Exploring the Impact of Salicylic Acid and Farmyard Manure on Soil Rhizospheric Properties and Cadmium Stress Alleviation in Maize (Zea mays L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crop Growth Conditions
2.2. Measured Parameters
2.3. Statistical Analysis
3. Results
3.1. Effect of Salicylic Acid (SA) and Farmyard Manure (FYM) on Root and Shoot Length of Maize Plant under Cd Stress
3.2. Effect of SA and FYM on Plant Height of Maize under Cd Stress
3.3. Effect of SA and FYM on Root, Shoot, and Plant Weight of Maize under Cd Stress
3.4. Effect of SA and FYM on Length and Number of Leaves of Maize under Cd Stress
3.5. Effect of SA and FYM on Chlorophyll Content of Maize under Cd Stress
3.6. Effect of SA and FYM on NPK Uptake of Maize under Cd Stress
3.7. Effect of SA and FYM on Crop Growth Rate (CGR) and Net Assimilation Rate (NAR) of Maize under Cd Stress
3.8. Effect of SA and FYM on Soil Organic Carbon (SOC), Dissolved Organic Nitrogen (DON), Dissolved Organic Carbon (DOC), Nitrogen Mineralization (NMIN), and Soil Respiration (SR) of Maize-Grown Soil under Cd Stress
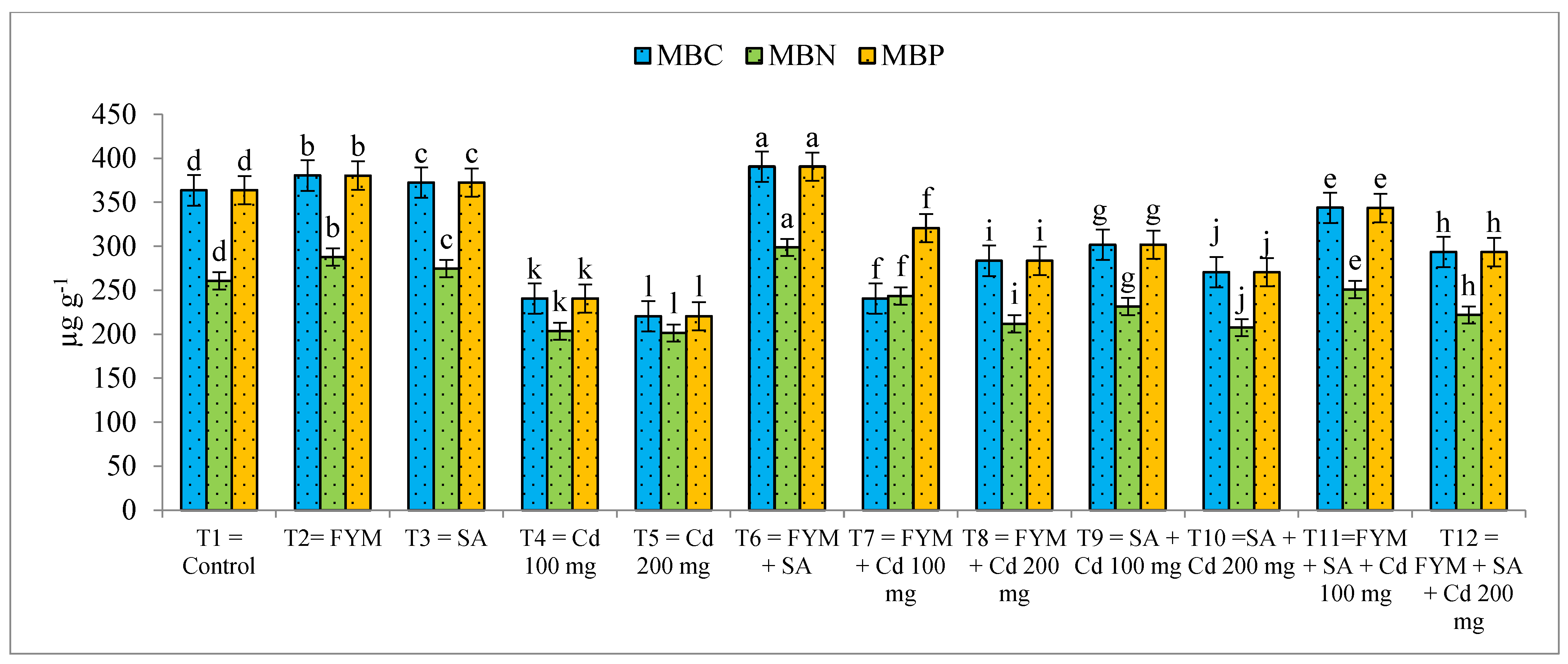
3.9. Effect of SA and FYM on Microbial Biomass Carbon (MBC), Phosphorus (MBP), and Nitrogen (MBN) of Maize-Grown Soil under Cd Stress
3.10. Effect of Farmyard Manure and Salicylic Acid on Soil pH, Mineral N, Bray P, and Exchangeable-K under Cadmium Stress Condition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Ali, A.; Ullah Khan, M.A.; Khan, T.A. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities—A review. Environ. Sci. Pollut. Res. 2019, 26, 12673–12688. [Google Scholar] [CrossRef]
- Imran, K.; Seleiman, M.F.; Chattha, M.U.; Jalal, R.S.; Mahmood, F.; Hassan, F.A.; Izzet, W.; Alhammad, B.A.; Rana, R.; Hassan, M.U. Enhancing antioxidant defense system of mung bean with a salicylic acid exogenous application to mitigate cadmium toxicity. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12303. [Google Scholar]
- Seleiman, M.F.; Selim, S.; Jaakkola, S.; Mäkelä, P.S. Chemical composition and in vitro digestibility of whole-crop maize fertilized with synthetic fertilizer or digestate and harvested at two maturity stages in Boreal growing conditions. Agric. Food Sci. 2017, 26, 47–55. [Google Scholar] [CrossRef]
- Sang, L.; Zhu, G.; Xu, Y.; Sun, Z.; Zhang, Z.; Tong, H. Effects of Agricultural Large-And Medium-Sized Reservoirs on Hydrologic Processes in the Arid Shiyang River Basin, Northwest China. Water Resour. Res. 2023, 59, e2022WR033519. [Google Scholar] [CrossRef]
- PARC (Pakistan Agricultural Research Council). 2023. (n.d.). Home. Retrieved May 8. Available online: https://www.parc.gov.pk/ (accessed on 1 January 2023).
- FAO (Food and Agriculture Organization of the United Nations). 2023. (n.d.). FAO Country Profiles: Pakistan. Retrieved May 8. Available online: http://www.fao.org/countryprofiles/index/en/?iso3=PAK (accessed on 1 March 2020).
- Aslam, M.M.; Okal, E.J.; Waseem, M. Cadmium toxicity impacts plant growth and plant remediation strategies. Plant Growth Regul. 2023, 99, 397–412. [Google Scholar] [CrossRef]
- Wei, X.; Bai, X.; Wen, X.; Liu, L.; Xiong, J.; Yang, C. A large and overlooked Cd source in karst areas: The migration and origin of Cd during soil formation and erosion. Sci. Total Environ. 2023, 895, 165126. [Google Scholar] [CrossRef]
- Malik, R.N.; Husain, S.Z.; Nazir, I. Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pak. J. Bot. 2010, 42, 291–301. [Google Scholar]
- Ali, H.Q.; Yasir, M.U.; Farooq, A.; Khan, M.; Salman, M.; Waqar, M. Tanneries impact on groundwater quality: A case study of Kasur city in Pakistan. Environ. Monit. Assess. 2022, 194, 823. [Google Scholar] [CrossRef]
- Qiu, D.; Zhu, G.; Bhat, M.A.; Wang, L.; Liu, Y.; Sang, L.; Lin, X.; Zhang, W.; Sun, N. Water use strategy of nitraria tangutorum shrubs in ecological water delivery area of the lower inland river: Based on stable isotope data. J. Hydrol. 2023, 624, 129918. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lu, D.; Sheng, H.; Xia, J.; Kan, P.; Yao, Z.; Chen, H.; Li, G.; Zhu, D.Z.; Liu, H. Constructed wetlands as hotspots of antibiotic resistance genes and pathogens: Evidence from metagenomic analysis in Chinese rural areas. J. Hazard. Mater. 2023, 447, 130778. [Google Scholar] [CrossRef] [PubMed]
- Noriega, G.; Caggiano, E.; Lecube, M.L.; Cruz, D.S.; Batlle, A.; Tomaro, M.; Balestrasse, K.B. The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. BioMetals 2012, 25, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Mateo, A.; Funck, D.; Mühlenbock, P.; Kular, B.; Mullineaux, P.M.; Karpiński, S. Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J. Exp. Bot. 2006, 57, 1795–1807. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Gao, F.; Liu, Y.; Lang, S.; Wang, C.; Zhang, D. Effect of ultrasound combined with exogenous GABA treatment on polyphenolic metabolites and antioxidant activity of mung bean during germination. Ultrason. Sonochem. 2023, 94, 106311. [Google Scholar] [CrossRef]
- Hafez, E.H.; Seleiman, M.F. Response of barley quality traits, yield and antioxidant enzymes to water-stress and chemical inducers. Intern. J. Plant Prod. 2017, 11, 477–490. [Google Scholar]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K. Salicylic Acid Alleviates the Cadmium Toxicity in Barley Seedlings. Plant Phy. 2003, 132, 272–281. [Google Scholar] [CrossRef]
- Iqbal, N.; Fatma, M.; Gautam, H.; Sehar, Z.; Rasheed, F.; Khan, M.I.R.; Khan, N.A. Salicylic acid increases photosynthesis of drought-grown mustard plants effectively with sufficient N via regulation of ethylene, abscisic acid, and nitrogen-use efficiency. J. Plant Growth Regul. 2022, 41, 1966–1977. [Google Scholar] [CrossRef]
- Singh, T.B.; Ali, A.; Prasad, M.; Yadav, A.; Shrivastav, P.; Goyal, D.; Dantu, P.K. Role of Organic Fertilizers in Improving Soil Fertility. In Contaminants in Agriculture; Naeem, M., Ansari, A., Gill, S., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Majeed, A.; Niaz, A.; Rizwan, M.; Imran, M.; Alsahli, A.A.; Alyemeni, M.N.; Ali, S. Effects of biochar, farm manure, and pressmud on mineral nutrients and cadmium availability to wheat (Triticum aestivum L.) in Cd-contaminated soil. Physiol. Plant. 2021, 173, 191–200. [Google Scholar] [CrossRef]
- Reddy, Y.R.; Reddy, G.H. Principle of Agronomy, 3rd ed.; Kalyani Publishers: Ludhiana, India, 2003; pp. 203–253. [Google Scholar]
- Kumar, V.; Singh, R.K.; Kumar, D.M. Effect of farm yard manure and Sulphur on production of Indian mustard: A review. J. Pharmacogn. Phytochem. 2019, 8, 2290–2294. [Google Scholar]
- Rizwan, M.; Ali, S.; Akbar, M.; Shakoor, M.B.; Mahmood, A.; Ishaque, W.; Hussain, A. Foliar application of aspartic acid lowers cadmium uptake and Cd-induced oxidative stress in rice under Cd stress. Environ. Sci. Pollut. Res. 2017, 24, 21938–21947. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, F.; Kuzyakov, Y.; Xiao, H.; Hoang, D.T.T.; Pu, S.; Razavi, B.S. Nutrients in the rhizosphere: A meta-analysis of content, availability, and influencing factors. Sci. Total Environ. 2022, 826, 153908. [Google Scholar] [CrossRef]
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of Heavy Metal-Contaminated Sites: Eco-environmental Concerns, Field Studies, Sustainability Issues, and Future Prospects. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; p. 249. [Google Scholar] [CrossRef]
- Schagerl, M.; Künzl, G. Chlorophyll a extraction from freshwater algae—A reevaluation. Biologia 2007, 62, 270–275. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Barr, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Hunt, R. Plant Growth Analysis; Arnold, E., Ed.; Institute of Terrestrial Ecology: Edinburgh, UK, 1978; pp. 26–38. [Google Scholar]
- Smolander, A.; Kitunen, V. Soil microbial activities and characteristics of dissolved organic C and N in relation to tree species. Soil Biol. Biochem. 2002, 34, 651–660. [Google Scholar] [CrossRef]
- Sofy, M.R.; Seleiman, M.F.; Alhammad, B.A.; Alharbi, B.M.; Mohamed, H.I. Minimizing adverse effects of Pb on maize plants by combined treatment with jasmonic and salicylic acids and proline. Agronomy 2020, 10, 699. [Google Scholar] [CrossRef]
- Bandyopadhyay, K.K.; Misra, A.K.; Ghosh, P.K.; Hati, K.M. Effect of integrated use of farmyard manure and chemical fertilizers on soil physical properties and productivity of soybean. Soil Tillage Res. 2010, 110, 115–125. [Google Scholar] [CrossRef]
- Nasiri, Y.; Zandi, H.; Morshedloo, M.R. Effect of salicylic acid and ascorbic acid on essential oil content and composition of dragonhead (Dracocephalum moldavica L.) under organic farming. J. Essent. Oil Bear. Pl. 2018, 21, 362–373. [Google Scholar] [CrossRef]
- Kashif, M.; Sang, Y.; Mo, S.; Rehman, S.U.; Khan, S.; Khan, M.R.; He, S.; Jiang, C. Deciphering the biodesulfurization pathway employing marine mangrove Bacillus aryabhattai strain NM1-A2 according to whole genome sequencing and transcriptome analyses. Genomics 2023, 115, 110635. [Google Scholar] [CrossRef]
- Tayyab, N.; Naz, R.; Yasmin, H.; Nosheen, A.; Keyani, R.; Sajjad, M.; Roberts, T.H. Combined seed and foliar pre-treatments with exogenous methyl jasmonate and salicylic acid mitigate drought-induced stress in maize. PLoS ONE 2020, 15, e0232269. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Hayat, S.; Khalique, G.; Wani, A.S.; Alyemeni, M.N.; Ahmad, A. Protection of growth in response to 28-homobrassinolide under the stress of cadmium and salinity in wheat. Int. J. Biol. Macromol. 2014, 64, 130–136. [Google Scholar] [CrossRef]
- Zaid, A.; Mohammad, F.; Fariduddin, Q. Plant growth regulators improve growth, photosynthesis, mineral nutrient and antioxidant system under cadmium stress in menthol mint (Mentha arvensis L.). Physiol. Mol. Biol. Plants. 2020, 26, 25–39. [Google Scholar] [CrossRef] [PubMed]
- El-Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2022, 52, 675–726. [Google Scholar] [CrossRef]
- Farzadfar, S.; Zarinkamar, F. Morphological and anatomical responses of Matricaria chamomilla plants to cadmium and calcium. Adv. Environ. Biol. 2012, 6, 1603–1609. [Google Scholar]
- Ali, S.; Abbas, Z.; Seleiman, M.F.; Rizwan, M.; YAVAŞ, İ.; Alhammad, B.A.; Shami, A.; Hasanuzzaman, M.; Kalderis, D. Glycine betaine accumulation, significance and interests for heavy metal tolerance in plants. Plants 2020, 9, 896. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931. [Google Scholar] [CrossRef]
- Chaffei, C.; Pageau, K.; Suzuki, A.; Gouia, H.; Ghorbel, M.H.; Masclaux-Daubresse, C. Cadmium toxicity induced changes in nitrogen management in Lycopersicon esculentum leading to a metabolic safeguard through an amino acid storage strategy. Plant Cell Physiol. 2004, 45, 1681–1693. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-ur-Rehman, M.; Hannan, F.; Keller, C.; Al-Wabel, M.I.; Ok, Y.S. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016, 130, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Alotaibi, M.; Alhammad, B.; Alharbi, B.; Refay, Y.; Badawy, S. Effects of ZnO nanoparticles and biochar of rice straw and cow manure on characteristics of contaminated soil and sunflower productivity, oil quality, and heavy metals uptake. Agronomy 2020, 10, 790. [Google Scholar] [CrossRef]
- Pan, J.; Guan, M.; Xu, P.; Chen, M.; Cao, Z. Salicylic acid reduces cadmium (Cd) accumulation in rice (Oryza sativa L.) by regulating root cell wall composition via nitric oxide signaling. Sci. Total Environ. 2021, 797, 149202. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, T.; Zia-ur-Rehman, M.; Naeem, A.; Nawaz, R.; Ali, S.; Murtaza, G.; Rizwan, M. Photosynthesis and growth response of maize (Zea mays L.) hybrids exposed to cadmium stress. Environ. Sci. Pollut. Res. 2017, 24, 5521–5529. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, C.; Liu, H.; Wang, W.; Sun, R.; Li, M.; Shi, Y.; Zhu, D.; Du, W.; Ma, L.; et al. Fine root biomass and morphology in a temperate forest are influenced more by canopy water addition than by canopy nitrogen addition. Front. Ecol. Evol. 2023, 11, 1132248. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Ali, H.H.; Iqbal, M.A.; Erinle, K.O.; Javed, T.; Iqbal, J.; Hashmi, M.I.U.; Mumtaz, M.Z.; Salama, E.A.A.; Kalaji, H.M.; et al. Cytokinin Production by Azospirillum brasilense Contributes to Increase in Growth, Yield, Antioxidant, and Physiological Systems of Wheat (Triticum aestivum L.). Front. Microbiol. 2022, 13, 886041. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, X.; Dungait, J.A.J.; Green, S.M.; Wen, X.; Quine, T.A. Contribution of soil microbial necromass to SOC stocks during vegetation recovery in a subtropical karst ecosystem. Sci. Total Environ. 2021, 761, 143945. [Google Scholar] [CrossRef]
- Meier, I.C.; Finzi, A.C.; Phillips, R.P. Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. Soil Biol. Biochem. 2017, 106, 119–128. [Google Scholar] [CrossRef]
- Nie, S.; Mo, S.; Gao, T.; Yan, B.; Shen, P.; Kashif, M.; Zhang, Z.; Li, J.; Jiang, C. Coupling effects of nitrate reduction and sulfur oxidation in a subtropical marine mangrove ecosystem with Spartina alterniflora invasion. Sci. Total Environ. 2023, 862, 160930. [Google Scholar] [CrossRef]
- Tanaka, H.; Kyaw, K.M.; Toyota, K.; Motobayashi, T. Influence of application of rice straw, farmyard manure, and municipal biowastes on nitrogen fixation, soil microbial biomass N, and mineral N in a model paddy microcosm. Biol. Fertil. Soils. 2005, 42, 501–505. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, Z.; Yin, X.; Zhu, Y. Impacts of biochars on bacterial community shifts and biodegradation of antibiotics in an agricultural soil during short-term incubation. Sci. Total Environ. 2021, 771, 144751. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Ali, H.H.; Erinle, K.O.; Wani, S.H.; Okon, O.G.; Nadeem, M.A.; Nawaz, M.; Bodlah, M.A.; Waqas, M.M.; Iqbal, J.; et al. Inoculation of Azospirillum brasilense and exogenous application of trans-zeatin riboside alleviates arsenic induced physiological damages in wheat (Triticum aestivum). Environ. Sci. Pollut. Res. 2022, 29, 33909–33919. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, X.; Shen, Z.; Gu, Y.; He, L.; Zhang, M.; Lu, N. Single-atom Fe nanozymes coupling with atomic clusters as superior oxidase mimics for ratiometric fluorescence detection. Chem. Eng. J. 2023, 469, 143923. [Google Scholar] [CrossRef]
- Saviozzi, A.; Biasci, A.; Riffaldi, R.; Levi-Minzi, R. Long-term effects of farmyard manure and sewage sludge on some soil biochemical characteristics. Biol. Fertil. Soils. 1999, 30, 100–106. [Google Scholar] [CrossRef]
- Joseph, B.; Jini, D.; Sujatha, S. Insight into the Role of Exogenous Salicylic Acid on Plants Grown under Salt Environment. Asian J. Crop Sci. 2020, 2, 226–235. [Google Scholar] [CrossRef]
- Moore, A.D.; Alva, A.K.; Collins, H.P.; Boydston, R.A. Mineralization of Nitrogen from Biofuel By-products and Animal Manures Amended to a Sandy Soil. Commun. Soil Sci. Plant Anal. 2010, 41, 1315–1326. [Google Scholar] [CrossRef]



| Physiochemical Properties of Soil | Values |
|---|---|
| Ph | 6.88 |
| EC | 4.97 dSm−1 |
| Organic matter | 4.93% |
| Moisture content | 19.01% |
| Concentration of nutrients | |
| Potassium K | 44.49 mg L−1 |
| Sodium Na | 25.01 mg L−1 |
| Magnesium Mg | 22.30 mg L−1 |
| Calcium Ca | 168.69 mg L−1 |
| Soil composition percentage | |
| Clay | 50% |
| Sand | 25% |
| Silt | 25% |
| Treatments | Root Length (cm) | Shoot Length (cm) | Plant Height (cm) |
|---|---|---|---|
| T1 = Control | 19.37 d | 52.43 d | 71.81 d |
| T2 = Farmyard manure | 21.26 b | 54.93 b | 76.19 b |
| T3 = Salicylic acid | 20.73 c | 53.15 c | 73.88 c |
| T4 = Cadmium (100 mg/kg) | 12.43 k | 45.55 k | 57.98 k |
| T5 = Cadmium (200 mg/kg) | 11.33 l | 44.13 l | 55.46 l |
| T6 = Farmyard manure + Salicylic acid | 22.43 a | 55.25 a | 77.68 a |
| T7 = FYM + Cd (100 mg/kg) | 17.92 f | 50.74 f | 50.74 f |
| T8 = FYM + Cd (200 mg/kg) | 14.57 i | 47.15 i | 47.15 i |
| T9 = Salicylic acid + Cd (100 mg/kg) | 16.63 g | 49.22 g | 49.22 g |
| T10 = Salicylic acid + Cd (200 mg/kg) | 13.64 j | 46.64 j | 60.28 j |
| T11 = FYM + Salicylic acid + Cd (100 mg/kg) | 18.35 e | 51.28 e | 69.63 e |
| T12 = FYM + Salicylic acid + Cd (200 mg/kg) | 15.72 h | 48.23 h | 48.23 h |
| Treatments | Root Fresh Weight (g) | Shoot Fresh Weight (g) | Plant Fresh Weight (g) | Plant Dry Weight (g) |
|---|---|---|---|---|
| T1 = Control | 08.75 d | 24.43 d | 33.18 d | 19.26 d |
| T2 = Farmyard manure | 10.27 b | 26.40 b | 36.67 b | 22.38 b |
| T3 = Salicylic acid | 09.87 c | 25.26 c | 35.14 c | 21.08 c |
| T4 = Cadmium (100 mg/kg) | 03.95 k | 17.66 k | 21.61 k | 10.39 k |
| T5 = Cadmium (200 mg/kg) | 03.42 l | 16.53 l | 19.95 l | 08.87 l |
| T6 = Farmyard manure + Salicylic acid | 10.95 a | 27.63 a | 38.58 a | 23.09 a |
| T7 = FYM + Cd (100 mg/kg) | 06.73 f | 22.43 f | 29.16 f | 17.21 f |
| T8 = FYM + Cd (200 mg/kg) | 05.65 i | 19.56 i | 25.21 i | 13.12 i |
| T9 = Salicylic acid + Cd (100 mg/kg) | 06.45 g | 21.66 g | 28.12 g | 15.93 g |
| T10 = Salicylic acid + Cd (200 mg/kg) | 04.66 j | 18.26 j | 22.93 j | 12.10 j |
| T11 = FYM + Salicylic acid + Cd (100 mg/kg) | 07.84 e | 23.73 e | 31.58 e | 18.38 e |
| T12 = FYM + Salicylic acid + Cd (200 mg/kg) | 06.14 h | 20.66 h | 26.80 h | 14.31 h |
| Treatments | Length of Leaf (cm) | Number of Leaves (n) |
|---|---|---|
| T1 = Control | 14.65 d | 08.66 d |
| T2 = Farmyard manure | 16.54 b | 10.66 b |
| T3 = Salicylic acid | 15.94 c | 10.66 c |
| T4 = Cadmium (100 mg/kg) | 09.65 k | 01.66 k |
| T5 = Cadmium (200 mg/kg) | 08.92 l | 01.66 l |
| T6 = Farmyard manure + Salicylic acid | 17.82 a | 11.66 a |
| T7 = FYM + Cd (100 mg/kg) | 13.14 f | 06.66 f |
| T8 = FYM + Cd (200 mg/kg) | 10.46 i | 03.66 i |
| T9 = Salicylic acid + Cd (100 mg/kg) | 12.86 g | 05.66 g |
| T10 = Salicylic acid + Cd (200 mg/kg) | 10.12 j | 02.66 j |
| T11 = FYM + Salicylic acid + Cd (100 mg/kg) | 13.43 e | 07.66 e |
| T12 = FYM + Salicylic acid + Cd (200 mg/kg) | 13.14 h | 04.66 h |
| Treatments | SOC (g kg−1) | DOC (g kg−1) | DON (g kg−1) | NMIN | SR (g CO2/m2/day) |
|---|---|---|---|---|---|
| T1 = Control | 1.333 d | 137.6 d | 17.663 d | 69.740 d | 22.77 d |
| T2 = Farmyard manure | 1.480 b | 141.7 b | 19.463 b | 71.710 b | 24.72 b |
| T3 = Salicylic acid | 1.380 c | 140.7 c | 18.857 c | 70.77 c | 23.66 c |
| T4 = Cadmium (100 mg/kg) | 0.740 k | 120.3 k | 10.823 k | 62.287 k | 15.69 k |
| T5 = Cadmium (200 mg/kg) | 0.666 l | 118.5 l | 09.620 l | 61.693 l | 14.57 l |
| T6 = Farmyard manure + Salicylic acid | 1.540 a | 143.8 a | 20.643 a | 72.853 a | 25.62 a |
| T7 = FYM + Cd (100 mg/kg) | 1.220 f | 135.5 f | 15.857 f | 67.493 f | 20.37 f |
| T8 = FYM + Cd (200 mg/kg) | 0.933 i | 128.7 i | 12.500 i | 64.737 i | 17.62 i |
| T9 = Salicylic acid + Cd (100 mg/kg) | 1.180 g | 133.6 g | 14.773 g | 66.557 g | 19.52 g |
| T10 = Salicylic acid + Cd (200 mg/kg) | 0.856 j | 126.7 j | 11.407 j | 63.243 j | 16.53 j |
| T11 = FYM + Salicylic acid + Cd (100 mg/kg) | 1.280 e | 136.6 e | 16.467 e | 68.460 e | 21.61 e |
| T12 = FYM + Salicylic acid + Cd (200 mg/kg) | 1.080 h | 130.6 h | 13.827 h | 65.323 h | 18.52 h |
| Treatments | Soil pH | Mineral N (mg kg−1) | Bray P (mg kg−1) | Exchangeable-K (mg kg−1) |
|---|---|---|---|---|
| T1 = Control | 6.960 d | 80.04 d | 7.526 d | 170.77 d |
| T2 = Farmyard manure | 7.136 b | 82.93 b | 7.746 b | 172.52 b |
| T3 = Salicylic acid | 7.026 c | 81.64 c | 7.640 c | 171.59 c |
| T4 = Cadmium (100 mg/kg) | 6.213 k | 69.49 k | 6.536 k | 159.23 k |
| T5 = Cadmium (200 mg/kg) | 6.143 l | 68.39 l | 6.446 l | 158.53 l |
| T6 = Farmyard manure + Salicylic acid | 7.246 a | 83.59 a | 7.846 a | 173.58 a |
| T7 = FYM + Cd (100 mg/kg) | 6.743 f | 78.79 f | 7.343 f | 168.53 f |
| T8 = FYM + Cd (200 mg/kg) | 6.436 i | 74.48 i | 6.960 i | 165.48 i |
| T9 = Salicylic acid + Cd (100 mg/kg) | 6.653 g | 77.60 g | 7.256 g | 167.84 g |
| T10 = Salicylic acid + Cd (200 mg/kg) | 6.340 j | 73.69 j | 6.853 j | 163.68 j |
| T11 = FYM + Salicylic acid + Cd (100 mg/kg) | 6.836 e | 79.55 e | 7.466 e | 169.75 e |
| T12 = FYM + Salicylic acid + Cd (200 mg/kg) | 6.563 h | 76.45 h | 7.153 h | 166.51 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, H.H.; Shehzadi, N.; Zaheer, M.S.; Seleiman, M.F.; Aldhuwaib, K.J.; Din Khan, W.u.; Raza, A. Exploring the Impact of Salicylic Acid and Farmyard Manure on Soil Rhizospheric Properties and Cadmium Stress Alleviation in Maize (Zea mays L.). Plants 2023, 12, 3115. https://doi.org/10.3390/plants12173115
Ali HH, Shehzadi N, Zaheer MS, Seleiman MF, Aldhuwaib KJ, Din Khan Wu, Raza A. Exploring the Impact of Salicylic Acid and Farmyard Manure on Soil Rhizospheric Properties and Cadmium Stress Alleviation in Maize (Zea mays L.). Plants. 2023; 12(17):3115. https://doi.org/10.3390/plants12173115
Chicago/Turabian StyleAli, Hafiz Haider, Nimra Shehzadi, Muhammad Saqlain Zaheer, Mahmoud F. Seleiman, Khalid J. Aldhuwaib, Waqas ud Din Khan, and Ali Raza. 2023. "Exploring the Impact of Salicylic Acid and Farmyard Manure on Soil Rhizospheric Properties and Cadmium Stress Alleviation in Maize (Zea mays L.)" Plants 12, no. 17: 3115. https://doi.org/10.3390/plants12173115
APA StyleAli, H. H., Shehzadi, N., Zaheer, M. S., Seleiman, M. F., Aldhuwaib, K. J., Din Khan, W. u., & Raza, A. (2023). Exploring the Impact of Salicylic Acid and Farmyard Manure on Soil Rhizospheric Properties and Cadmium Stress Alleviation in Maize (Zea mays L.). Plants, 12(17), 3115. https://doi.org/10.3390/plants12173115









