Comprehensive Investigation of Die-Back Disease Caused by Fusarium in Durian
Abstract
:1. Introduction
2. Results
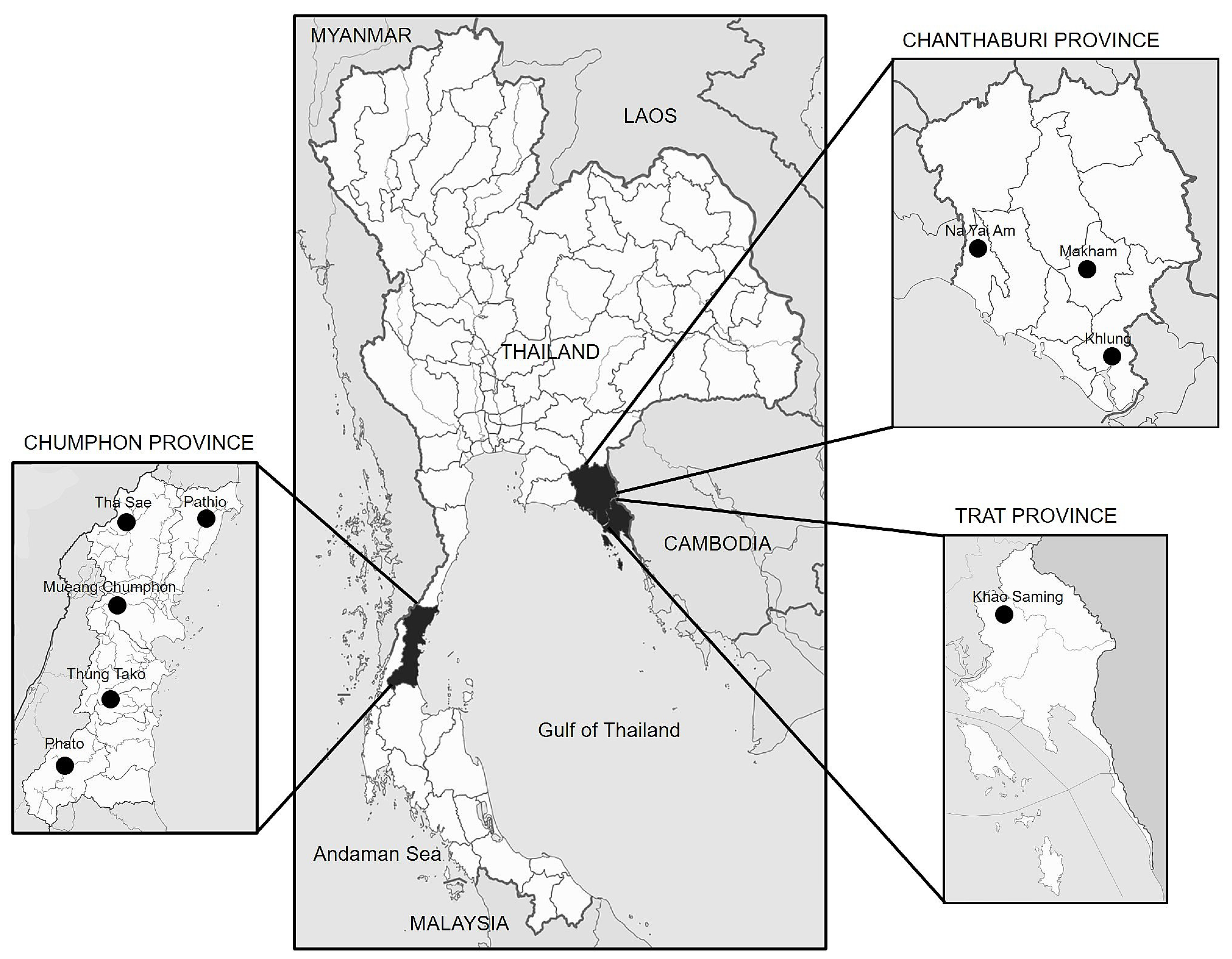
2.1. Fungal Isolation and Purification
2.2. DNA Marker and Fungal Identification
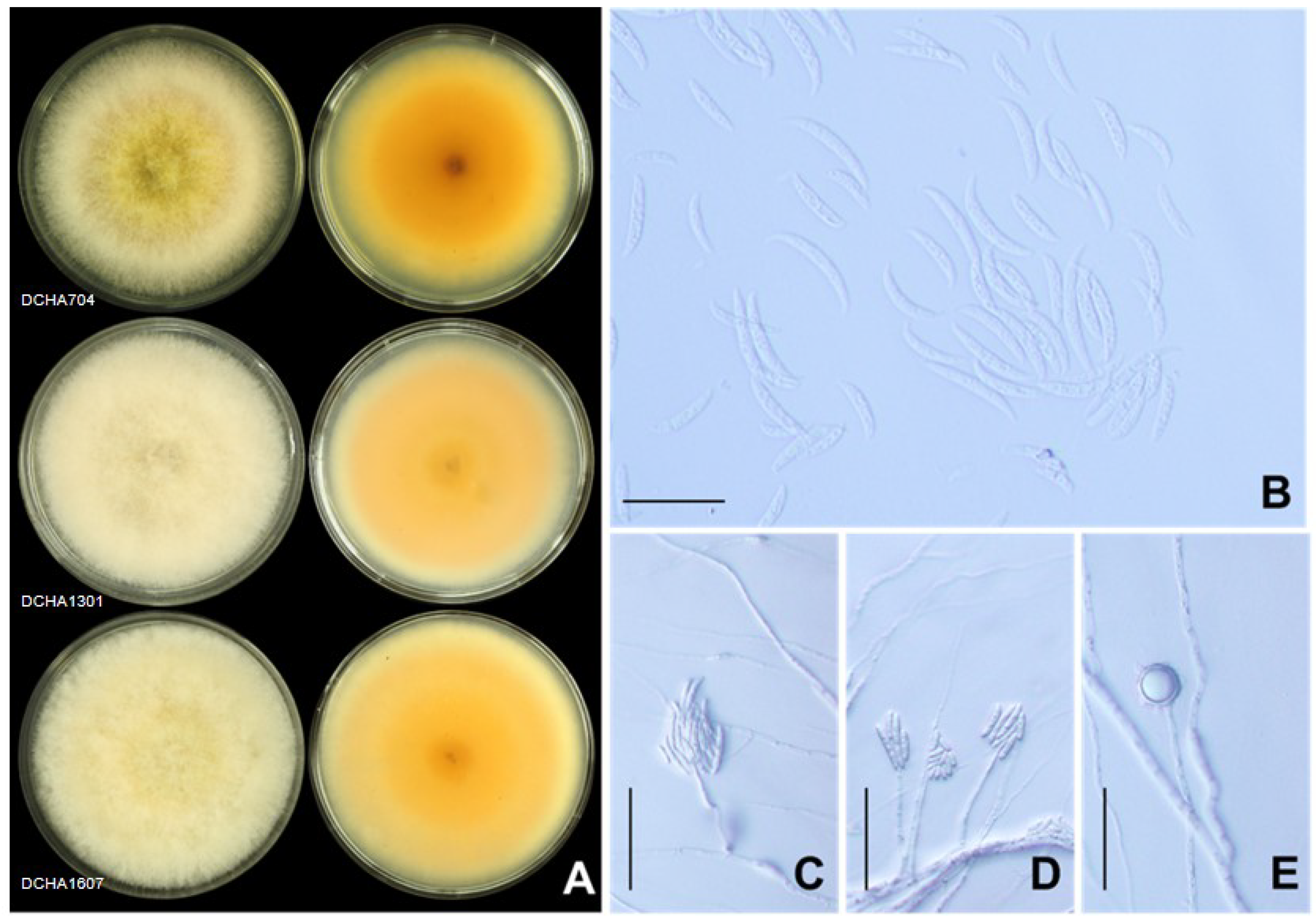
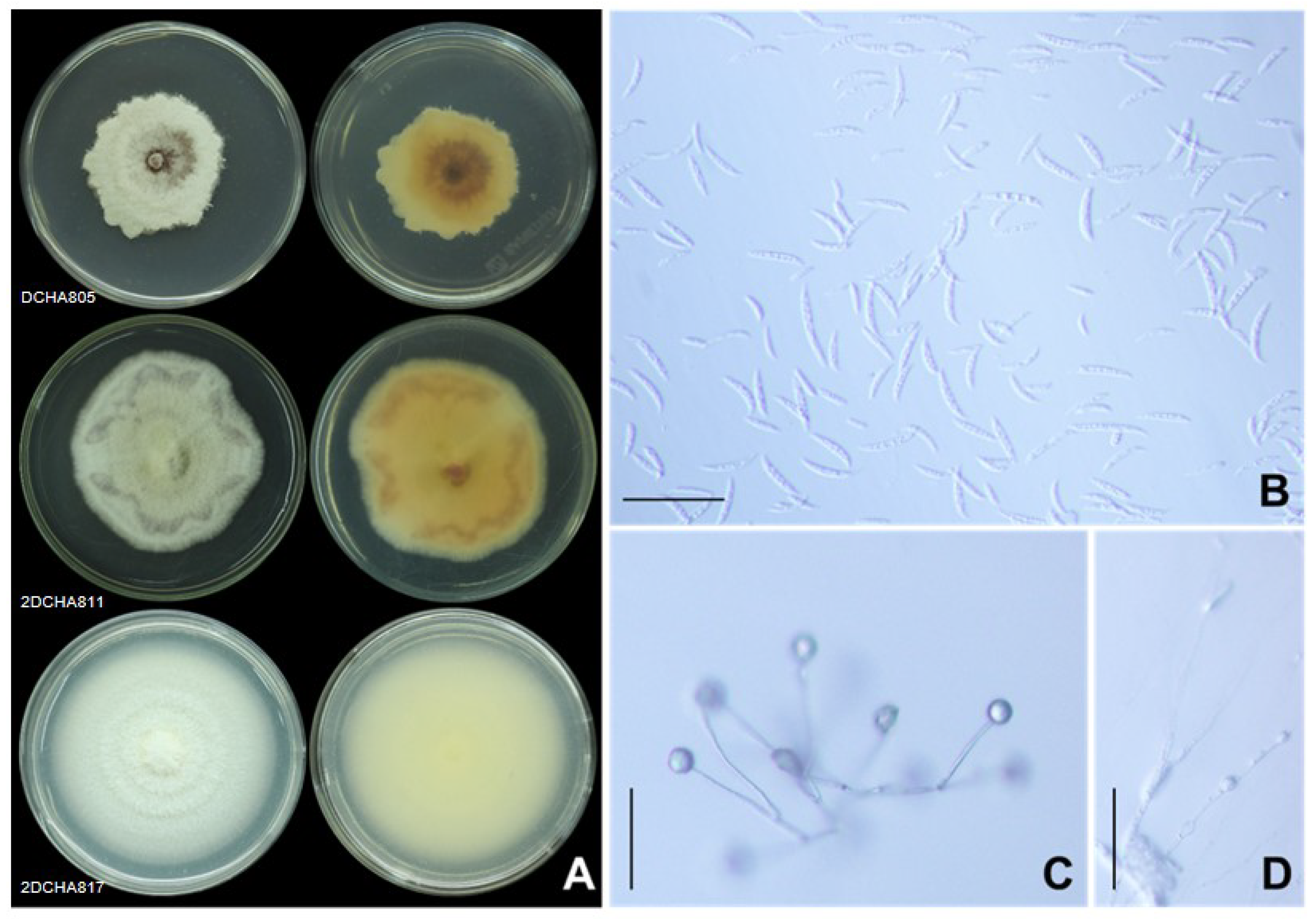
2.3. Morphological Characteristics
2.4. Pathogenicity Test
2.5. Fusarium–Ambrosia Beetle Association
3. Discussion
4. Materials and Methods
4.1. Fungal Isolation and Purification
4.2. DNA Extraction and Molecular Identification
4.3. Morphological Characteristics
4.4. Phylogenetic Analysis
4.5. Pathogenicity Test
4.6. Fusarium–Ambrosia Beetle Association
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fresh Durian. Available online: https://www.tridge.com/intelligences/durian (accessed on 31 March 2023).
- World Food and Agriculture—Statistical Yearbook 2022. Available online: https://www.fao.org/statistics/en/ (accessed on 31 March 2023).
- Thailand, Vietnam, and the Philippines Compete for Durian Share in China. Available online: https://www.globaltimes.cn/page/202305/1291568.shtml (accessed on 1 June 2023).
- Agricultural Economics Information 2021. Available online: https://www.oae.go.th/assets/portals/1/ebookcategory/67_commodity2564/ (accessed on 1 March 2023).
- Drenth, A.; Guest, D. Diversity and Management of Phytophthora in Southeast Asia; ACIAR Monograph 114: Melbourne, Australia, 2004. [Google Scholar]
- Pongpisutta, R.; Sangchote, S. Phytophthora fruit rot of durian (Durio zibethinus). In Proceedings of the International Conference. ACIAR Proceedings no. 50′; Champ, B.R., Highley, E., Johnson, G.I., Eds.; Arawang Communications Group: Canberra, Australia, 1993; pp. 460–461. [Google Scholar]
- Lim, T.K.; Sangchote, S. Diseases of Durian. In Diseases of Tropical Fruit Crops; Ploetz, R.C., Ed.; CABI Publishing: Wallingford, UK, 2003; pp. 241–251. [Google Scholar]
- Pongpisutta, R.; Rattanakreetakul, C.; Bincader, S.; Chatchaisiri, K.; Boonruanfrod, P. Detection of fungal pathogen causing durian dieback disease. Khon Kaen Agr. J. 2020, 48, 703–714. [Google Scholar] [CrossRef]
- Gadd, C.; Loos, C.A. The ambrosia fungus of Xyleborus fornicatus Eich. Trans. Br. Mycol. Soc. 1947, 31, 13–18. [Google Scholar] [CrossRef]
- Norris, D.M.; Baker, J.K. Symbiosis: Effects of a mutualistic fungus upon the growth and reproduction of Xyleborus ferrugineus. Science 1967, 156, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Mueller, U.G.; Gerardo, N. Fungus-farming insects: Multiple origins and diverse evolutionary histories. Proc. Natl. Acad. Sci. USA 2002, 99, 15247–15249. [Google Scholar] [CrossRef]
- Ranger, C.M.; Biedermann, P.H.W.; Phuntumart, V.; Beligala, G.U.; Ghosh, S.; Palmquist, D.E.; Mueller, R.; Barnett, J.; Schultz, P.B.; Reding, M.E.; et al. Symbiont selection via alcohol benefits fungus farming by ambrosia beetles. Proc. Natl. Acad. Sci. USA 2018, 115, 4447–4452. [Google Scholar] [CrossRef]
- Hung, T.X.; Thu, P.Q.; Chi, N.M.; Binh, L.V.; Dell, B. Impacts and trapping of ambrosia beetles Euwallacea fornicatus and E. similis in Acacia plantations in Vietnam. South. For. 2022, 84, 242–252. [Google Scholar] [CrossRef]
- Mathew, G.; Shamsudeen, R.S.M.; Chandran, R. Insect fauna of Peechi-Vazhani Wildlife Sanctuary, Kerala, India. ZOO’s Print J. 2005, 20, 1955–1960. [Google Scholar] [CrossRef]
- Hulcr, J.; Stelinski, L.L. The Ambrosia symbiosis: From evolutionary ecology to practical management. Annu. Rev. Entomol. 2017, 62, 285–303. [Google Scholar] [CrossRef]
- Lynn, K.M.T.; Wingfield, M.J.; Durán, A.; Oliveira, L.S.S.; de Beer, Z.W.; Barnes, I. Novel Fusarium mutualists of two Euwallacea species infesting Acacia crassicarpa in Indonesia. Mycologia 2021, 113, 536–558. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Fothergill, A.; McCarthy, D.; Rinaldi, M.G.; Brandt, M.E.; Zhang, N.; Geiser, D.M. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J. Clin. Microbiol. 2008, 46, 2477–2490. [Google Scholar] [CrossRef]
- Xia, J.W.; Sandoval-Denis, M.; Crous, P.W.; Zhang, X.G.; Lombard, L. Numbers to names—Restyling the Fusarium incarnatum-equiseti species complex. Persoonia 2019, 43, 186–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; O’Donnell, K.; Sutton, D.A.; Nalim, F.A.; Summerbell, R.C.; Padhye, A.A.; Geiser, D.M. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 2006, 44, 2186–2190. [Google Scholar] [CrossRef]
- Chang, D.C.; Grant, G.B.; O’Donnell, K.; Wannemuehler, K.A.; Noble-Wang, J.; Rao, C.Y.; Jacobson, L.M.; Crowell, C.S.; Sneed, R.S.; Lewis, F.M.T.; et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA 2006, 296, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Kasson, M.T.; O’Donnell, K.; Rooney, A.P.; Sink, S.; Ploetz, R.C.; Ploetz, J.N.; Konkol, J.L.; Carrillo, D.; Freeman, S.; Mendel, Z.; et al. An inordinate fondness for Fusarium: Phylogenetic diversity of fusaria cultivated by ambrosia beetles in the genus Euwallacea on avocado and other plant hosts. Fungal. Genet. Biol. 2013, 56, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.; Groenewald, M.; de Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef]
- Rattanakreetakul, C.; Keawmanee, P.; Bincader, S.; Mongkolporn, O.; Phuntumart, V.; Chiba, S.; Pongpisutta, R. Two newly identified Colletotrichum species associated with mango anthracnose in central Thailand. Plants 2023, 12, 1130. [Google Scholar] [CrossRef]
- Mirghasempour, S.A.; Studholme, D.J.; Chen, W.; Zhu, W.; Mao, B. Molecular and pathogenic characterization of Fusarium species associated with corm rot disease in saffron from China. J. Fungi. 2022, 8, 515. [Google Scholar] [CrossRef]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef]
- McTaggart, A.R.; James, T.Y.; Shivas, R.G.; Drenth, A.; Wingfield, B.D.; Summerell, B.A.; Duong, T.A. Population genomics reveals historical and ongoing recombination in the Fusarium oxysporum species complex. Stud. Mycol. 2021, 99, 100132. [Google Scholar] [CrossRef]
- Yilmaz, N.; Sandoval-Denis, M.; Lombard, L.; Visagie, C.M.; Wingfield, B.D.; Crous, P.W. Redefining species limits in the Fusarium fujikuroi species complex. Persoonia 2021, 46, 129–162. [Google Scholar] [CrossRef]
- Lombard, L.; Sandoval-Denis, M.; Lamprecht, S.C.; Crous, P.W. Epitypification of Fusarium oxysporum—Clearing the taxonomic chaos. Persoonia 2019, 43, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, L. Fusarium species associated with diseases of major tropical fruit crops. Horticulturae 2023, 9, 322. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006. [Google Scholar]
- Mendel, Z.; Protasov, A.; Sharon, M.; Zveibil, A.; Yehuda, S.B.; O’Donnell, K.; Rabaglia, R.; Wysoki, M.; Freeman, S. An Asian ambrosia beetle Euwallacea fornicatus and its novel symbiotic fungus Fusarium sp. pose a serious threat to the Israeli avocado industry. Phytoparasitica 2012, 40, 235–238. [Google Scholar] [CrossRef]
- O’Donnell, K.; Ward, T.J.; Robert Vincent, A.R.G.; Crous, P.W.; Geiser, D.M.; Kang, S. DNA sequence-based identification of Fusarium: Current status and future directions. Phytoparasitica 2015, 43, 583–595. [Google Scholar] [CrossRef]
- Swain, D.M.; Yadav, S.K.; Tyagi, I.; Kumar, R.; Kumar, R.; Ghosh, S.; Das, J.; Jha, G. A prophage tail-like protein is deployed by Burkholderia bacteria to feed on fungi. Nat. Commun. 2017, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, L.; Odegaard, F. Ongoing invasions of old-growth tropical forests: Establishment of three incestuous beetle species in Central America. Zootaxa 2007, 1588, 53–62. [Google Scholar] [CrossRef]
- Carrillo, D.; Duncan, R.E.; Peña, J.E. Ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) that breed in avocado wood in Florida. Fla. Entomol. 2012, 95, 573–579. [Google Scholar] [CrossRef]
- Aoki, T.; Kasson, M.T.; Berger, M.C.; Freeman, S.; Geiser, D.M.; O’Donnell, K. Fusarium oligoseptatum sp. nov., a mycosymbiont of the ambrosia beetle Euwallacea validus in the Eastern U.S. and typification of F. ambrosium. Fungal. Syst. Evol. 2018, 1, 23–39. [Google Scholar] [CrossRef]
- Aoki, T.; Smith, J.A.; Kasson, M.T.; Freeman, S.; Geiser, D.M.; Geering, A.D.W.; O’Donnell, K. Three novel Ambrosia Fusarium Clade species producing clavate macroconidia known (F. floridanum and F. obliquiseptatum) or predicted (F. tuaranense) to be farmed by Euwallacea spp. (Coleoptera: Scolytinae) on woody hosts. Mycologia 2019, 111, 919–935. [Google Scholar] [CrossRef]
- Cognato, A.I.; Hoebeke, E.R.; Kajimura, H.; Smith, S.M. History of the exotic ambrosia beetles Euwallacea interjectus and Euwallacea validus (Coleoptera: Curculionidae: Xyleborini) in the United States. J. Econ. Entomol. 2015, 108, 1129–1135. [Google Scholar] [CrossRef]
- Li, Y.; Simmons, D.R.; Bateman, C.C.; Short, D.P.; Kasson, M.T.; Rabaglia, R.J.; Hulcr, J. New fungus-insect symbiosis: Culturing, molecular, and histological methods determine saprophytic Polyporales mutualists of ambrosiodmus ambrosia beetles. PLoS ONE 2015, 10, e0137689. [Google Scholar] [CrossRef] [PubMed]
- Stouthamer, R.; Rugman-Jones, P.; Thu, P.Q.; Eskalen, A.; Thibault, T.; Hulcr, J.; Wang, L.-J.; Chen, C.-Y.; Cooperband, M.; Hsu, J.-C.; et al. Tracing the origin of a cryptic invader: Phylogeography of the Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) species complex. Agric. For. Entomol. 2017, 19, 366–375. [Google Scholar] [CrossRef]
- Dzurenko, M.; Ranger, C.M.; Hulcr, J.; Galko, J.; Kaňuch, P. Origin of non-native Xylosandrus germanus, an invasive pest ambrosia beetle in Europe and North America. J. Pest. Sci. 2021, 94, 553–562. [Google Scholar] [CrossRef]
- Carrillo, J.; Rugman-Jones, P.; Husein, D.; Stajich, J.; Kasson, M.; Carrillo, D.; Stouthamer, R.; Eskalen, A. Members of the Euwallacea fornicatus species complex exhibit promiscuous mutualism with ambrosia fungi in Taiwan. Fungal Genet. Biol. 2019, 133, 103269. [Google Scholar] [CrossRef]
- Carrillo, J.D.; Dodge, C.; Stouthamer, R.; Eskalen, A. Fungal symbionts of the polyphagous and Kuroshio shot hole borers (Coleoptera: Scolytinae, Euwallacea spp.) in California can support both ambrosia beetle systems on artificial media. Symbiosis 2020, 80, 155–168. [Google Scholar] [CrossRef]
- Zimand, G.; Valinsky, L.; Elad, Y.; Chet, I.; Manulis, S. Use of the RAPD procedure for the identification of Trichoderma strains. Mycol. Res. 1994, 98, 531–534. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- O’Donnell, K.; Cigelnik, E.; Nirenberg, H.I. Molecular systematics and phy-logeography of the Gibberella fujikuroi species complex. Mycologia 1998, 90, 465–493. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Booth, C. Fusarium: Laboratory Guide to the Identification of the Major Species; The Common Wealth Mycological Institute: London, UK, 1971; p. 237. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, L.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 10 October 2022).
- Statistical Procedures for Agricultural Research, R Package Version 1.4.0. Available online: https://myaseen208.github.io/agricolae (accessed on 10 October 2022).
- Biedermann, P.H.W.; Klepzig, K.D.; Michael, T. Fungus cultivation by ambrosia beetles: Behavior and laboratory breeding success in three Xyleborine species. Environ. Entomol. 2009, 38, 1096–1105. [Google Scholar] [CrossRef] [PubMed]


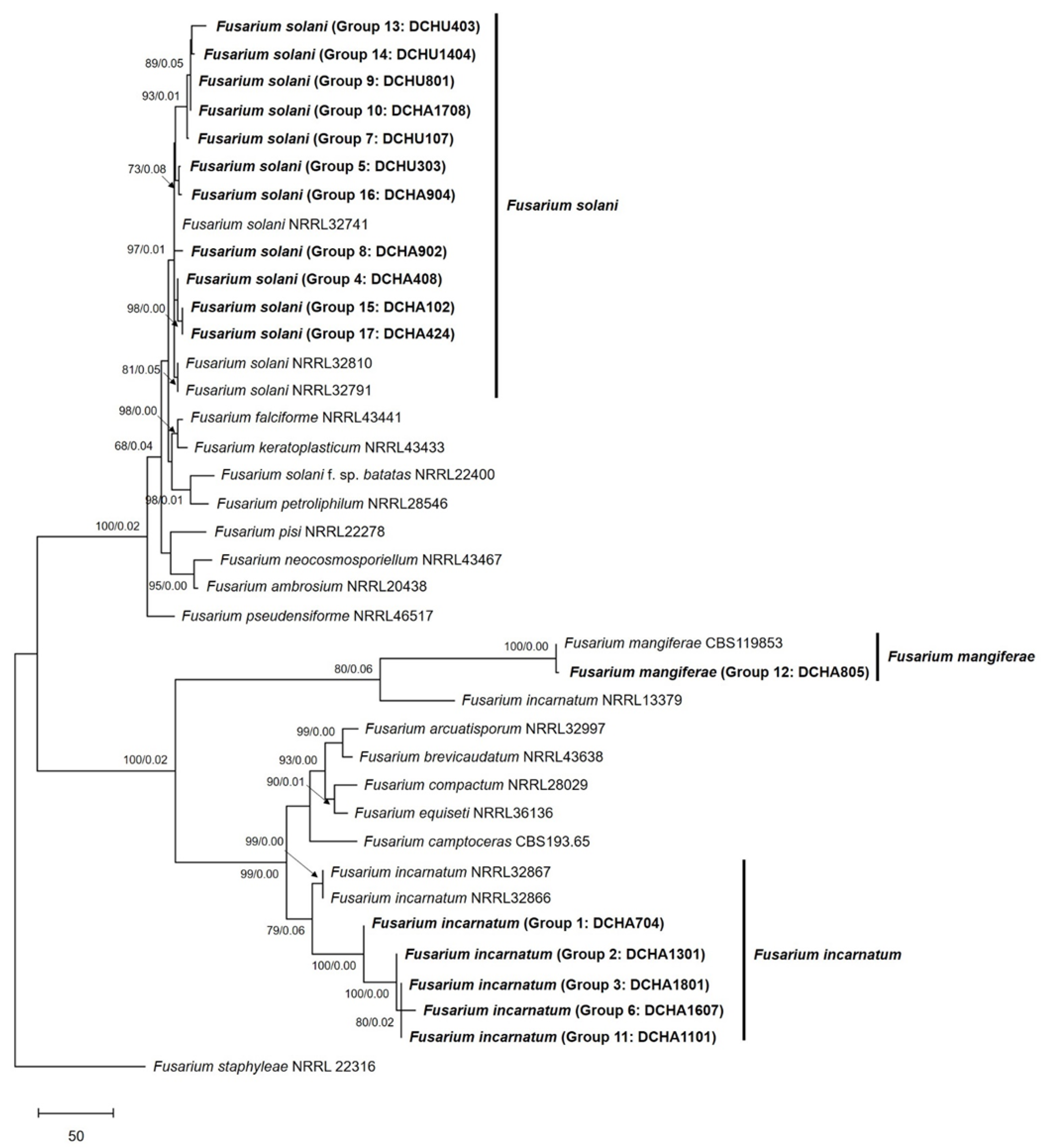



| Species | Collection 1 | GenBank Accession Numbers | Identification (GenBank References at 99–100% Similarity) | ||
|---|---|---|---|---|---|
| ITS | TEF1-α | RPB2 | |||
| Fusarium Group 1 | DCHA704 | LC745744.1 | LC745761.1 | LC745778.1 | F. incarnatum (NRRL32867, NRRL32866) |
| Fusarium Group 2 | DCHA1301 | LC745745.1 | LC745762.1 | LC745779.1 | F. incarnatum (NRRL32867, NRRL32866) |
| Fusarium Group 3 | DCHA1801 | LC745746.1 | LC745763.1 | LC745780.1 | F. incarnatum (NRRL32867, NRRL32866) |
| Fusarium Group 4 | DCHA408 | LC745747.1 | LC745764.1 | LC745781.1 | F. solani (NRRL32810, NRRL32791) |
| Fusarium Group 5 | DCHU303 | LC745748.1 | LC745765.1 | LC745782.1 | F. solani NRRL32741 |
| Fusarium Group 6 | DCHA1607 | LC745749.1 | LC745766.1 | LC745783.1 | F. incarnatum (NRRL32867, NRRL32866) |
| Fusarium Group 7 | DCHU107 | LC745750.1 | LC745767.1 | LC745784.1 | F. solani (NRRL32741) |
| Fusarium Group 8 | DCHA902 | LC745751.1 | LC745768.1 | LC745785.1 | F. solani (NRRL32810, NRRL32791) |
| Fusarium Group 9 | DCHU801 | LC745752.1 | LC745769.1 | LC745786.1 | F. solani (NRRL32741) |
| Fusarium Group 10 | DCHA1708 | LC745753.1 | LC745770.1 | LC745787.1 | F. solani (NRRL32741) |
| Fusarium Group 11 | DCHA1101 | LC745754.1 | LC745771.1 | LC745788.1 | F. incarnatum (NRRL32867, NRRL32866) |
| Fusarium Group 12 | DCHA805 | LC745755.1 | LC745772.1 | LC745789.1 | F. mangiferae (CBS119853) |
| Fusarium Group 13 | DCHU403 | LC745756.1 | LC745773.1 | LC745790.1 | F. solani (NRRL32741) |
| Fusarium Group 14 | DCHU1404 | LC745757.1 | LC745774.1 | LC745791.1 | F. solani (NRRL32741) |
| Fusarium Group 15 | DCHA102 | LC745758.1 | LC745775.1 | LC745792.1 | F. solani (NRRL32810, NRRL32791) |
| Fusarium Group 16 | DCHA904 | LC745759.1 | LC745776.1 | LC745793.1 | F. solani (NRRL32741) |
| Fusarium Group 17 | DCHA424 | LC745760.1 | LC745777.1 | LC745794.1 | F. solani (NRRL32810, NRRL32791) |
| Group | Species | Representative Isolate | Mean Lesion Size (cm) ± SD |
|---|---|---|---|
| 1 | F. incarnatum | DCHA704 | 3.35 ± 0.32 e |
| 2 | F. incarnatum | DCHA1301 | 2.38 ± 0.34 f |
| 3 | F. incarnatum | DCHA1801 | 1.76 ± 0.14 g |
| 4 | F. solani | DCHA408 | 3.49 ± 0.27 e |
| 5 | F. solani | DCHU303 | 3.94 ± 0.13 d |
| 6 | F. incarnatum | DCHA1607 | 2.42 ± 0.11 f |
| 7 | F. solani | DCHU107 | 5.05 ± 0.26 c |
| 8 | F. solani | DCHA902 | 5.38 ± 0.19 b |
| 9 | F. solani | DCHU801 | 5.98 ± 0.08 a |
| 10 | F. solani | DCHA1708 | 5.90 ± 0.22 a |
| 11 | F. incarnatum | DCHA1101 | 3.98 ± 0.17 d |
| 12 | F. mangiferae | DCHA805 | 0.00 ± 0.00 h |
| 13 | F. solani | DCHU403 | 3.88 ± 0.17 d |
| 14 | F. solani | DCHU1404 | 3.60 ± 0.29 e |
| 15 | F. solani | DCHA102 | 4.06 ± 0.07 d |
| 16 | F. solani | DCHA904 | 3.94 ± 0.07 d |
| 17 | F. solani | DCHA424 | 4.05 ± 0.07 d |
| F-test | *** | ||
| C.V. (%) | 5.2863 | ||
| MSE | 0.039 | ||
| Gene/DNA Region | Primer Details | |||
|---|---|---|---|---|
| Name | Name | Sequence (5′→3′) | Ta (°C) | References |
| ITS region | ITS5 | GGAAGTAAAAGTCGTAACAAGG | 56 | [45] |
| ITS4 | TCCTCCGCTTATTGATATGC | |||
| TEF1-α gene | EF1-F | ATGGGTAAGGARGACAAGAC | 56 | [46] |
| EF2-R | GGARGTACCAGTSATCATGTT | |||
| RPB2 gene | fRPB2-7cf | ATGGGYAARCAAGCYATGGG | 57.2 | [47] |
| fRPB2-7cr | CCCATRGCTTGYTTRCCCAT | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pongpisutta, R.; Keawmanee, P.; Sanguansub, S.; Dokchan, P.; Bincader, S.; Phuntumart, V.; Rattanakreetakul, C. Comprehensive Investigation of Die-Back Disease Caused by Fusarium in Durian. Plants 2023, 12, 3045. https://doi.org/10.3390/plants12173045
Pongpisutta R, Keawmanee P, Sanguansub S, Dokchan P, Bincader S, Phuntumart V, Rattanakreetakul C. Comprehensive Investigation of Die-Back Disease Caused by Fusarium in Durian. Plants. 2023; 12(17):3045. https://doi.org/10.3390/plants12173045
Chicago/Turabian StylePongpisutta, Ratiya, Pisut Keawmanee, Sunisa Sanguansub, Paradorn Dokchan, Santiti Bincader, Vipaporn Phuntumart, and Chainarong Rattanakreetakul. 2023. "Comprehensive Investigation of Die-Back Disease Caused by Fusarium in Durian" Plants 12, no. 17: 3045. https://doi.org/10.3390/plants12173045
APA StylePongpisutta, R., Keawmanee, P., Sanguansub, S., Dokchan, P., Bincader, S., Phuntumart, V., & Rattanakreetakul, C. (2023). Comprehensive Investigation of Die-Back Disease Caused by Fusarium in Durian. Plants, 12(17), 3045. https://doi.org/10.3390/plants12173045







