Lemnaceae as Novel Crop Candidates for CO2 Sequestration and Additional Applications
Abstract
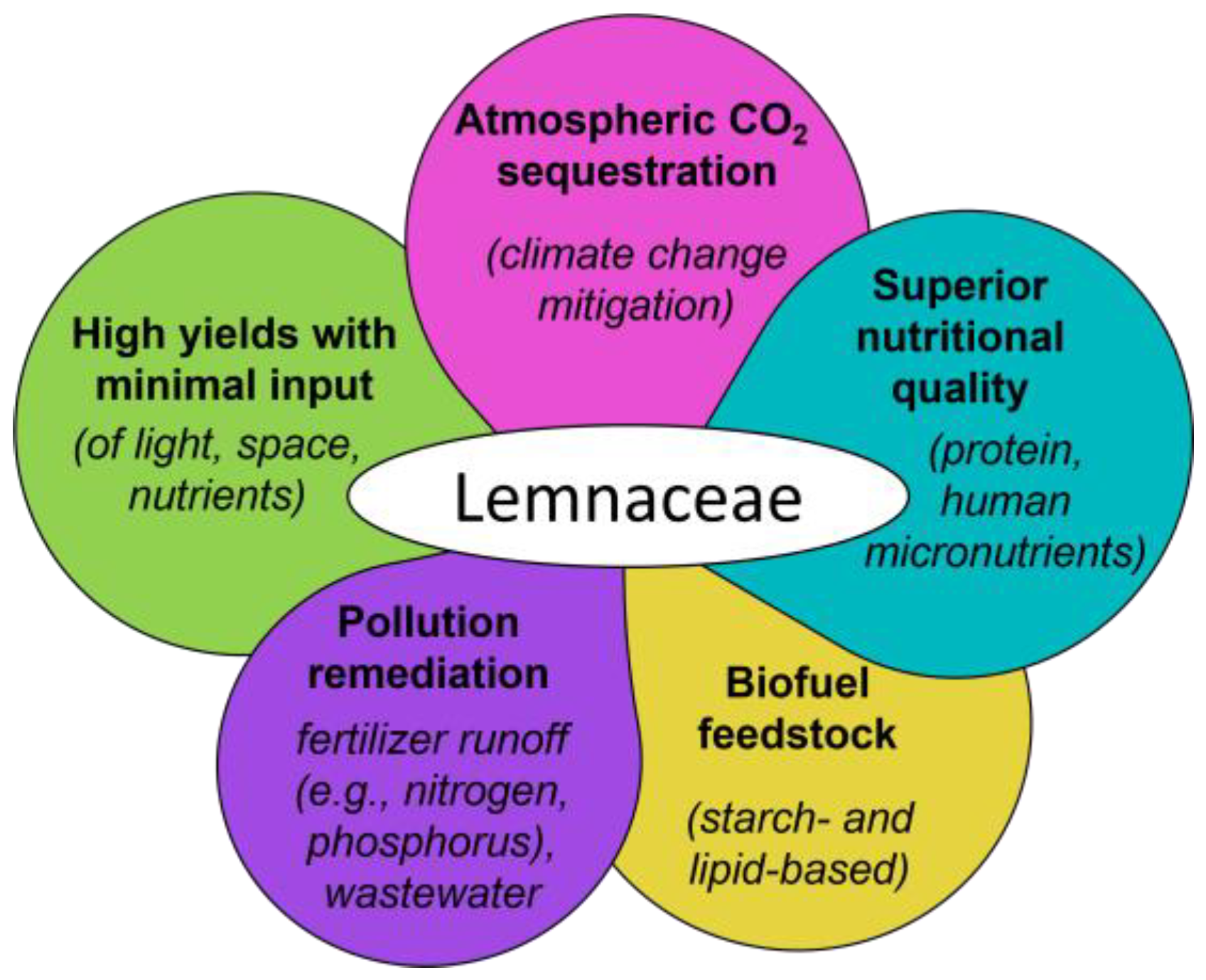
:1. Overview: Lemnaceae for CO2 Mitigation Coupled with Additional Attractive Features
1.1. Harvest Index
1.2. The Plant Microbiome’s Multiple Roles
1.3. Growth Rate, Sink Strength, and Response to CO2
2. Context-Dependent Response of Duckweed to Elevated CO2: Role of Nutrients, Light, and Microbiome
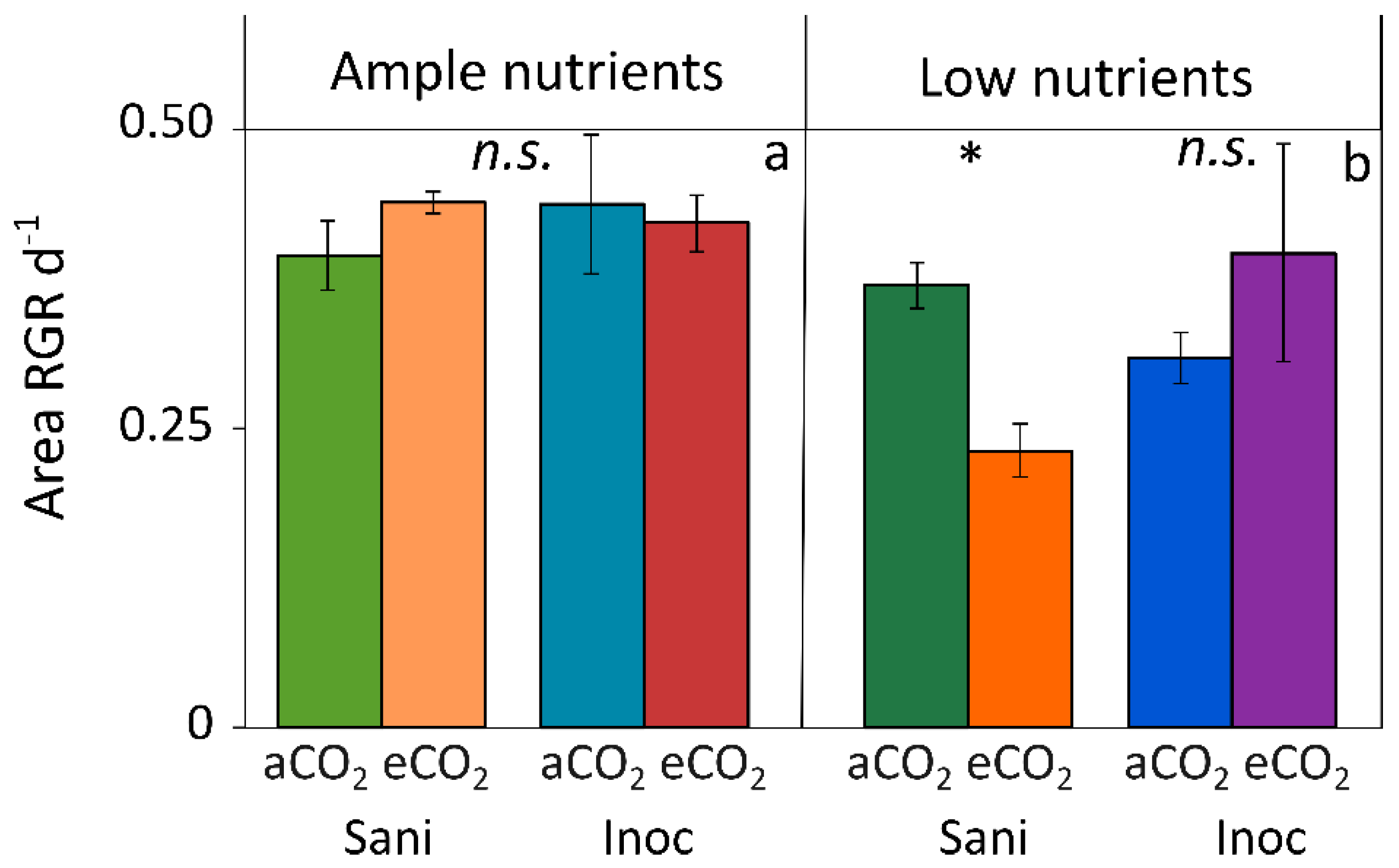
2.1. Effect of Nutrient Supply and Plant Microbiome under Moderate Light Supply
2.2. Response of Inoculated Lemna Plants under High Light and Ample Nutrient Supply
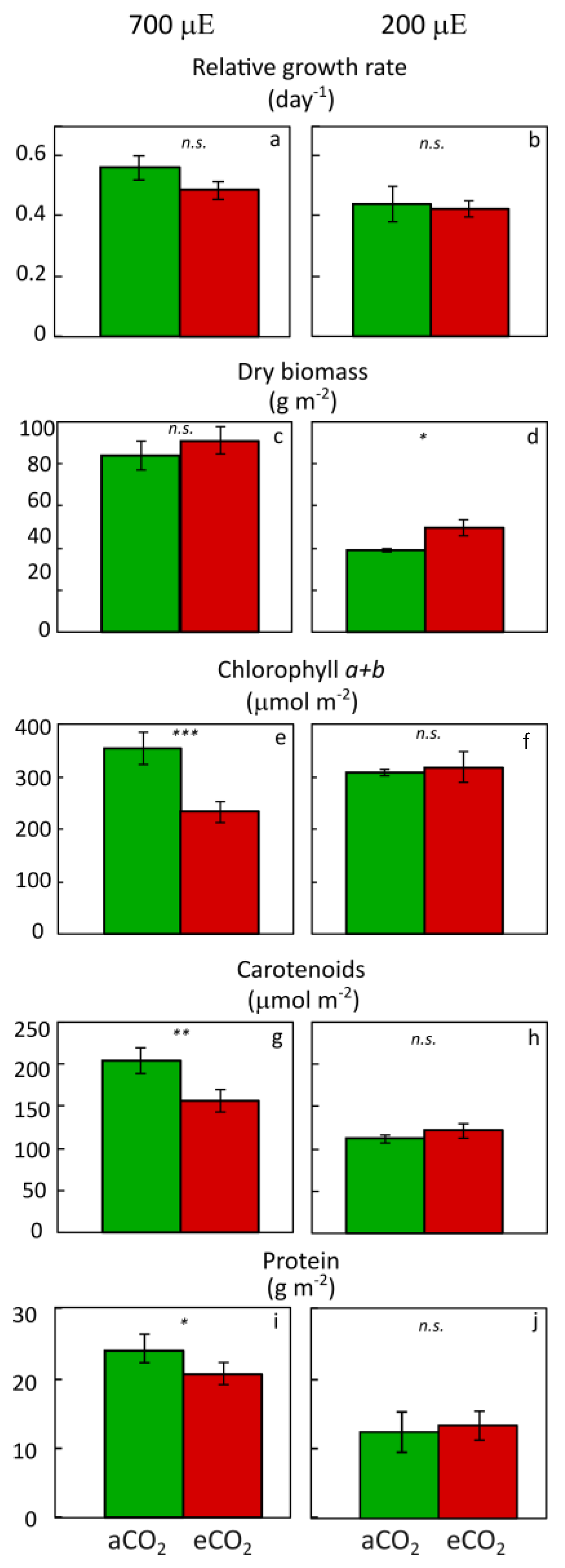
3. Multiple Advantages of Growing Duckweed under Low Light Intensity
4. Light Quality Effects
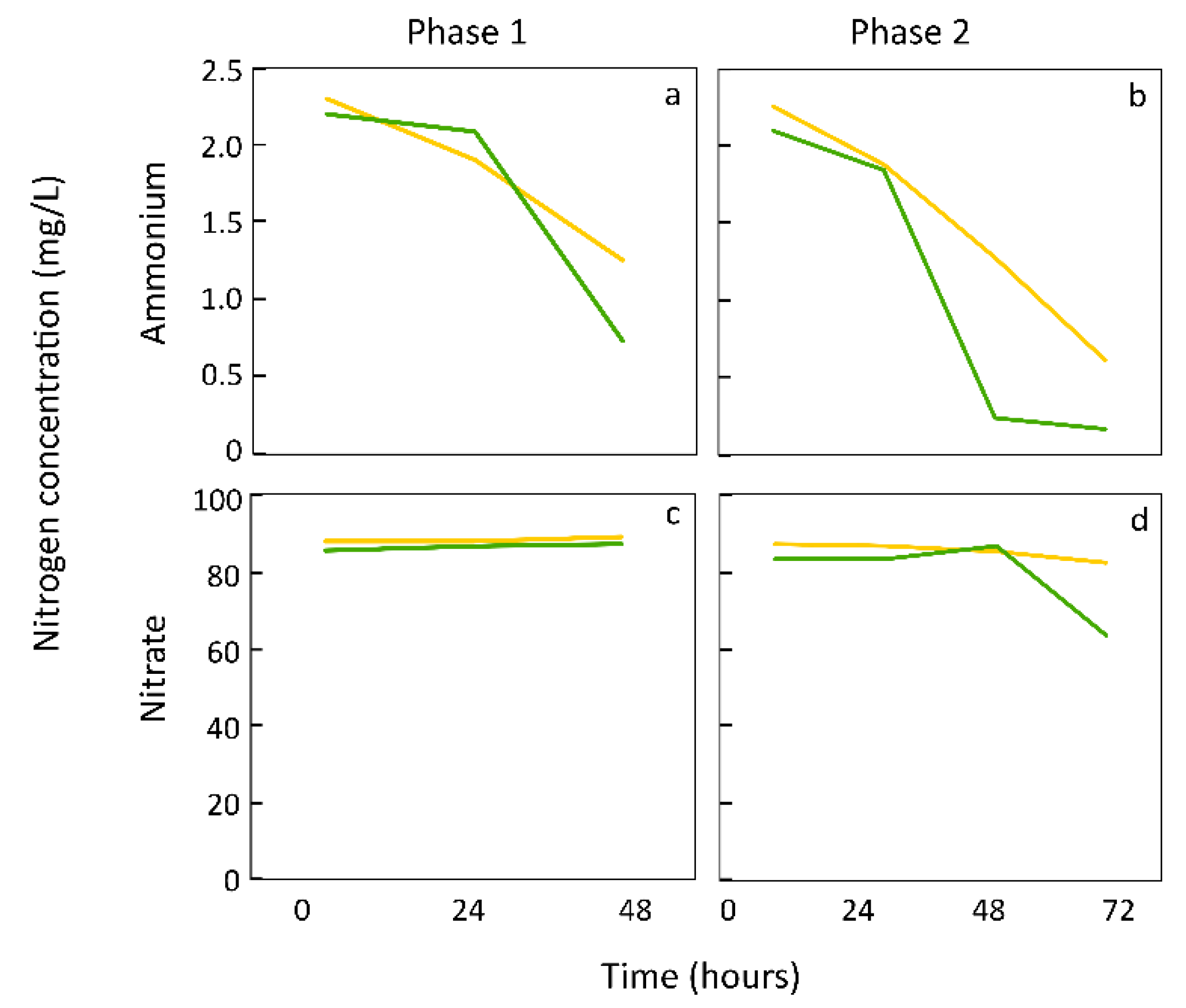
5. Duckweed Preference for Ammonium versus Nitrate
6. Interactions among Nutrient Transporters, Hormones, Elevated CO2, and the Microbiome
7. Commercial Applications of Duckweed
7.1. Human and Livestock Nutrition
7.2. Feedstock for Biofuels
8. Agricultural Technology: Hydroponics and Vertical Farming
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In IPCC, 2013: Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar] [CrossRef]
- Liu, K.; Harrison, M.T.; Yan, H.; Liu, D.L.; Meinke, H.; Hoogenboom, G.; Wang, B.; Peng, B.; Guan, K.; Jaegermeyr, J.; et al. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023, 14, 765. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.J.; Stomp, A.M. Growing duckweed to recover nutrients from wastewaters and for production of fuel ethanol and animal feed. Clean—Soil Air Water 2009, 37, 17–26. [Google Scholar] [CrossRef]
- Mohedano, R.A.; Costa, R.H.R.; Filho, P.B. Effects of CO2 concentration on nutrient uptake and starch accumulation by duckweed used for wastewater treatment and bioethanol production. Rev. Latinoam. Biotecnol. Ambient. Algal 2016, 7, 30–41. [Google Scholar] [CrossRef]
- Baek, G.Y.; Saeed, M.; Choi, H.K. Duckweeds: Their utilization, metabolites and cultivation. Appl. Biol. Chem. 2021, 64, 73. [Google Scholar] [CrossRef]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364–371. [Google Scholar] [CrossRef]
- Rabaey, J.; Cotner, J. Pond greenhouse gas emissions controlled by duckweed coverage. Front. Environ. Sci. 2022, 10, 889289. [Google Scholar] [CrossRef]
- Valenzuela-Aragon, B.; Parra-Cota, F.I.; Santoyo, G.; Arellano-Wattenbarger, G.L.; de los Santos-Villalobos, S. Plant-assisted selection: A promising alternative for in vivo identification of wheat (Triticum turgidum L. subsp. Durum) growth promoting bacteria. Plant Soil 2019, 435, 367–384. [Google Scholar] [CrossRef]
- Kong, Z.; Glick, B.R. The role of bacteria in phytoremediation. In Applied Bioengineering: Innovations and Future Directions; Yoshida, T., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 327–353. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Sen, S. Role of biological nitrogen fixation (BNF) in sustainable agriculture: A Review. Int. J. Adv. Life Sci. Res. 2021, 4, 1–7. [Google Scholar] [CrossRef]
- Alori, E.T.; Dare, M.O.; Babalola, O.O. Microbial inoculants for soil quality and plant health. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Cham, Switzerland, 2017; Volume 22, pp. 281–307. [Google Scholar] [CrossRef]
- Emami, S.; Alikhani, H.A.; Pourbabaee, A.A.; Etesami, H.; Motasharezadeh, B.; Sarmadian, F. Consortium of endophyte and rhizosphere phosphate solubilizing bacteria improves phosphorous use efficiency in wheat cultivars in phosphorus deficient soils. Rhizosphere 2020, 14, 100196. [Google Scholar] [CrossRef]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Roy, S.; Roy, M. Bioleaching of heavy metals by sulfur oxidizing bacteria: A Review. Int. Res. J. Environ. Sci. 2015, 4, 75–79. [Google Scholar]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K.; et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef]
- Anfang, M.; Shani, E. Transport mechanisms of plant hormones. Curr. Opin. Plant Biol. 2021, 63, 102055. [Google Scholar] [CrossRef] [PubMed]
- Romero-Contreras, Y.J.; Ramírez-Valdespino, C.A.; Guzmán-Guzmán, P.; Macías-Segoviano, J.I.; Villagómez-Castro, J.C.; Olmedo-Monfil, V. Tal6 from Trichoderma atroviride is a LysM effector involved in mycoparasitism and plant association. Front. Microbiol. 2019, 10, 2231. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Mekureyaw, M.F.; Pandey, C.; Roitsch, T. Role of cytokinins for interactions of plants with microbial pathogens and pest insects. Front. Plant Sci. 2020, 10, 1777. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Pathania, P.; Rajta, A.; Singh, P.C.; Bhatia, R. Role of plant growth-promoting bacteria in sustainable agriculture. Biocatal. Agric. Biotechnol. 2020, 30, 101842. [Google Scholar] [CrossRef]
- Glick, B.R. Beneficial Plant-Bacterial Interactions, 2nd ed.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, T.; Yu, X.; Yang, Y.; Wang, C.; Yang, Q.; Wang, X. Enhanced sugar accumulation and regulated plant hormone signalling genes contribute to cold tolerance in hypoploid Saccharum spontaneum. BMC Genom. 2020, 21, 507. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A.; Nelson, R.; Long, S.P. Testing the “source-sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric. For. Meteorol. 2004, 122, 85–94. [Google Scholar] [CrossRef]
- Asha, A.D.; Nivetha, N.; Krishna, G.K.; Thakur, J.K.; Rathi, M.S.; Manjunatha, B.S.; Chinnusamy, V.; Paul, S. Amelioration of short-term drought stress during different growth stages in Brassica juncea by rhizobacteria mediated maintenance of ROS homeostasis. Physiol. Plant. 2021, 172, 1880–1893. [Google Scholar] [CrossRef] [PubMed]
- Poupin, M.J.; Timmermann, T.; Vega, A.; Zuñiga, A.; González, B. Effects of the plant growth-promoting bacterium Burkholderia phytofirmans PsJN throughout the life cycle of Arabidopsis thaliana. PLoS ONE 2013, 8, e69435. [Google Scholar] [CrossRef] [PubMed]
- Salwan, R.; Sharma, M.; Sharma, A.; Sharma, V. Insights into plant beneficial microorganism-triggered induced systemic resistance. Plant Stress 2023, 7, 100140. [Google Scholar] [CrossRef]
- Glick, B.R. Soil microbes and sustainable agriculture. Pedosphere 2018, 28, 167–169. [Google Scholar] [CrossRef]
- Körner, C. Carbon limitation in trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Cabrera-Bosquet, L.; Morcuende, R.; Avice, J.C.; Nogués, S.; Araus, J.L.; Martínez-Carrasco, R.; Pérez, P. Does ear C sink strength contribute to overcoming photosynthetic acclimation of wheat plants exposed to elevated CO2? J. Exp. Bot. 2011, 62, 3957–3969. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Polutchko, S.K.; Zenir, M.C.; Fourounjian, P.; Stewart, J.J.; López-Pozo, M.; Adams, W.W., III. Intersections: Photosynthesis, abiotic stress, and the plant microbiome. Photosynthetica 2022, 60, 59–69. [Google Scholar] [CrossRef]
- Pourtau, N.; Jennings, R.; Pelzer, E.; Pallas, J.; Wingler, A. Effect of sugar-induced senescence on gene expression and implications for the regulation of senescence in Arabidopsis. Planta 2006, 224, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Sree, K.S.; Sudakaran, S.; Appenroth, K.J. How fast can angiosperms grow? Species and clonal diversity of growth rates in the genus Wolffia (Lemnaceae). Acta Physiol. Plant. 2015, 37, 31–39. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; López-Pozo, M.; Polutchko, S.K.; Fourounjian, P.; Stewart, J.J.; Zenir, M.C.; Adams, W.W., III. Growth and nutritional quality of Lemnaceae viewed comparatively in an ecological and evolutionary context. Plants 2022, 11, 145. [Google Scholar] [CrossRef]
- Stewart, J.J.; Adams, W.W., III; Escobar, C.M.; López-Pozo, M.; Demmig-Adams, B. Growth and essential carotenoid micronutrients in Lemna gibba as a function of growth light intensity. Front. Plant Sci. 2020, 11, 480. [Google Scholar] [CrossRef]
- Zenir, M.C.; López-Pozo, M.; Polutchko, S.K.; Stewart, J.J.; Adams, W.W., III; Escobar, A.; Demmig-Adams, B. Productivity and nutrient quality of Lemna minor as affected by microbiome, CO2 level, and nutrient supply. Stresses 2023, 3, 69–85. [Google Scholar] [CrossRef]
- López-Pozo, M.; Zenir, M.C.; Polutchko, S.K.; Stewart, J.J.; Adams, W.W., III; Escobar, A.; Demmig-Adams, B. Effects of growth environment on Lemna minor. E Sch. Community Encycl. 2023, 40481. Available online: https://encyclopedia.pub/entry/40481 (accessed on 24 August 2023).
- Agüera, E.; De la Haba, P. Leaf senescence in response to elevated atmospheric CO2 concentration and low nitrogen supply. Biol. Plant. 2018, 62, 401–408. [Google Scholar] [CrossRef]
- Poorter, H.; Van Berkel, Y.; Baxter, R.; Den Hertog, J.; Dijkstra, P.; Gifford, R.M.; Griffin, K.L.; Roumet, C.; Roy, J.; Wong, S.C. The effect of elevated CO2 on the chemical composition and construction costs of leaves of 27 C3 species. Plant Cell Environ. 1997, 20, 472–482. [Google Scholar] [CrossRef]
- Louback, E.; Batista, D.S.; Pereira, T.A.R.; Mamedes-Rodrigues, T.C.; Silva, T.D.; Felipe, S.H.S.; Rocha, D.I.; Steinmacher, D.A.; Otoni, W.C. CO2 enrichment leads to altered cell wall composition in plants of Pfaffia glomerata (Spreng.) Pedersen (Amaranthaceae). Plant Cell Tissue Organ Cult. 2021, 145, 603–613. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Björndahl, G.; Nilsen, S. Growth potential of Lemna gibba: Effect of CO2 enrichment at high photon flux rate. Aquat. Bot. 1985, 22, 79–82. [Google Scholar] [CrossRef]
- Smernoff, D.T. Interactions between Carbon Assimilation and Nitrogen Metabolism at Elevated Carbon Dioxide in Duckweed Species and Aphanizomenon flos-aquae; Stanford University ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 1992. [Google Scholar]
- Toyama, T.; Mori, K.; Tanaka, Y.; Ike, M.; Morikawa, M. Growth promotion of giant duckweed Spirodela polyrhiza (Lemnaceae) by Ensifer sp. SP4 through enhancement of nitrogen metabolism and photosynthesis. Mol. Plant-Microbe Interact. 2022, 35, 28–38. [Google Scholar] [CrossRef]
- Gale, J.; Smernoff, D.T.; Macler, B.A.; MacElroy, R.D. Carbon balance and productivity of Lemna gibba, a candidate plant for CELSS. Adv. Space Res. 1989, 9, 43–52. [Google Scholar] [CrossRef]
- Krapp, A.; Stitt, M. An evaluation of direct and indirect mechanisms for the “sink-regulation” of photosynthesis in spinach: Changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 1995, 195, 313–323. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; López-Pozo, M.; Stewart, J.J.; Adams, W.W., III. Zeaxanthin and lutein: Photoprotectors, anti-inflammatories, and brain food. Molecules 2020, 25, 3607. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z. Effects of elevated CO2 on nutritional quality of vegetables: A review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef]
- Pal, M.; Karthikeyapandian, V.; Jain, V.; Srivastava, A.C.; Raj, A.; Sengupta, U.K. Biomass production and nutritional levels of berseem (Trifolium alexandrium) grown under elevated CO2. Agric. Ecosyst. Environ. 2004, 101, 37–38. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef]
- López-Pozo, M.; Adams, W.W., III; Polutchko, S.K.; Demmig-Adams, B. Terrestrial and floating aquatic plants differ in acclimation to light environment. Plants 2023, 12, 1928. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Stewart, J.J.; Mcnamara, M.; Garcia, N.D.; López-Pozo, M.; Adams, W.W., III; Demmig-Adams, B. Lemna as a sustainable, highly nutritious crop: Nutrient production in different light environments. Nutraceuticals 2022, 2, 350–364. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Stewart, J.J.; Demmig-Adams, B. Integrative view of the nutrition of the eye. In Nutraceuticals and Functional Foods in Human Health and Disease Prevention; CRC Press: Boca Raton, FL, USA, 2015; pp. 407–418. [Google Scholar] [CrossRef]
- Stewart, J.J.; Adams, W.W., III; López-Pozo, M.; Doherty Garcia, N.; McNamara, M.; Escobar, C.M.; Demmig-Adams, B. Features of the duckweed Lemna that support rapid growth under extremes of light intensity. Cells 2021, 10, 1481. [Google Scholar] [CrossRef]
- Martindale, W.; Bowes, G. The effects of irradiance and CO2 on the activity and activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in the aquatic plant Spirodela polyrhiza. J. Exp. Bot. 1996, 47, 781–784. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Adams, W.W., III; Escobar, C.M.; Demmig-Adams, B. Conquering space with crops that produce ample oxygen and antioxidants. Oxygen 2022, 2, 211–226. [Google Scholar] [CrossRef]
- Sree, K.S.; Dahse, H.M.; Chandran, J.N.; Schneider, B.; Jahreis, G.; Appenroth, K.J. Duckweed for human nutrition: No cytotoxic and no anti-proliferative effects on human cell lines. Plant Foods Hum. Nutr. 2019, 74, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.W., III; Demmig-Adams, B.; Barker, D.H.; Kiley, S. Carotenoids and photosystem II characteristics of upper and lower halves of leaves acclimated to high light. Aust. J. Plant Physiol. 1996, 23, 669–677. [Google Scholar] [CrossRef]
- Terashima, I.; Inoue, Y. Vertical gradient in photosynthetic properties of spinach chloroplast dependent on intra-leaf light environment. Plant Cell Physiol. 1985, 26, 781–785. [Google Scholar] [CrossRef]
- Hommel, E.; Liebers, M.; Offermann, S.; Pfannschmidt, T. Effectiveness of light-quality and dark-white growth light shifts in short-term light acclimation of photosynthesis in Arabidopsis. Front. Plant Sci. 2022, 12, 615253. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W., III. Zeaxanthin, a molecule for photoprotection in many different environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef]
- Parker, G.G.; Fitzjarrald, D.R.; Gonçalves Sampaio, I.C. Consequences of environmental heterogeneity for the photosynthetic light environment of a tropical forest. Agric. For. Meteorol. 2019, 278, 107661. [Google Scholar] [CrossRef]
- Zhou, Y.; Kishchenko, O.; Stepanenko, A.; Chen, G.; Wang, W.; Zhou, J.; Pan, C.; Borisjuk, N. The dynamics of NO3− and NH4+ uptake in duckweed are coordinated with the expression of major nitrogen assimilation genes. Plants 2022, 11, 11. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Babourina, O.; Rengel, Z.; Yang, X.E.; Pu, P.M. Ammonium and nitrate uptake by the floating plant Landoltia punctata. Ann. Bot. 2007, 99, 365–370. [Google Scholar] [CrossRef]
- Gallego, L.M.; Chien, Y.H.; Angeles, I.P. Effects of light source and photoperiod on growth of duckweed Landoltia punctata and its water quality. Aquac. Res. 2022, 53, 398–408. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Guo, B.; Liu, C.; Liu, J.; Qiu, G.; Fu, Q.; Li, H. Alleviation of aqueous nitrogen loss from paddy fields by growth and decomposition of duckweed (Lemna minor L.) after fertilization. Chemosphere 2023, 311, 137073. [Google Scholar] [CrossRef] [PubMed]
- Cedergreen, N.; Madsen, T.V. Nitrogen uptake by the floating macrophyte Lemna minor. New Phytol. 2002, 155, 285–292. [Google Scholar] [CrossRef]
- Petersen, F.; Demann, J.; Restemeyer, D.; Ulbrich, A.; Olfs, H.W.; Westendarp, H.; Appenroth, K.J. Influence of the nitrate-N to ammonium-N ratio on relative growth rate and crude protein content in the duckweeds Lemna minor and Wolffiella hyalina. Plants 2021, 10, 1741. [Google Scholar] [CrossRef] [PubMed]
- Petersen, F.; Demann, J.; von Salzen, J.; Olfs, H.W.; Westendarp, H.; Wolf, P.; Appenroth, K.J.; Ulbrich, A. Re-circulating indoor vertical farm: Technicalities of an automated duckweed biomass production system and protein feed product quality evaluation. J. Clean. Prod. 2022, 380, 134894. [Google Scholar] [CrossRef]
- Mehrer, I.; Mohr, H. Ammonium toxicity: Description of the syndrome in Sinapis alba and the search for its causation. Physiol. Plant 1989, 77, 545–554. [Google Scholar] [CrossRef]
- Hecht, U.; Mohr, H. Factors controlling nitrate and ammonium accumulation in mustard (Sinapis alba) seedlings. Physiol. Plant. 1990, 78, 379–387. [Google Scholar] [CrossRef]
- Wang, W.; Haberer, G.; Gundlach, H.; Gläßer, C.; Nussbaumer, T.; Luo, M.C.; Lomsadze, A.; Borodovsky, M.; Kerstetter, R.A.; Shanklin, J.; et al. The Spirodela polyrhiza genome reveals insights into its neotenous reduction fast growth and aquatic lifestyle. Nat. Commun. 2014, 5, 3311. [Google Scholar] [CrossRef]
- Ziegler, P.; Adelmann, K.; Zimmer, S.; Schmidt, C.; Appenroth, K.J. Relative in vitro growth rates of duckweeds (Lemnaceae)—The most rapidly growing higher plants. Plant Biol. 2015, 17, 33–41. [Google Scholar] [CrossRef]
- Vega, A.; O’Brien, J.A.; Gutiérrez, R.A. Nitrate and hormonal signaling crosstalk for plant growth and development. Curr. Opin. Plant Biol. 2019, 52, 155–163. [Google Scholar] [CrossRef]
- Cassan, O.; Pimparé, L.L.; Dubos, C.; Gojon, A.; Bach, L.; Lèbre, S.; Martin, A. A gene regulatory network in Arabidopsis roots reveals features and regulators of the plant response to elevated CO2. New Phytol. 2023, 239, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Yang, Z.; Hao, T.; Zhuang, L.; Xu, Q.; Yu, J. Differential effects of elevated atmosphere CO2 concentration on root growth in association with regulation of auxin and cytokinins under different nitrate supply. Environ. Exp. Bot. 2022, 201, 104943. [Google Scholar] [CrossRef]
- Mantelin, S.; Touraine, B. Plant growth-promoting bacteria and nitrate availability: Impacts on root development and nitrate uptake. J. Exp. Bot. 2004, 394, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.; Degon, Z.; Ruiz, D.; Pope, J.; Rahmatallah, Y.; Mukherjee, A. The plant growth-promoting bacteria, Azospirillum brasilense, induce a diverse array of genes in rice shoots and promote their growth. Plant Growth Regul. 2022, 97, 143–155. [Google Scholar] [CrossRef]
- Saini, M.R.; Chandran, L.P.; Barbadikar, K.M.; Sevanthi, A.M.V.; Chawla, G.; Kaushik, M.; Mulani, E.; Phule, A.S.; Govindannagari, R.; Sonth, B.; et al. Understanding plant–microbe interaction of rice and soybean with two contrasting diazotrophic bacteria through comparative transcriptome analysis. Front. Plant Sci. 2022, 13, 939395. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Irineu, L.E.S.; da Silva Irineu, C.; Soares, T.S.; de Almeida, F.A.; Almeida-Silva, F.; Gazara, R.K.; Meneses, C.H.S.G.; Canellas, L.P.; Silveira, V.; Venancio, T.M.; et al. Multiomic approaches reveal hormonal modulation and nitrogen uptake and assimilation in the initial growth of maize inoculated with Herbaspirillum seropedicae. Plants 2023, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Shuvro, S.K.; Jog, R.; Morikawa, M. Diazotrophic bacterium Azotobacter vinelandii as a mutualistic growth promoter of an aquatic plant: Lemna minor. Plant Growth Regul. 2023, 100, 171–180. [Google Scholar] [CrossRef]
- Friel, C.A.; Friesen, M.L. Legumes modulate allocation to rhizobial nitrogen fixation in response to factorial light and nitrogen manipulation. Front. Plant Sci. 2019, 10, 1316. [Google Scholar] [CrossRef]
- Kittiwongwattana, C.; Thawai, C. Rhizobium lemnae sp. nov., a bacterial endophyte of Lemna aequinoctialis. Int. J. Syst. Evol. Microbiol. 2014, 64, 2455–2460. [Google Scholar] [CrossRef]
- Nakei, M.D.; Venkataramana, P.B.; Ndakidemi, P.A. Soybean-nodulating rhizobia: Ecology, characterization, diversity, and growth promoting functions. Front. Sustain. Food Syst. 2022, 6, 824444. [Google Scholar] [CrossRef]
- Oikawa, S.; Miyagi, K.M.; Hikosaka, K.; Okada, M.; Matsunami, T.; Kokubun, M.; Kinugasa, T.; Hirose, T. Interactions between elevated CO2 and N2-fixation determine soybean yield-a test using a non-nodulated mutant. Plant Soil 2010, 330, 163–172. [Google Scholar] [CrossRef]
- Sree, K.S.; Maheshwari, S.C.; Boka, K.; Khurana, J.P.; Keresztes, Á.; Appenroth, K.J. The duckweed Wolffia microscopica: A unique aquatic monocot. Flora Morphol. Distrib. Funct. Ecol. Plants 2015, 210, 31–39. [Google Scholar] [CrossRef]
- Sanz-sáez, Á.; Heath, K.D.; Burke, P.V.; Ainsworth, E.A. Inoculation with an enhanced N2-fixing Bradyrhizobium japonicum strain (USDA110) does not alter soybean (Glycine max Merr.) response to elevated [CO2]. Plant Cell Environ. 2015, 38, 2589–2602. [Google Scholar] [CrossRef] [PubMed]
- Soba, D.; Aranjuelo, I.; Gakière, B.; Gilard, F.; Pérez-López, U.; Mena-Petite, A.; Muñoz-Rueda, A.; Lacuesta, M.; Sanz-Saez, A. Soybean inoculated with one Bradyrhizobium strain isolated at elevated [CO2] show an impaired C and N metabolism when grown at ambient [CO2]. Front. Plant Sci. 2021, 12, 656961. [Google Scholar] [CrossRef] [PubMed]
- Mohedano, R.A.; Costa, R.H.R.; Tavares, F.A.; Belli Filho, P. High nutrient removal rate from swine wastes and protein biomass production by full-scale duckweed ponds. Bioresour. Technol. 2012, 112, 98–104. [Google Scholar] [CrossRef] [PubMed]
- De Beukelaar, M.F.A.; Zeinstra, G.G.; Mes, J.J.; Fischer, A.R.H. Duckweed as human food. The influence of meal context and information on duckweed acceptability of Dutch consumers. Food Qual. Prefer. 2019, 71, 76–86. [Google Scholar] [CrossRef]
- Kaplan, A.; Zelicha, H.; Tsaban, G.; Meir, A.Y.; Rinott, E.; Kovsan, J.; Novack, L.; Thiery, J.; Ceglarek, U.; Burkhardt, R.; et al. Protein bioavailability of Wolffia globosa duckweed, a novel aquatic plant—A randomized controlled trial. Clin. Nutr. 2019, 38, 2576–2582. [Google Scholar] [CrossRef]
- Skillicorn, P.; Spira, W.; Journey, W. Duckweed Aquaculture: A New Aquatic Farming System for Developing Countries; World Bank: Washington, DC, USA, 1993. [Google Scholar]
- Chakrabarti, R.; Clark, W.D.; Sharma, J.G.; Goswami, R.K.; Shrivastav, A.K.; Tocher, D.R. Mass production of Lemna minor and its amino acid and fatty acid profiles. Front. Chem. 2018, 6, 479. [Google Scholar] [CrossRef]
- Yahaya, N.; Hamdan, N.H.; Zabidi, A.R.; Mohamad, A.M.; Suhaimi, M.L.H.; Johari, M.A.A.M.; Yahya, H.N.; Yahya, H. Duckweed as a future food: Evidence from metabolite profile, nutritional and microbial analyses. Futur. Foods 2022, 5, 100128. [Google Scholar] [CrossRef]
- Noonan, S.C. Oxalate content of foods and its effect on humans. Asia Pac. J. Clin. Nutr. 1999, 8, 64–74. [Google Scholar] [CrossRef]
- Hu, Z.; Fang, Y.; Yi, Z.; Tian, X.; Li, J.; Jin, Y.; He, K.; Liu, P.; Du, A.; Huang, Y.; et al. Determining the nutritional value and antioxidant capacity of duckweed (Wolffia arrhiza) under artificial conditions. LWT 2022, 153, 112477. [Google Scholar] [CrossRef]
- Kumar, V.; Irfan, M.; Datta, A. Manipulation of oxalate metabolism in plants for improving food quality and productivity. Phytochemistry 2019, 158, 103–109. [Google Scholar] [CrossRef]
- Yang, J.; Fu, M.; Ji, C.; Huang, Y.; Wu, Y. Maize oxalyl-coa decarboxylase1 degrades oxalate and affects the seed metabolome and nutritional quality. Plant Cell 2018, 30, 2447–2462. [Google Scholar] [CrossRef] [PubMed]
- Natesh, H.N.; Abbey, L.; Asiedu, S.K. An overview of nutritional and anti nutritional factors in green leafy vegetables. Hortic. Int. J. 2017, 1, 58–65. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Sree, S.K.; Bog, M.; Ecker, J.; Seeliger, C.; Böhm, V.; Lorkowski, S.; Sommer, K.; Vetter, W.; Tolzin-Banasch, K.; et al. Nutritional value of the duckweed species of the genus Wolffia (Lemnaceae) as human food. Front. Chem. 2018, 6, 483. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, J.; Dugmore, T.I.J.; Matharu, A.; Martinez-Hernandez, E.; Aburto, J.; Rahman, P.K.S.M.; Lynch, J. Perspectives on “game changer” global challenges for sustainable 21st century: Plant-based diet, unavoidable food waste biorefining, and circular economy. Sustainability 2020, 12, 1976. [Google Scholar] [CrossRef]
- Coughlan, N.E.; Walsh, É.; Bolger, P.; Burnell, G.; O’Leary, N.; O’Mahoney, M.; Paolacci, S.; Wall, D.; Jansen, M.A.K. Duckweed bioreactors: Challenges and opportunities for large-scale indoor cultivation of Lemnaceae. J. Clean. Prod. 2022, 336, 130285. [Google Scholar] [CrossRef]
- Markou, G.; Wang, L.; Ye, J.; Unc, A. Using agro-industrial wastes for the cultivation of microalgae and duckweeds: Contamination risks and biomass safety concerns. Biotechnol. Adv. 2018, 36, 1238–1254. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Strategy for Food Safety 2022–2030—Towards Stronger Food Safety Systems and Global Cooperation; World Health Organization: Geneva, Switzerland, 2022; pp. 1–86. [Google Scholar]
- Sela, I.; Meir, A.Y.; Brandis, A.; Krajmalnik-Brown, R.; Zeibich, L.; Chang, D.; Dirks, B.; Tsaban, G.; Kaplan, A.; Rinott, E.; et al. Wolffia globosa–mankai plant-based protein contains bioactive vitamin B12 and is well absorbed in humans. Nutrients 2020, 12, 3067. [Google Scholar] [CrossRef]
- Green, R.; Miller, J.W. Vitamin B12 deficiency. In Vitamins and Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 119, pp. 405–439. [Google Scholar] [CrossRef]
- U.S. Department of Health & Human Services. National Institutes of Health. Division of Program Coordination, Planning, and S.I. Vitamin B12 Fact Sheet for Health Professionals. 2023. Available online: https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/ (accessed on 24 August 2023).
- Yokoyama, Y.; Levin, S.M.; Barnard, N.D. Association between plant-based diets and plasma lipids: A systematic review and meta-analysis. Nutr. Rev. 2017, 75, 683–698. [Google Scholar] [CrossRef]
- Sukumar, N.; Saravanan, P. Investigating vitamin B12 deficiency. BMJ 2019, 365, 1865. [Google Scholar] [CrossRef]
- Watanabe, F.; Takenaka, S.; Kittaka-Katsura, H.; Ebara, S.; Miyamoto, E. Characterization and bioavailability of vitamin B12-compounds from edible algae. J. Nutr. Sci. Vitaminol. 2002, 48, 325–331. [Google Scholar] [CrossRef]
- Meir, A.Y.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT plus randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef] [PubMed]
- Meir, A.Y.; Tsaban, G.; Zelicha, H.; Rinott, E.; Kaplan, A.; Youngster, I.; Rudich, A.; Shelef, I.; Tirosh, A.; Brikner, D.; et al. A Green-Mediterranean diet, supplemented with mankai duckweed, preserves iron-homeostasis in humans and is efficient in reversal of anemia in rats. J. Nutr. 2019, 149, 1004–1011. [Google Scholar] [CrossRef]
- Rinott, E.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J.; et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: A randomized controlled trial. Genome Med. 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Tsaban, G.; Yaskolka Meir, A.; Rinott, E.; Zelicha, H.; Kaplan, A.; Shalev, A.; Katz, A.; Rudich, A.; Tirosh, A.; Shelef, I.; et al. The effect of green Mediterranean diet on cardiometabolic risk; A randomised controlled trial. Heart 2021, 107, 1054–1061. [Google Scholar] [CrossRef]
- Zelicha, H.; Kaplan, A.; Meir, A.Y.; Tsaban, G.; Rinott, E.; Shelef, I.; Tirosh, A.; Brikner, D.; Pupkin, E.; Qi, L.; et al. The effect of Wolffia globosa mankai, a green aquatic plant, on postprandial glycemic response: A randomized crossover controlled trial. Diabetes Care 2019, 42, 1162–1169. [Google Scholar] [CrossRef]
- Kaplan, A.; Zelicha, H.; Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Levakov, G.; Prager, O.; Salti, M.; Yovell, Y.; Ofer, J.; et al. The effect of a high-polyphenol Mediterranean diet (Green-MED) combined with physical activity on age-related brain atrophy: The Dietary Intervention Randomized Controlled Trial Polyphenols Unprocessed Study (DIRECT PLUS). Am. J. Clin. Nutr. 2022, 115, 1270–1281. [Google Scholar] [CrossRef]
- Meir, A.Y.; Tuohy, K.; von Bergen, M.; Krajmalnik-Brown, R.; Heinig, U.; Zelicha, H.; Tsaban, G.; Rinott, E.; Kaplan, A.; Aharoni, A.; et al. The metabolomic-gut-clinical axis of mankai plant-derived dietary polyphenols. Nutrients 2021, 13, 1866. [Google Scholar] [CrossRef]
- Rinott, E.; Youngster, I.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Fava, F.; Scholz, M.U.; et al. Effects of diet-modulated autologous fecal microbiota transplantation on weight gain. Gastroenterology 2021, 160, 158–173. [Google Scholar] [CrossRef]
- Papong, S.; Rewlay-ngoen, C.; Itsubo, N.; Malakul, P. Environmental life cycle assessment and social impacts of bioethanol production in Thailand. J. Clean. Prod. 2017, 157, 254–266. [Google Scholar] [CrossRef]
- Cui, W.; Cheng, J.J. Growing duckweed for biofuel production: A review. Plant Biol. 2015, 17, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Fang, Y.; Li, Q.; Yang, G.L.; Guo, L.; Chen, G.K.; Tan, L.; He, K.Z.; Jin, Y.L.; Zhao, H. Turion, an innovative duckweed-based starch production system for economical biofuel manufacture. Ind. Crops Prod. 2018, 124, 108–114. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, W.; Zhao, X.; Chen, Y.; Yang, J.; Xu, S.; Hou, H. Sulfur limitation boosts more starch accumulation than nitrogen or phosphorus limitation in duckweed (Spirodela polyrhiza). Ind. Crops Prod. 2022, 185, 115098. [Google Scholar] [CrossRef]
- Li, J.M.; Du, A.P.; Liu, P.H.; Tian, X.P.; Jin, Y.L.; Yi, Z.L.; He, K.Z.; Fang, J.; Zhao, H. High starch accumulation mechanism and phosphorus utilization efficiency of duckweed (Landoltia punctata) under phosphate starvation. Ind. Crops Prod. 2021, 167, 113529. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, X.H.; Anaokar, S.; Shi, H.; Dahl, W.B.; Cai, Y.; Luo, G.; Chai, J.; Cai, Y.; Mollá-Morales, A.; et al. Engineering triacylglycerol accumulation in duckweed (Lemna japonica). Plant Biotechnol. J. 2023, 21, 317–330. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa-Rodríguez, R.; Lara-Herrera, A.; Trejo-Téllez, L.I.; Padilla-Bernal, L.E.; Solis-Sánchez, L.O.; Ortiz-Rodríguez, J.M. Water and fertilizers use efficiency in two hydroponic systems for tomato production. Hortic. Bras. 2020, 38, 47–52. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Takagaki, M. (Eds.) Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- AlShrouf, A. Hydroponics, aeroponic and aquaponic as compared with conventional farming. Am. Sci. Res. J. Eng. Technol. Sci. 2017, 27, 247–255. [Google Scholar] [CrossRef]
- Keuter, V.; Deck, S.; Giesenkamp, H.; Gonglach, D.; Katayama, V.T.; Liesegang, S.; Petersen, F.; Schwindenhammer, S.; Steinmetz, H.; Ulbrich, A. Significance and vision of nutrient recovery for sustainable city food systems in Germany by 2050. Sustainability 2021, 13, 10772. [Google Scholar] [CrossRef]
- Escobar, C.M.; Escobar, A.C.; Power, G.J.; Nabity, J.A. μG-LilyPondTM: Preliminary design of a floating plant pond for microgravity. Int. Conf. Environ. Syst. 2020, 1–12. [Google Scholar]
- Fan, C.; Manivannan, A.; Wei, H. Light quality-mediated influence of morphogenesis in micropropagated horticultural crops: A comprehensive overview. Biomed Res. Int. 2022, 2022, 4615079. [Google Scholar] [CrossRef]
- Naoya Fukuda, M.E.; Yoshida, H.; Kusano, M. Effects of light quality, photoperiod, CO2 concentration, and air temperature on chlorogenic acid and rutin accumulation in young lettuce plants. Plant Physiol. Biochem. 2022, 186, 290–298. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Pozo, M.; Adams, W.W., III; Demmig-Adams, B. Lemnaceae as Novel Crop Candidates for CO2 Sequestration and Additional Applications. Plants 2023, 12, 3090. https://doi.org/10.3390/plants12173090
López-Pozo M, Adams WW III, Demmig-Adams B. Lemnaceae as Novel Crop Candidates for CO2 Sequestration and Additional Applications. Plants. 2023; 12(17):3090. https://doi.org/10.3390/plants12173090
Chicago/Turabian StyleLópez-Pozo, Marina, William W. Adams, III, and Barbara Demmig-Adams. 2023. "Lemnaceae as Novel Crop Candidates for CO2 Sequestration and Additional Applications" Plants 12, no. 17: 3090. https://doi.org/10.3390/plants12173090
APA StyleLópez-Pozo, M., Adams, W. W., III, & Demmig-Adams, B. (2023). Lemnaceae as Novel Crop Candidates for CO2 Sequestration and Additional Applications. Plants, 12(17), 3090. https://doi.org/10.3390/plants12173090










