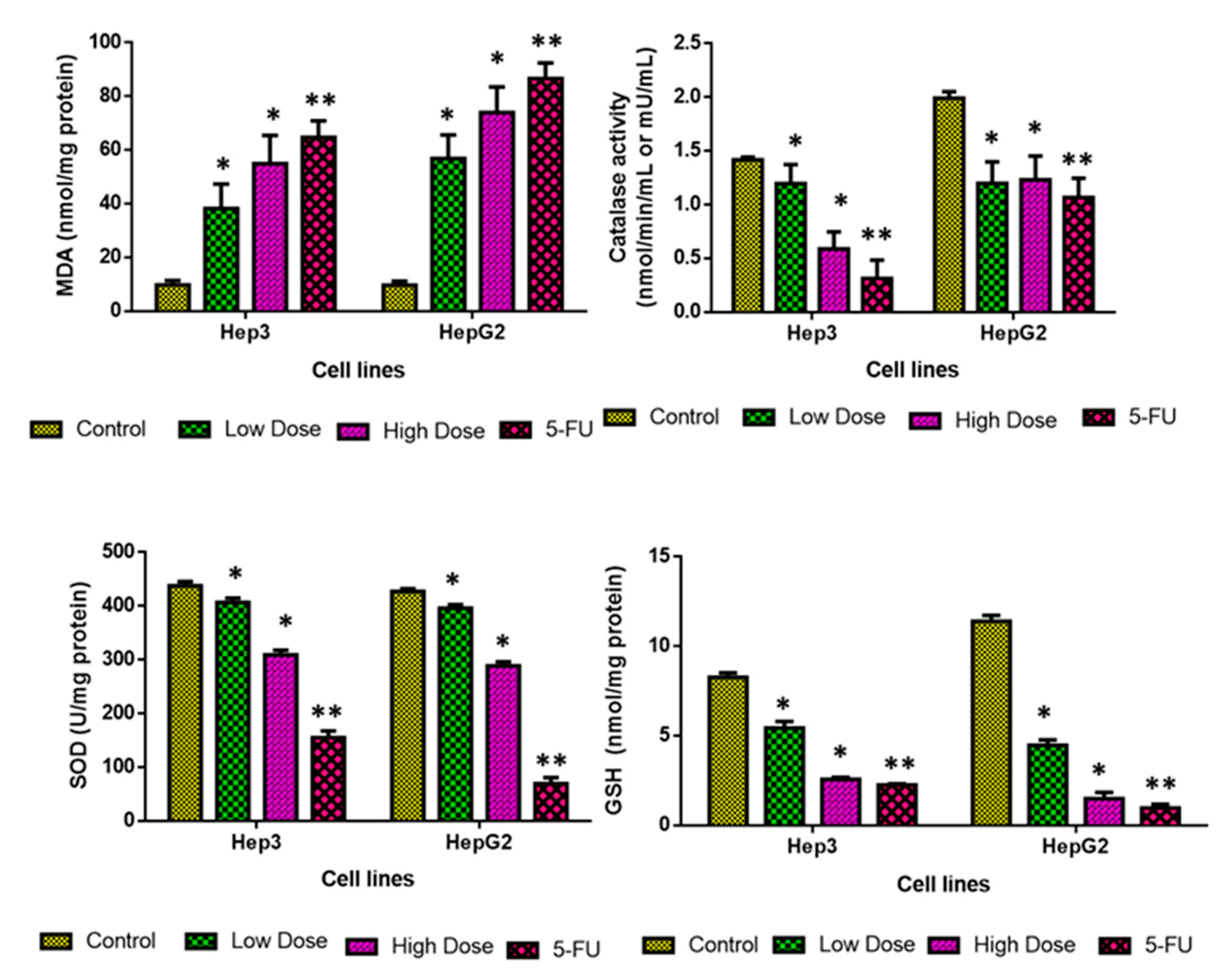
2.8. Analysis of Oxidative Stress Markers
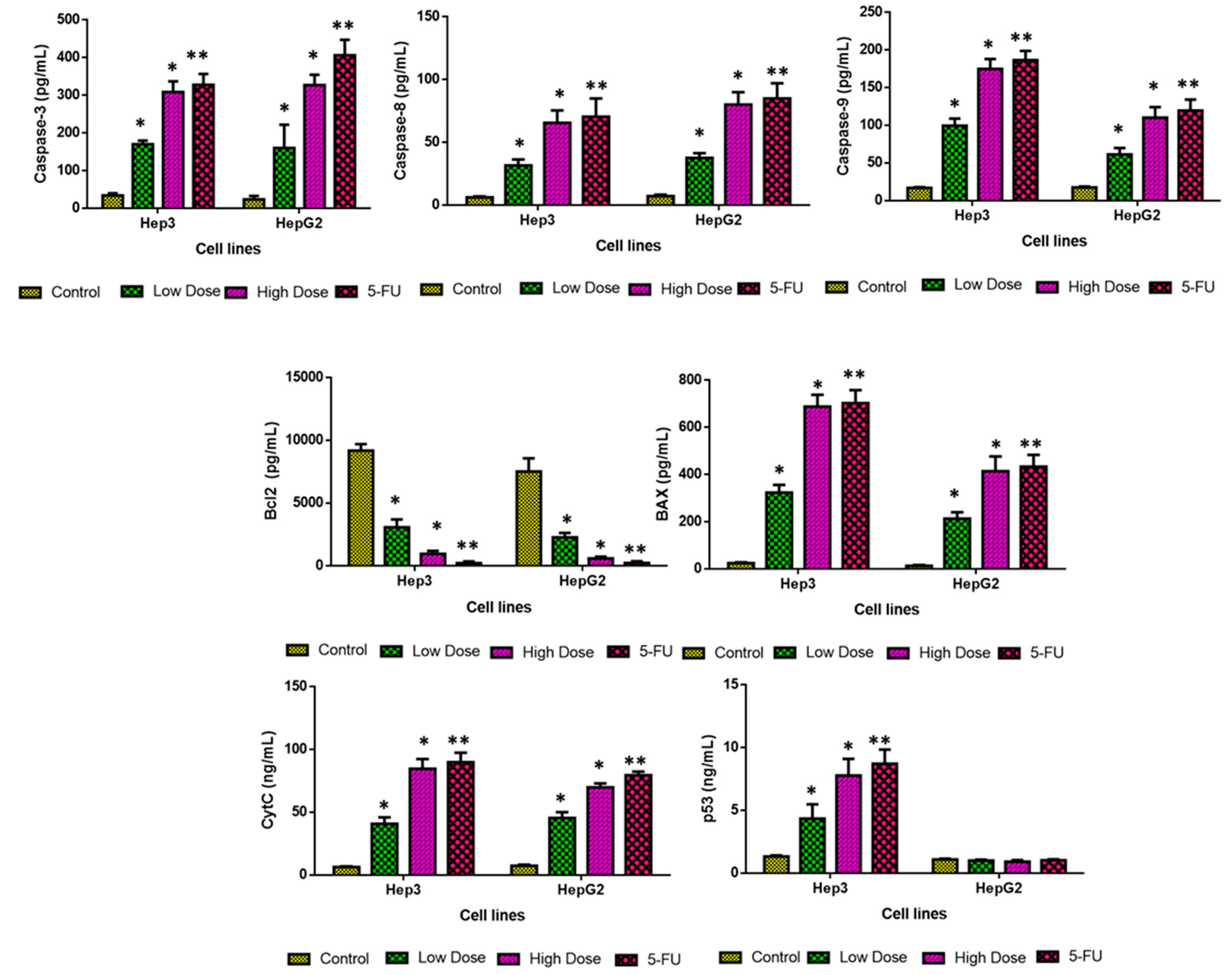
2.9. Analysis of Apoptotic Protein Levels
3. Materials and Methods
3.1. Syzygium Cumini Seed Extract Preparation
3.2. Characterization Techniques
3.3. TiO2 NPs Dispersion
3.4. MTT Assay
3.5. Dual Staining
3.6. Comet Assay
3.7. DAPI Staining
3.8. Rhodamine-123 Staining
3.9. Quantification of Oxidative Stress Markers
3.10. Measurement of Apoptotic Protein Levels
3.11. Statistical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metwally, A.A.; Abdel-Hady, A.N.A.A.; Haridy, M.A.M.; Ebnalwaled, K.; Saied, A.A.; Soliman, A.S. Wound healing properties of green (using Lawsonia inermis leaf extract) and chemically synthesized ZnO nanoparticles in albino rats. Environ. Sci. Pollut. Res. 2022, 29, 23975–23987. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Garg, P. Selective Preference of Antibody Mimetics over Antibody, as Binding Molecules, for Diagnostic and Therapeutic Applications in Cancer Therapy. Biointerface Res. Appl. Chem. 2021, 11, 10765–10775. [Google Scholar]
- Ikeda, K. Recent advances in medical management of hepatocellular carcinoma. Hepatol. Res. 2019, 49, 14–32. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World. J. Microbio. Biotech. 2021, 37, 108. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; González, B.; Izquierdo-Barba, I. Nanomaterials as promising alternative in the infection treatment. Int. J. Mol. Sci. 2019, 20, 3806. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomedicine. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef]
- Moosavi, M.A.; Sharifi, M.; Ghafary, S.M.; Mohammadalipour, Z.; Khataee, A.; Rahmati, M.; Hajjaran, S.; Łos, M.J.; Klonisch, T.; Ghavami, S. Photodynamic N-TiO2 Nanoparticle Treatment Induces Controlled ROS-mediated Autophagy and Terminal Differentiation of Leukemia Cells. Sci. Rep. 2016, 6, 34413. [Google Scholar] [CrossRef]
- Itoo, A.M.; Paul, M.; Padaga, S.G.; Ghosh, B.; Biswas, S. Nanotherapeutic Intervention in Photodynamic Therapy for Cancer. ACS Omega. 2022, 7, 45882–45909. [Google Scholar] [CrossRef]
- Zhu, Y.; Eaton, J.W.; Li, C. Titanium dioxide (TiO2) nanoparticles preferentially induce cell death in transformed cells in a Bak/Bax-independent fashion. PLoS ONE 2012, 7, e50607. [Google Scholar] [CrossRef]
- Thevenot, P.; Cho, J.; Wavhal, D.; Timmons, R.B.; Tang, L. Surface chemistry influences cancer killing effect of TiO2 nanoparticles. Nanomedicine 2008, 4, 226–236. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Mary, M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN. App. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Verma, V.; Al-Dossari, M.; Singh, J.; Rawat, M.; Kordy, M.G.; Shaban, M. A Review on Green Synthesis of TiO2 NPs: Photocatalysis and Antimicrobial Applications. Polymers 2022, 14, 1444. [Google Scholar] [CrossRef]
- Singh, S.; Maurya, I.C.; Tiwari, A.; Srivastava, P.; Bahadur, L. Green synthesis of TiO2 nanoparticles using Citrus limon juice extract as a bio-capping agent for enhanced performance of dye-sensitized solar cells. Surf. Interfaces 2022, 28, 101652. [Google Scholar] [CrossRef]
- Singh, T.; Pal, D.B.; Almalki, A.H.; Althobaiti, Y.S.; Alkhanani, M.F.; Haque, S.; Sharma, S.; Srivastava, N. Green synthesis of TiO2 bionanocomposite using waste leaves of water hyacinth: Application in antibacterial activity of toilet bacteria Serratia marcescens. Material. Lett. 2022, 316, 132012. [Google Scholar] [CrossRef]
- Erim, B.; Ciğeroğlu, Z.; Bayramoğlu, M. Green synthesis of TiO2/GO/chitosan by using leaf extract of Olea europaea as a highly efficient photocatalyst for the degradation of cefixime trihydrate under UV-A radiation exposure: An optimization study with D-optimal design. J. Mol. Struct. 2021, 1234, 130194. [Google Scholar] [CrossRef]
- Nabi, G.; Ain, Q.-U.; Tahir, M.B.; Riaz, K.N.; Iqbal, T.; Rafique, M.; Hussain, S.; Raza, W.; Aslam, I.; Rizwan, M. Green synthesis of TiO2 nanoparticles using lemon peel extract: Their optical and photocatalytic properties. Int. J. Environ. Anal. Chem. 2022, 102, 434–442. [Google Scholar] [CrossRef]
- Nabi, G.; Raza, W.; Tahir, M.B. Green synthesis of TiO2 nanoparticle using cinnamon powder extract and the study of optical properties. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1425–1429. [Google Scholar] [CrossRef]
- Amanulla, A.M.; Sundaram, R.J.M.T.P. Green synthesis of TiO2 nanoparticles using orange peel extract for antibacterial, cytotoxicity and humidity sensor applications. Mater. Today Proc. 2019, 8, 323–331. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem. Eng. J. 2018, 336, 386–396. [Google Scholar] [CrossRef]
- Abu-Dalo, M.; Jaradat, A.; Albiss, B.A.; Al-Rawashdeh, N.A. Green synthesis of TiO2 NPs/pristine pomegranate peel extract nanocomposite and its antimicrobial activity for water disinfection. J. Environ. Chem. Eng. 2019, 7, 103370. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Altynnikov, A.A.; Savinov, E.N.; Kurkin, E.N. Correlation of TiO2 photocatalytic activity and difuse refectance spectra. J. Photochem. Photobiol. A Chem. 2001, 144, 193–196. [Google Scholar] [CrossRef]
- Choudhury, B.; Dey, M.; Choudhury, A. Defect generation, d-d transition, and band gap reduction in Cu-doped TiO2 nanoparticles. Int. Nano. Lett. 2013, 3, 25. [Google Scholar] [CrossRef]
- Sivaranjani, V.; Philominathan, P.J.W.M. Synthesize of Titanium dioxide nanoparticles using Moringa oleifera leaves and evaluation of wound healing activity. Wound Med. 2016, 12, 1–5. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Characterisation of gold nanoparticles synthesised by leaf and seed extract of Syzygium cumini L. J. Exp. Nanosci. 2012, 7, 440–451. [Google Scholar] [CrossRef][Green Version]
- Ahmad, W.; Jaiswal, K.K.; Soni, S. Green synthesis of titanium dioxide (TiO2) nanoparticles by using Mentha arvensis leaves extract and its antimicrobial properties. Inorg. Nano-Met. Chem. 2020, 50, 1032–1038. [Google Scholar] [CrossRef]
- Loan, T.T.; Huong, V.H.; Huyen, N.T.; Van Quyet, L.; Bang, N.A.; Long, N.N. Anatase to rutile phase transformation of iron-doped titanium dioxide nanoparticles: The role of iron content. Optical. Mat. 2021, 111, 110651. [Google Scholar] [CrossRef]
- Narayanan, M.; Devi, P.G.; Natarajan, D.; Kandasamy, S.; Devarayan, K.; Alsehli, M.; Elfasakhany, A.; Pugazhendhi, A. Green. synthesis and characterization of titanium dioxide nanoparticles using leaf extract of Pouteria campechiana and lar-vicidal and pupicidal activity on Aedes aegypti. Environ. Res. 2021, 200, 111333. [Google Scholar] [PubMed]
- Santhoshkumar, T.; Rahuman, A.A.; Jayaseelan, C.; Rajakumar, G.; Marimuthu, S.; Kirthi, A.V.; Velayutham, K.; Thomas, J.; Venkatesan, J.; Kim, S.K. Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac. J. Trop. Med. 2014, 7, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Mehata, M.S. Phase-dependent optical and photocatalytic performance of synthesized titanium dioxide (TiO2) nanoparticles. Optik 2019, 193, 163011. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
- Egusa, S.; Ebrahem, Q.; Mahfouz, R.Z.; Saunthararajah, Y. Ligand exchange on gold nanoparticles for drug delivery and enhanced therapeutic index evaluated in acute myeloid leukemia models. Exp. Biol. Med. 2014, 239, 853–861. [Google Scholar] [CrossRef]
- Liao, J.; Jia, Y.; Wu, Y.; Shi, K.; Yang, D.; Li, P.; Qian, Z. Physical-, chemical-, and biological responsive nanomedicine for cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2020, 12, e1581. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer. Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Valentini, E.; D’Aguanno, S.; Di Martile, M.; Montesano, C.; Ferraresi, V.; Patsilinakos, A.; Sabatino, M.; Antonini, L.; Chiacchiarini, M.; Valente, S.; et al. Targeting the anti-apoptotic Bcl-2 family proteins: Machine learning virtual screening and biological evaluation of new small molecules. Theranostics 2022, 12, 2427–2444. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Chan, T.H.; Liu, M.; Kong, K.L.; Qiu, J.L.; Li, Y.; Yuan, Y.F.; Guan, X.Y. SPOCK1 is regulated by CHD1L and blocks apoptosis and promotes HCC cell invasiveness and metastasis in mice. Gastroenterology. 2013, 144, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Shaban, N.Z.; El-Kersh, M.A.R.; Bader-Eldin, M.M.; Kato, S.A.; Hamoda, A.F. Effect of Punica granatum (Pomegranate) juice extract on healthy liver and hepatotoxicity induced by diethylnitrosamine and phenobarbital in male rats. J. Med. Food 2014, 17, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, H.; Oppermann, S.; Osterlund, E.J.; Leber, B.; Andrews, D.W. Molecular pathways: Leveraging the BCL-2 interactome to kill cancer cellsmitochondrial outer membrane permeabilization and beyond. Clin. Cancer Res. 2015, 21, 2671–2676. [Google Scholar] [CrossRef] [PubMed]
- Bieging, K.T.; Mello, S.S.; Attardi, L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer. 2014, 14, 359–370. [Google Scholar] [CrossRef]
- McComb, S.; Chan, P.K.; Guinot, A.; Hartmannsdottir, H.; Jenni, S.; Dobay, M.P.; Bourquin, J.P.; Bornhauser, B.C. Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or-7. Sci. Adv. 2019, 5, eaau9433. [Google Scholar] [CrossRef]
- Souza, W.; Piperni, S.G.; Laviola, P.; Rossi, A.L.; Rossi, M.I.D.; Archanjo, B.S.; Leite, P.E.; Fernandes, M.H.; Rocha, L.A.; Granjeiro, J.M.; et al. The Two Faces of Titanium Dioxide Nanoparticles Bio-Camouflage in 3D Bone Spheroids. Sci. Rep. 2019, 9, 9309. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Gemini-Piperni, S.; Travassos, R.; Lemgruber, L.; Silva, C.R.; Rossi, A.L.; Farina, M.; Anselme, K.; Shokuhfar, T.; Shahbazian-Yassar, R.; et al. Trojan-Like Internalization of Anatase Titanium Dioxide Nanoparticles by Human Osteoblast Cells. Sci. Rep. 2016, 6, 23615. [Google Scholar] [CrossRef]
- Liu, K.; Liu, P.C.; Liu, R.; Wu, X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic. Res. 2015, 21, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Elje, E.; Hesler, M.; Rundén-Pran, E.; Mann, P.; Mariussen, E.; Wagner, S.; Dusinska, M.; Kohl, Y. The comet assay applied to HepG2 liver spheroids. Mutat. Res. /Genet. Toxicol. Environ. Mutagen. 2019, 845, 403033. [Google Scholar] [CrossRef]
- Bianco, G.; Fontanella, B.; Severino, L.; Quaroni, A.; Autore, G.; Marzocco, S. Nivalenol and deoxynivalenol affect rat intestinal epithelial cells: A concentration related study. PLoS ONE 2012, 7, e52051. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, L.; Wang, X.; Zhu, J.; Du, J.; Shen, B. Detection of Mitochondria Membrane Potential to Study CLIC4 Knockdown-induced HN4 Cell Apoptosis In Vitro. J. Vis. Exp. 2018, 137, 56317. [Google Scholar] [CrossRef]
- Hussein, M.M.A.; Gad, E.; Ahmed, M.M.; Arisha, A.H.; Mahdy, H.F.; Swelum, A.A.; Tukur, H.A.; Saadeldin, I.M. Amelioration of titanium dioxide nanoparticle reprotoxicity by the antioxidants morin and rutin. Environ. Sci. Pollut. Res. 2019, 26, 29074–29084. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.W.; Li, J.L.; Guo, B.B.; Fan, H.M.; Zhao, W.M.; Wang, H.Y. Chlorogenic acid analogues from Gynura nepalensis protect H9c2 cardiomyoblasts against H2O2-induced apoptosis. Acta Pharmacol. Sin. 2016, 37, 1413–1422. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayzah, M.; Elderdery, A.Y.; Alzerwi, N.A.N.; Alzahrani, B.; Alsrhani, A.; Alsultan, A.; Idrees, B.; Rayzah, F.; Bakhsh, Y.; Alzahrani, A.M.; et al. Syzygium cumini (L.) Extract-Derived Green Titanium Dioxide Nanoparticles Induce Caspase-Dependent Apoptosis in Hepatic Cancer Cells. Plants 2023, 12, 3174. https://doi.org/10.3390/plants12183174
Rayzah M, Elderdery AY, Alzerwi NAN, Alzahrani B, Alsrhani A, Alsultan A, Idrees B, Rayzah F, Bakhsh Y, Alzahrani AM, et al. Syzygium cumini (L.) Extract-Derived Green Titanium Dioxide Nanoparticles Induce Caspase-Dependent Apoptosis in Hepatic Cancer Cells. Plants. 2023; 12(18):3174. https://doi.org/10.3390/plants12183174
Chicago/Turabian StyleRayzah, Musaed, Abozer Y. Elderdery, Nasser A. N. Alzerwi, Badr Alzahrani, Abdullah Alsrhani, Afnan Alsultan, Bandar Idrees, Fares Rayzah, Yaser Bakhsh, Ahmed M. Alzahrani, and et al. 2023. "Syzygium cumini (L.) Extract-Derived Green Titanium Dioxide Nanoparticles Induce Caspase-Dependent Apoptosis in Hepatic Cancer Cells" Plants 12, no. 18: 3174. https://doi.org/10.3390/plants12183174
APA StyleRayzah, M., Elderdery, A. Y., Alzerwi, N. A. N., Alzahrani, B., Alsrhani, A., Alsultan, A., Idrees, B., Rayzah, F., Bakhsh, Y., Alzahrani, A. M., Subbiah, S. K., & Mok, P. L. (2023). Syzygium cumini (L.) Extract-Derived Green Titanium Dioxide Nanoparticles Induce Caspase-Dependent Apoptosis in Hepatic Cancer Cells. Plants, 12(18), 3174. https://doi.org/10.3390/plants12183174







