Chemical Variability of the Essential Oils from Two Portuguese Apiaceae: Coriandrum sativum L. and Foeniculum vulgare Mill.
Abstract
1. Introduction
2. Results
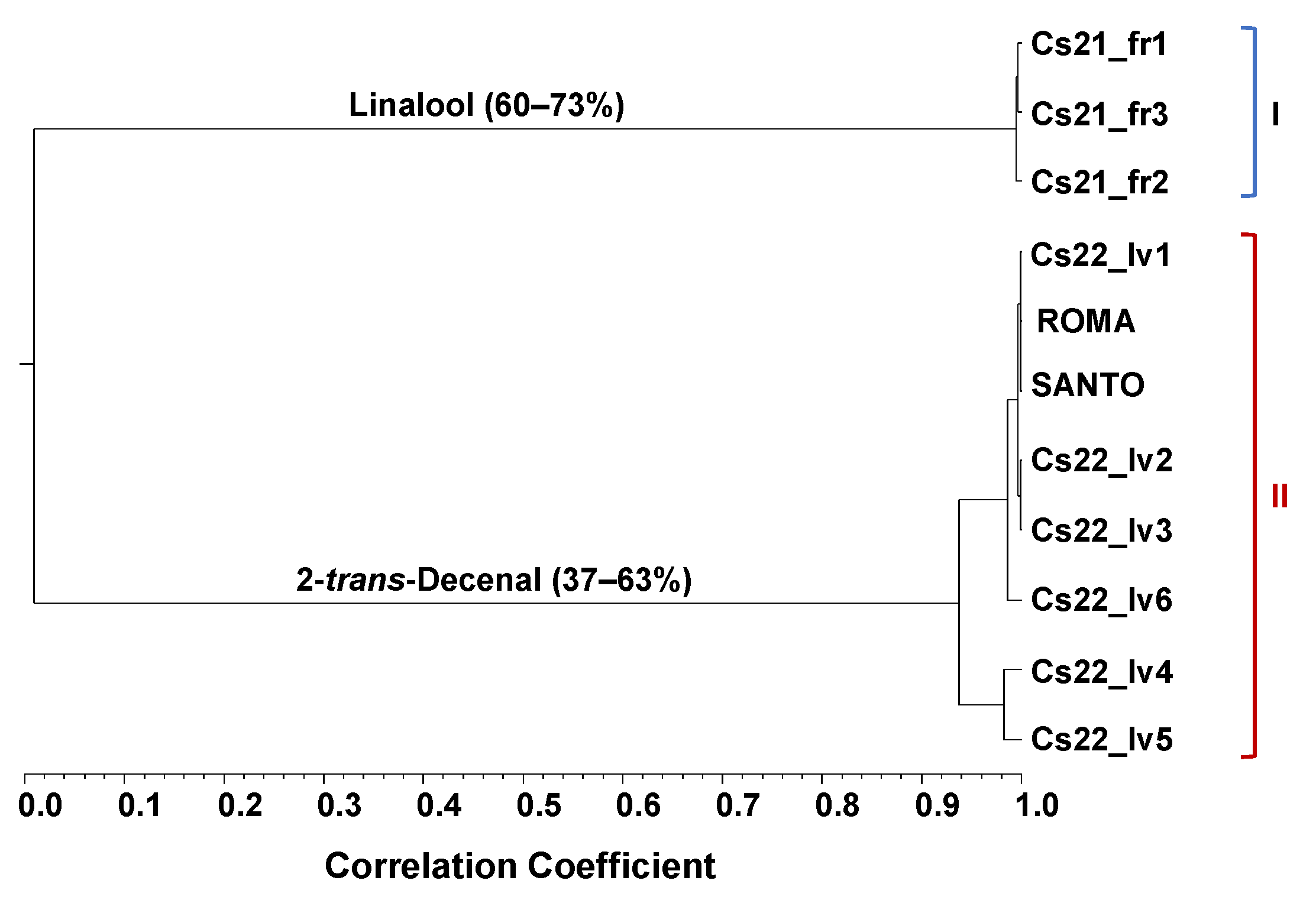
2.1. Coriandrum sativum Fruits and Vegetative Aerial Parts—EO Profile and Cluster Analysis
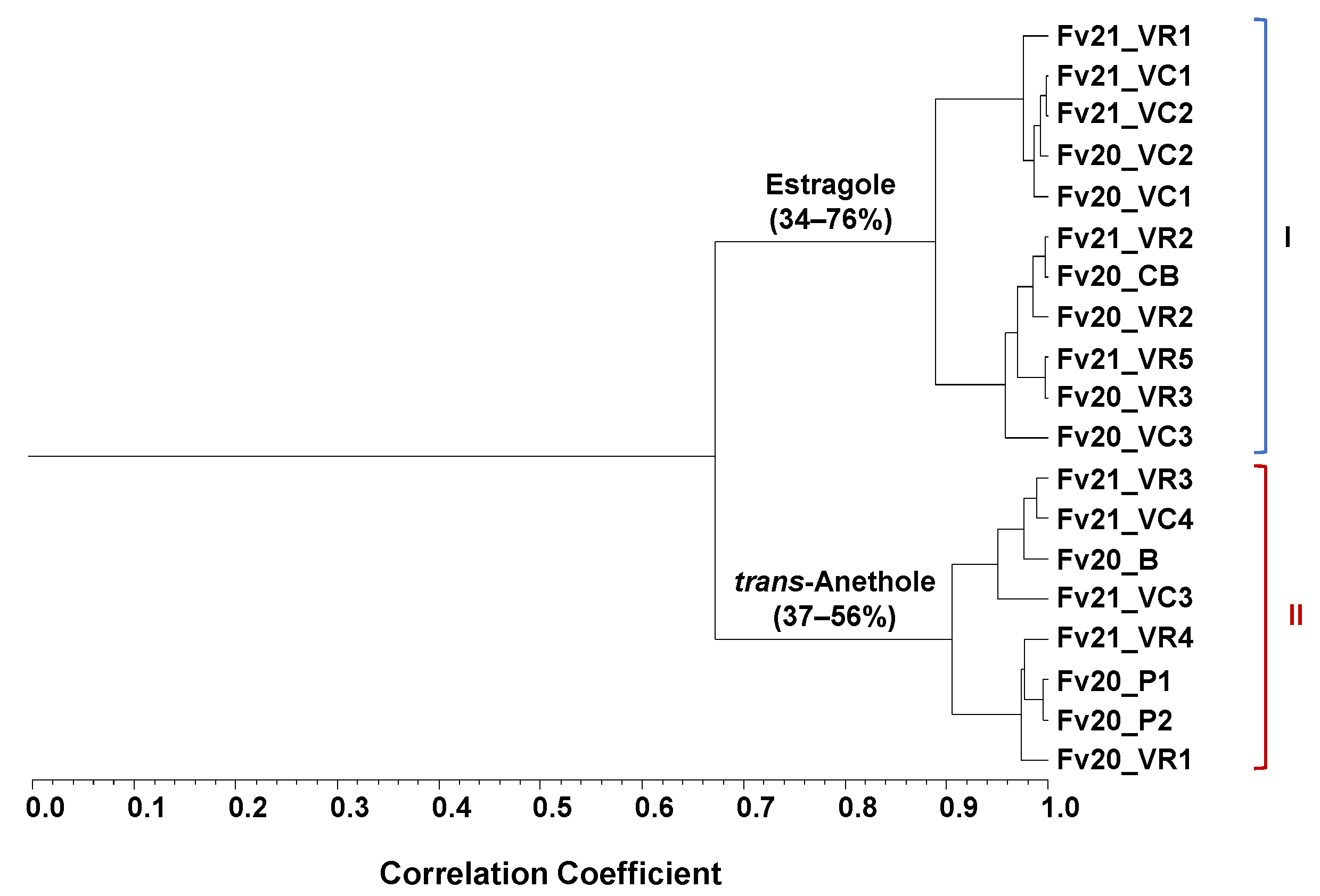
2.2. Foeniculum vulgare Mill. Fruits—EO Profile and Cluster Analysis
3. Discussion
3.1. Coriandrum sativum EOs Isolated from Fruits and Vegetative Aerial Parts
3.2. Foeniculum vulgare EOs Isolated from Fruits
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oils Isolation
4.3. Analysis and Compound Quantification of the EOs
4.3.1. Gas Chromatography with Flame Ionization Detection (GC-FID)
4.3.2. Gas Chromatography–Mass Spectrometry (GC-MS)
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Z.; Chang, L. Apiaceae or Umbelliferae. In Identification and Control of Common Weeds: Volume 3; Xu, Z., Chang, L., Eds.; Springer: Singapore, 2017; pp. 3–49. [Google Scholar]
- Spinozzi, E.; Maggi, F.; Bonacucina, G.; Pavela, R.; Boukouvala, M.C.; Kavallieratos, N.G.; Canale, A.; Romano, D.; Desneux, N.; Wilke, A.B.B.; et al. Apiaceae Essential Oils and Their Constituents as Insecticides against Mosquitoes—A Review. Ind. Crops Prod. 2021, 171, 113892. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Piumali, D.; Merah, O.; Madhujith, T. Apiaceae as an Important Source of Antioxidants and Their Applications. Cosmetics 2021, 8, 111. [Google Scholar] [CrossRef]
- Plunkett, G.M.; Pimenov, M.G.; Reduron, J.P.; Kljuykov, E.V.; van Wyk, B.E.; Ostroumova, T.A.; Henwood, M.J.; Tilney, P.M.; Spalik, K.; Watson, M.F.; et al. Apiaceae. In Flowering Plants. Eudicots. The Families and Genera of Vascular Plants; Kadereit, J., Bittrich, V., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 15, pp. 9–206. [Google Scholar]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal Family as Source for Industrial Uses. Ind. Crops Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- Ngahang Kamte, S.L.; Ranjbarian, F.; Cianfaglione, K.; Sut, S.; Dall’Acqua, S.; Bruno, M.; Afshar, F.H.; Iannarelli, R.; Benelli, G.; Cappellacci, L.; et al. Identification of Highly Effective Antitrypanosomal Compounds in Essential Oils from the Apiaceae Family. Ecotoxicol. Environ. Saf. 2018, 156, 154–165. [Google Scholar] [CrossRef]
- Zengin, G.; Stojković, D.; Mahomoodally, M.F.; Jugreet, B.S.; Paksoy, M.Y.; Ivanov, M.; Gašić, U.; Gallo, M.; Montesano, D. Comprehensive Biological and Chemical Evaluation of Two Seseli Species (S. gummiferum and S. transcaucasicum). Antioxidants 2021, 10, 1510. [Google Scholar] [CrossRef]
- Simpson, M.G. Diversity and Classification of Flowering Plants: Eudicots. In Plant Systematics, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 275–448. [Google Scholar]
- Mahleyuddin, N.N.; Moshawih, S.; Ming, L.C.; Zulkifly, H.H.; Kifli, N.; Loy, M.J.; Sarker, M.M.R.; Al-Worafi, Y.M.; Goh, B.H.; Thuraisingam, S.; et al. Coriandrum Sativum L.: A Review on Ethnopharmacology, Phytochemistry, and Cardiovascular Benefits. Molecules 2021, 27, 209. [Google Scholar] [CrossRef]
- Soares, B.V.; Morais, S.M.; Dos Santos Fontenelle, R.O.; Queiroz, V.A.; Vila-Nova, N.S.; Pereira, C.M.C.; Brito, E.S.; Neto, M.A.S.; Brito, E.H.S.; Cavalcante, C.S.P.; et al. Antifungal Activity, Toxicity and Chemical Composition of the Essential Oil of Coriandrum sativum L. Fruits. Molecules 2012, 17, 8439–8448. [Google Scholar] [CrossRef]
- Samojlik, I.; Lakić, N.; Mimica-Dukić, N.; Đaković-Švajcer, K.; Božin, B. Antioxidant and Hepatoprotective Potential of Essential Oils of Coriander (Coriandrum sativum L.) and Caraway (Carum carvi L.) (Apiaceae). J. Agric. Food Chem. 2010, 58, 8848–8853. [Google Scholar] [CrossRef]
- Cioanca, O.; Hancianu, M.; Mircea, C.; Trifan, A.; Hritcu, L. Essential Oils from Apiaceae as Valuable Resources in Neurological Disorders: Foeniculi vulgare aetheroleum. Ind. Crops Prod. 2016, 88, 51–57. [Google Scholar] [CrossRef]
- Mota, A.S.; Martins, M.R.; Arantes, S.; Lopes, V.R.; Bettencourt, E.; Pombal, S.; Gomes, A.C.; Silva, L.A. Antimicrobial Activity and Chemical Composition of the Essential Oils of Portuguese Foeniculum vulgare Fruits. Nat. Prod. Commun. 2015, 10, 673–676. [Google Scholar] [CrossRef]
- Mata, A.T.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and Antiacetylcholinesterase Activities of Five Plants Used as Portuguese Food Spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Dias dos Santos, S.M.; Pintado, M.E.; Pérez-Álvarez, J.A.; Fernández-López, J.; Viuda-Martos, M. Chemical Composition and in vitro Antimicrobial, Antifungal and Antioxidant Properties of Essential Oils Obtained from Some Herbs Widely Used in Portugal. Food Control 2013, 32, 371–378. [Google Scholar] [CrossRef]
- Alves, S.; Duarte, A.; Sousa, S.; Domingues, F.C. Study of the Major Essential Oil Compounds of Coriandrum sativum against Acinetobacter baumannii and the Effect of Linalool on Adhesion, Biofilms and Quorum Sensing. Biofouling 2016, 32, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Lo Cantore, P.; Iacobellis, N.S.; De Marco, A.; Capasso, F.; Senatore, F. Antibacterial Activity of Coriandrum sativum L. and Foeniculum vulgare Miller var. Vulgare (Miller) Essential Oils. J. Agric. Food Chem. 2004, 52, 7862–7866. [Google Scholar] [CrossRef] [PubMed]
- Garzoli, S.; Božović, M.; Baldisserotto, A.; Sabatino, M.; Cesa, S.; Pepi, F.; Vicentini, C.B.; Manfredini, S.; Ragno, R. Essential Oil Extraction, Chemical Analysis and Anti-Candida Activity of Foeniculum vulgare Miller—New Approaches. Nat. Prod. Res. 2018, 32, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G.; Cruz, C.; Faleiro, L.; Simões, M.T.F.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Foeniculum vulgare Essential Oils: Chemical Composition, Antioxidant and Antimicrobial Activities. Nat. Prod. Commun. 2010, 5, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.K.; Matosc, O.; Novoa, M.T.; Figueiredo, A.C.; Delgado, M.; Moiteiro, C. Larvicidal Activity against Aedes aegypti of Foeniculum vulgare Essential Oils from Portugal and Cape Verde. Nat. Prod. Commun. 2015, 10, 677–682. [Google Scholar] [PubMed]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum Vulgare Mill: A Review of Its Botany, Phytochemistry, Pharmacology, Contemporary Application, and Toxicology. BioMed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef]
- Tavares, A.C.; Salgueiro, L.; Canhoto, J.; Paiva, J.A.R. Iberian endemic “Apiaceae”: A reassessment for conservation purposes in Portugal. Stud. Bot. 2010, 29, 13–33. [Google Scholar]
- Cabral, C.; Poças, J.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Ridolfia segetum (L.) Moris (Apiaceae) from Portugal: A Source of Safe Antioxidant and Anti-Inflammatory Essential Oil. Ind. Crops Prod. 2015, 65, 56–61. [Google Scholar] [CrossRef]
- Vilar, L. Coriandrum L. In Flora Iberica Plantas Vasculares de la Peninsula Iberica e Islas Baleares; Castroviejo, S., Laínz, M., González, L.G., Montserrat, P., Garmendia, F.M., Paiva, J., Villar, L., Eds.; Real Jardín Botánico, CSIC: Madrid, Spain, 2010; Volume XII, CXL, pp. 136–138. [Google Scholar]
- Tutin, T.G. Foeniculum Mill. In Flora Europaea Vol. 2. Rosaceae to Umbelliferae; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: London, UK, 1968; p. 341. [Google Scholar]
- Burdock, G.A.; Carabin, I.G. Safety Assessment of Coriander (Coriandrum sativum L.) Essential Oil as a Food Ingredient. Food Chem. Toxicol. 2009, 47, 22–34. [Google Scholar] [CrossRef]
- Nguyen, Q.-H.; Talou, T.; Cerny, M.; Evon, P.; Merah, O. Oil and Fatty Acid Accumulation during Coriander (Coriandrum sativum L.) Fruit Ripening under Organic Cultivation. Crop J. 2015, 3, 366–369. [Google Scholar] [CrossRef]
- Satyal, P.; Setzer, W.N. Chemical Compositions of Commercial Essential Oils from Coriandrum sativum Fruits and Aerial Parts. Nat. Prod. Commun. 2020, 15, 1934578X20933067. [Google Scholar] [CrossRef]
- Nurzynska-Wierdak, R. Essential Oil Composition of the Coriander (Coriandrum sativum L.) Herb Depending on the Development Stage. Acta Agrobot. 2013, 66, 53–60. [Google Scholar] [CrossRef]
- European Pharmacopoeia (Ph. Eur.) 10th Edition-European Directorate for the Quality of Medicines & HealthCare-EDQM. Available online: https://www.edqm.eu/en/ (accessed on 12 June 2023).
- Instituto Nacional da Farmácia e do Medicamento. Farmacopeia Portuguesa 9.0; Autoridade Nacional do Medicamento e Produtos de Saúde: Lisboa, Portugal, 2008. [Google Scholar]
- ISO 3516:1997; Oil of Coriander Fruits (Coriandrum sativum L.). ISO: Geneva, Switzerland, 1997.
- Pujadas-Salvá, A.J.; Triano, E.; Anaya, J.; Grande, M.; Raposo, C.; Torres, P.; Hernández Molina, P. Foeniculum sanguineum Triano & A. Pujadas (Apiaceae) New Species from the South Western Mediterranean Region. Acta Bot. Malacit. 2015, 40, 71–88. [Google Scholar]
- Ilardi, V.; Troia, A. Re-evaluation and Typification of Foeniculum piperitum (Apiaceae), an Underknown Medicinal Plant and Crop Wild Relative. Phytotaxa 2021, 508, 197–205. [Google Scholar] [CrossRef]
- Bernáth, J.; Németh, É.; Kattaa, A.; Héthelyi, É. Morphological and Chemical Evaluation of Fennel (Foeniculum vulgare Mill.) Populations of Different Origin. J. Essent. Oil Res. 1996, 8, 247–253. [Google Scholar] [CrossRef]
- ISO 17412:2007; Oil of Bitter Fennel (Foeniculum vulgare Mill. ssp. vulgare var. vulgare). ISO: Geneva, Switzerland, 2007.
- César, B.; Placido, J.; Lopes, V.; Barata, A.; Serrano, M.; Póvoa, O.; Farinha, N.; Figueiredo, A. Funcho (Foeniculum vulgare Mill.). A Planta, Usos Tradicionais e Óleos Essenciais Em Portugal. Voz do Campo. 2021, 252, 75–77. [Google Scholar]
- Lopes, V.R.; Barata, A.M.; Rocha, F.; Bettencourt, E.; Mota, A.S.; Silva, L.; Figueiredo, A.C. Seed Progeny of Portuguese Fennel Wild Populations: Morphological and Essentials Oils Variability. In Proceedings of the 8th CMAPSEEC (8th Conference on Medicinal and Aromatic Plants of Southeast European Countries), Durrës, Albania, 19–22 May 2014; Section II. pp. 265–275. [Google Scholar]
- Search Accessions GRIN-Global, Banco Português de Germoplasma Vegetal. Available online: http://bpgv.iniav.pt/gringlobal/ (accessed on 15 July 2023).
- Póvoa, O.; Lopes, V.; Barata, A.M.; Farinha, N. Monitoring Genetic Erosion of Aromatic and Medicinal Plant Species in Alentejo (South Portugal). Plants 2023, 12, 2588. [Google Scholar] [CrossRef]
- Rocha, F.; Gaspar, C. Medicinal and Aromatic Plants Collecting Missions in Portugal. Arab. J. Med. Aromat. Plants 2017, 3, 19–27. [Google Scholar]
- Msaada, K.; Hosni, K.; Taarit, M.B.; Chahed, T.; Kchouk, M.E.; Marzouk, B. Changes on Essential Oil Composition of Coriander (Coriandrum sativum L.) Fruits during Three Stages of Maturity. Food Chem. 2007, 102, 1131–1134. [Google Scholar] [CrossRef]
- Ravi, R.; Prakash, M.; Bhat, K.K. Aroma Characterization of Coriander (Coriandrum sativum L.) Oil Samples. Eur. Food Res. Technol. 2007, 225, 367–374. [Google Scholar] [CrossRef]
- Shahwar, M.K.; El-Ghorab, A.H.; Anjum, F.M.; Butt, M.S.; Hussain, S.; Nadeem, M. Characterization of Coriander (Coriandrum sativum L.) Seeds and Leaves: Volatile and Non Volatile Extracts. Int. J. Food Prop. 2012, 15, 736–7478. [Google Scholar] [CrossRef]
- Chahal, K.K.; Singh, R.; Kumar, A.; Bhardwaj, U. Chemical Composition and Biological Activity of Coriandrum sativum L.: A Review. Indian J. Nat. Prod. Resour 2017, 8, 193–203. [Google Scholar]
- Orav, A.; Arak, E.; Raal, A. Essential Oil Composition of Coriandrum sativum L. Fruits from Different Countries. J. Essent. Oil Bear. Plants 2011, 14, 118–123. [Google Scholar] [CrossRef]
- Nejad Ebrahimi, S.; Hadian, J.; Ranjbar, H. Essential Oil Compositions of Different Accessions of Coriandrum sativum L. from Iran. Nat. Prod. Res. 2010, 24, 1287–1294. [Google Scholar] [CrossRef]
- Bhuiyan, M.N.I.; Begum, J.; Sultana, M. Chemical Composition of Leaf and Seed Essential Oil of Coriandrum sativum L. from Bangladesh. Bangladesh J. Pharmacol. 2009, 4, 150–153. [Google Scholar] [CrossRef]
- Silva, F.; Ferreira, S.; Duarte, A.; Mendonça, D.I.; Domingues, F.C. Antifungal Activity of Coriandrum sativum Essential Oil, Its Mode of Action against Candida Species and Potential Synergism with Amphotericin B. Phytomedicine 2011, 19, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) Essential Oil: Chemistry and Biological Activity. Asian Pac. J. Trop. Biomed. 2015, 5, 421–428. [Google Scholar] [CrossRef]
- Sahib, N.G.; Anwar, F.; Gilani, A.-H.; Hamid, A.A.; Saari, N.; Alkharfy, K.M. Coriander (Coriandrum sativum L.): A Potential Source of High-Value Components for Functional Foods and Nutraceuticals—A Review. Phytother. Res. 2013, 27, 1439–1456. [Google Scholar] [CrossRef]
- Furletti, V.F.; Teixeira, I.P.; Obando-Pereda, G.; Mardegan, R.C.; Sartoratto, A.; Figueira, G.M.; Duarte, R.M.T.; Rehder, V.L.G.; Duarte, M.C.T.; Höfling, J.F. Action of Coriandrum Sativum L. Essential Oil upon Oral Candida Albicans Biofilm Formation. Evid. Based Complement. Alternat. Med. 2011, 2011, 985832. [Google Scholar] [CrossRef] [PubMed]
- Matasyoh, J.C.; Maiyo, Z.C.; Ngure, R.M.; Chepkorir, R. Chemical Composition and Antimicrobial Activity of the Essential Oil of Coriandrum sativum. Food Chem. 2009, 113, 526–529. [Google Scholar] [CrossRef]
- Telci, I.; Toncer, O.G.; Sahbaz, N. Yield, Essential Oil Content and Composition of Coriandrum sativum Varieties (var. vulgare Alef and var. microcarpum DC.) Grown in Two Different Locations. J. Essent. Oil Res. 2006, 18, 189–193. [Google Scholar]
- Miraldi, E. Comparison of the Essential Oils from Ten Foeniculum vulgare Miller Samples of Fruits of Different Origin. Flavour Fragr. J. 1999, 14, 379–382. [Google Scholar] [CrossRef]
- Cabral, C.; Miranda, M.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Assessment of Safe Bioactive Doses of Foeniculum vulgare Mill. Essential Oil from Portugal. Nat. Prod. Res. 2017, 31, 2654–2659. [Google Scholar] [CrossRef]
- Cavaleiro, C.M.F.; Roque, O.L.; da Cunha, A.P. Contribution for the Characterization of Portuguese Fennel Chemotypes. J. Essent. Oil Res. 1993, 5, 223–225. [Google Scholar] [CrossRef]
- Lopes, V.R.; Barata, A.M.; Farias, R.; Mendes, M.D.; Lima, A.S.; Pedro, L.G.; Barroso, J.G.; Figueiredo, A.C. Morphological and Essential Oil Variability from Nine Portuguese Fennel (Foeniculum vulgare Mill.) Accessions. Acta Hortic. 2010, 860, 33–49. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barbosa, P.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from Essential Oils, Essential Oils Fractions and Decoction Waters. Phytochemistry 2013, 94, 220–228. [Google Scholar] [CrossRef]
- Martins, M.R.; Tinoco, M.T.; Almeida, A.S.; Cruz-Morais, J. Chemical Composition, Antioxidant and Antimicrobial Properties of Three Essential Oils from Portuguese Flora. J. Pharmacogn. 2012, 3, 39–44. [Google Scholar]
- Hassan, O.M.; Elhassan, I.A. Characterization of Essential Oils from Fruits of Umbelliferous Crop Cultivated in Sudan. Coriandrum sativum L. (Coriander) and Foeniculum vulgare Mill (Fennel). J. Pharmacogn. Phytochem. 2017, 6, 113–116. [Google Scholar]
- Olle, M.; Bender, I. The Content of Oils in Umbelliferous Crops and Its Formation. Agron. Res. 2010, 8, 687–696. [Google Scholar]
- Türkmenoğlu, A.; Özmen, D. Allergenic Components, Biocides, and Analysis Techniques of Some Essential Oils Used in Food Products. J. Food Sci. 2021, 86, 2225–2241. [Google Scholar]
- Instituto Português do Mar e da Atmosfera, I.P. Divisão Clima e Alterações Climáticas. Versão 1.0. Boletim. Anual. Resumo. 2021, Portugal. Available online: https://www.ipma.pt/resources.www/docs/im.publicacoes/edicoes.online/20220114/OsDbAwhZGBQbebLJSLoA/cli_20211201_20211231_pcl_aa_co_pt.pdf (accessed on 12 July 2023).
- Farinha, N.; Churra, M.; Paulo, M.; Lopes, E.; Barata, A.; Lopes, V.; Figueiredo, A.C.; Serrano, C.; Póvoa, O. Plant Breeding of Coriandrum Sativum from Landraces Collected in Alentejo (Portugal). Acta Hortic. 2023, 1358, 57–64. [Google Scholar] [CrossRef]
- Council of Europe; European Pharmacopoeia Commission; European Directorate for the Quality of Medicines & Healthcare. European Directorate for the Quality of Medicines, in European Pharmacopoeia, 7th ed.; Council of Europe, European Directorate for the Quality of Medicines and Healthcare: Strasbourg, France, 2010; p. 241. [Google Scholar]
- ISO 7609:1985; Essential Oils-Analysis by Gas Chromatography on Capillary Columns-General Method. ISO: Geneva, Switzerland, 1985.
- Santos, P.M.; Figueiredo, A.C.; Oliveira, M.M.; Barroso, J.G.; Pedro, L.G.; Deans, S.G.; Younus, A.K.M.; Scheffer, J.J.C. Essential Oils from Hairy Root Cultures and from Fruits and Roots of Pimpinella anisum. Phytochemistry 1998, 48, 455–460. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Sim-Sim, M.; Barroso, J.G.; Pedro, L.G.; Santos, P.A.G.; Fontinha, S.S.; Schripsema, J.; Deans, S.G.; Scheffer, J.J.C. Composition of the Essential Oil from the Liverwort Marchesinia mackaii (Hook.) S. F. Gray Grown in Portugal. J. Essent. Oil Res. 2002, 14, 439–442. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pais, M.S.S.; Scheffer, J.J.C. Composition of the Essential Oils from Leaves and Flowers of Achillea millefolium L. ssp. millefolium. Flavour Fragr. J. 1992, 7, 219–222. [Google Scholar] [CrossRef]
- Ascensão, L.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Schripsema, J.; Deans, S.G.; Scheffer, J.J.C. Plectranthus madagascariensis: Morphology of the Glandular Trichomes, Essential Oil Composition, and Its Biological Activity. Int. J. Plant Sci. 1998, 159, 31–38. [Google Scholar] [CrossRef]
- Santos, P.A.G.; Figueiredo, A.C.; Lourenço, P.M.L.; Barroso, J.G.; Pedro, L.G.; Oliveira, M.M.; Schripsema, J.; Deans, S.G.; Scheffer, J.J.C. Hairy Root Cultures of Anethum graveolens (dill): Establishment, Growth, Time-Course Study of Their Essential Oil and Its Comparison with Parent Plant Oils. Biotechnol. Lett. 2002, 24, 1031–1036. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Sena, I.; Moiteiro, C.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Nematotoxic and Phytotoxic Activity of Satureja montana and Ruta graveolens Essential Oils on Pinus pinaster Shoot Cultures and P. pinaster with Bursaphelenchus xylophilus in vitro Co-cultures. Ind. Crops Prod. 2015, 77, 59–65. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Rodrigues, A.M.; Sena, I.; Moiteiro, C.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity of Ruta graveolens and Satureja montana Essential Oils on Solanum tuberosum Hairy Roots and Solanum tuberosum Hairy Roots with Meloidogyne chitwoodi Co-cultures. J. Agric. Food Chem. 2016, 64, 7452–7458. [Google Scholar] [CrossRef]
- Salgueiro, L.R.; Vila, R.; Tomi, F.; Figueiredo, A.C.; Barroso, J.G.; Cañigueral, S.; Casanova, J.; Proença da Cunha, A.; Adzet, T. Variability of Essential Oils of Thymus caespititius from Portugal. Phytochemistry 1997, 45, 307–311. [Google Scholar] [CrossRef]
- Cavaleiro, C.; Salgueiro, L.; Barroso, J.G.; Figueiredo, A.C.; Pedro, L.G.; Fontinha, S.S.; Bighelli, A.; Casanova, J.; Looman, A.; Scheffer, J.J.C. Composition of the Essential Oil of Juniperus cedrus Webb & Berth. Grown on Madeira. Flavour Fragr. J. 2002, 17, 111–114. [Google Scholar]
- Figueiredo, A.C.; Moiteiro, C.; Rodrigues, M.C.S.M.; Almeida, A.J.R.M. Essential Oil Composition from Cryptomeria japonica D.Don Grown in Azores: Biomass Valorization from Forest Management. Nat. Prod. Commun. 2021, 16, 1934578X211038431. [Google Scholar] [CrossRef]
- Rohlf, J.F. NTSYS-pc, Numerical Taxonomy and Multivariate Analysis System. Version 2.1. User Guide; Exeter Publishing Setauket: New York, NY, USA, 2000. [Google Scholar]
- Pestana, M.H.; Gageiro, J.N. Análise De Dados Para Ciências Sociais: A Complementaridade do SPSS.; Edições Sílabo: Lisboa, Portugal, 2000. [Google Scholar]
- DGAV, Direção Geral de Alimentação e Veterinária. Catálogo Nacional de Variedades. Espécies Agrícolas e Hortícolas; Direção de Serviços de Sanidade Vegetal e Direção Geral de Alimentação e Veterinária: Lisboa, Portugal, 2023; p. 53. [Google Scholar]
 coriander fruits,
coriander fruits,  vegetative aerial parts, and
vegetative aerial parts, and  fennel fruits.
fennel fruits.
 coriander fruits,
coriander fruits,  vegetative aerial parts, and
vegetative aerial parts, and  fennel fruits.
fennel fruits.
| Experimental Field | BPGV Accession | Accession Origin Municipality/District | Plant Material | Plantation Date (Month/Year) | Harvest Date (Month/Year) | EO Yield (%, v/w) | Accession Code |
|---|---|---|---|---|---|---|---|
| Coriandrum sativum | |||||||
| ESAE/IPP | BPGV08514 | Elvas, Portalegre | Fruits | 03/21 | 06/21 | <0.05 | Cs21_fr1 |
| ESAE/IPP | BPGV19290 | Alcácer do Sal, Setúbal | Fruits | 03/21 | 06/21 | <0.05 | Cs21_fr2 |
| ESAE/IPP | BPGV28150 | Campo Maior, Portalegre | Fruits | 03/21 | 06/21 | <0.05 | Cs21_fr3 |
| ESAE/IPP | BPGV08514 | Elvas, Portalegre | VAPs | 02/22 | 05/22 | 0.11 | Cs22_lv1 |
| ESAE/IPP | BPGV19290 | Alcácer do Sal, Setúbal | VAPs | 02/22 | 05/22 | 0.09 | Cs22_lv2 |
| ESAE/IPP | BPGV28150 | Campo Maior, Portalegre | VAPs | 02/22 | 05/22 | 0.06 | Cs22_lv3 |
| ESAE/IPP | - | CV | VAPs | 02/22 | 05/22 | 0.07 | ROMA |
| ESAE/IPP | - | CV | VAPs | 02/22 | 05/22 | 0.07 | SANTO |
| ESAE/IPP | BPGV19280 | Amareleja, Beja | VAPs | 02/22 | 05/22 | <0.05 | Cs22_lv4 |
| ESAE/IPP | BPGV19282 | Castro Verde, Beja | VAPs | 02/22 | 05/22 | <0.05 | Cs22_lv5 |
| ESAE/IPP | BPGV19284 | Vidigueira, Beja | VAPs | 02/22 | 05/22 | 0.10 | Cs22_lv6 |
| Foeniculum vulgare | |||||||
| BPGV | BPGV10429 | Avis, Portalegre | Fruits | 04/20 | 10/20 | 3.00 | Fv20_P1 |
| BPGV | BPGV10439 | Sousel, Portalegre | Fruits | 04/20 | 10/20 | 4.00 | Fv20_P2 |
| BPGV | BPGV11263 | Bragança, Bragança | Fruits | 04/20 | 10/20 | 4.50 | Fv20_B |
| BPGV | BPGV12149 | Vila Real, Vila Real | Fruits | 04/20 | 10/20 | 4.00 | Fv20_VR1 |
| BPGV | BPGV12172 | Vila Real, Vila Real | Fruits | 04/20 | 10/20 | 4.50 | Fv20_VR2 |
| BPGV | BPGV12179 | Mesão Frio, Vila Real | Fruits | 04/20 | 10/20 | 4.00 | Fv20_VR3 |
| BPGV | BPGV16265 | Monção, Viana do Castelo | Fruits | 04/20 | 10/20 | 4.00 | Fv20_VC1 |
| BPGV | BPGV16268 | Valença, Viana do Castelo | Fruits | 04/20 | 10/20 | 3.00 | Fv20_VC2 |
| BPGV | BPGV16271 | Viana do Castelo, Viana do Castelo | Fruits | 04/20 | 10/20 | 4.50 | Fv20_VC3 |
| BPGV | BPGV16428 | Fundão, Castelo Branco | Fruits | 04/20 | 10/20 | 4.00 | Fv20_CB |
| BPGV | BPGV12198 | Montalegre, Vila Real | Fruits | 05/21 | 11/21 | 2.79 | Fv21_VR1 |
| BPGV | BPGV12221 | Vila Real, Vila Real | Fruits | 05/21 | 11/21 | 3.67 | Fv21_VR2 |
| BPGV | BPGV12225 | Valpaços, Vila Real | Fruits | 05/21 | 11/21 | 4.69 | Fv21_VR3 |
| BPGV | BPGV12231 | Chaves, Vila Real | Fruits | 05/21 | 11/21 | 3.25 | Fv21_VR4 |
| BPGV | BPGV12233 | Chaves, Vila Real | Fruits | 05/21 | 11/21 | 4.42 | Fv21_VR5 |
| BPGV | BPGV16276 | Ponte da Barca, Viana do Castelo | Fruits | 05/21 | 11/21 | 3.49 | Fv21_VC1 |
| BPGV | BPGV16279 | Paredes de Coura, Viana do Castelo | Fruits | 05/21 | 11/21 | 4.80 | Fv21_VC2 |
| BPGV | BPGV16285 | Ponte da Barca, Viana do Castelo | Fruits | 05/21 | 11/21 | 2.58 | Fv21_VC3 |
| BPGV | BPGV16294 | Ponte de Lima, Viana do Castelo | Fruits | 05/21 | 11/21 | 2.88 | Fv21_VC4 |
| Components | RI | Cluster I | Cluster II | ||
|---|---|---|---|---|---|
| Min | Max | Min | Max | ||
| n-Nonane | 900 | t | t | 0.3 | 3.1 |
| α-Pinene | 930 | 1.7 | 4.3 | t | 0.3 |
| γ-Terpinene | 1035 | 8.1 | 12.0 | t | 0.3 |
| Linalool | 1074 | 59.6 | 72.6 | t | 0.4 |
| n-Decanal | 1180 | 0.7 | 1.9 | 13.2 | 30.2 |
| 2-trans-Decenal | 1236 | 1.4 | 3.3 | 36.7 | 63.3 |
| 2-trans-Undecenal | 1334 | t | 0.1 | 2.6 | 6.2 |
| Geranyl acetate | 1370 | 1.4 | 4.5 | ||
| 2-trans-Dodecenal | 1446 | 0.3 | 1.0 | 6.0 | 11.8 |
| 2-trans-Tetradecenal * | 1643 | 0.1 | 0.2 | 2.5 | 4.1 |
| % Identification | 98.9 | 99.7 | 96.9 | 98.3 | |
| Grouped components | |||||
| Monoterpene hydrocarbons | 16.9 | 22.0 | 0.6 | 1.3 | |
| Oxygen-containing monoterpenes | 66.3 | 76.7 | 0.1 | 0.5 | |
| Sesquiterpene hydrocarbons | t | 0.1 | t | t | |
| Oxygen-containing sesquiterpenes | t | 0.3 | t | 0.1 | |
| Oxygen-containing diterpenes | 0.2 | 0.4 | |||
| Fatty acids | 0.4 | 4.8 | |||
| Fatty acid derivatives | 4.0 | 9.2 | 91.8 | 96.6 | |
| Others | t | t | 0.4 | 3.5 | |
| Components | RI | Cluster I | Cluster II | ||
|---|---|---|---|---|---|
| Min | Max | Min | Max | ||
| α-Pinene | 930 | 0.4 | 2.7 | 0.5 | 1.2 |
| β-Myrcene | 975 | 0.4 | 1.5 | 0.7 | 1.5 |
| 1,8-Cineole | 1005 | 0.3 | 1.6 | 0.3 | 2.0 |
| Limonene | 1009 | 1.3 | 3.5 | 2.2 | 7.1 |
| γ-Terpinene | 1035 | 0.3 | 2.4 | 0.3 | 2.2 |
| Fenchone | 1050 | 15.8 | 29.6 | 13.6 | 34.1 |
| Estragole (=Methyl chavicol) | 1163 | 34.0 | 75.5 | 3.2 | 35.1 |
| trans-Anethole | 1254 | 1.0 | 32.0 | 37.0 | 56.3 |
| % Identification | 99.8 | 99.9 | 99.8 | 99.9 | |
| Grouped components | |||||
| Monoterpene hydrocarbons | 3.5 | 9.4 | 6.6 | 13.2 | |
| Oxygen-containing monoterpenes | 16.7 | 31.3 | 15.1 | 35.3 | |
| Phenylpropanoids | 62.2 | 77.9 | 56.3 | 75.2 | |
| Others | t | t | t | t | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, A.M.; Lopes, V.; Barata, A.M.; Póvoa, O.; Farinha, N.; Figueiredo, A.C. Chemical Variability of the Essential Oils from Two Portuguese Apiaceae: Coriandrum sativum L. and Foeniculum vulgare Mill. Plants 2023, 12, 2749. https://doi.org/10.3390/plants12142749
Machado AM, Lopes V, Barata AM, Póvoa O, Farinha N, Figueiredo AC. Chemical Variability of the Essential Oils from Two Portuguese Apiaceae: Coriandrum sativum L. and Foeniculum vulgare Mill. Plants. 2023; 12(14):2749. https://doi.org/10.3390/plants12142749
Chicago/Turabian StyleMachado, Alexandra M., Violeta Lopes, Ana Maria Barata, Orlanda Póvoa, Noémia Farinha, and Ana Cristina Figueiredo. 2023. "Chemical Variability of the Essential Oils from Two Portuguese Apiaceae: Coriandrum sativum L. and Foeniculum vulgare Mill." Plants 12, no. 14: 2749. https://doi.org/10.3390/plants12142749
APA StyleMachado, A. M., Lopes, V., Barata, A. M., Póvoa, O., Farinha, N., & Figueiredo, A. C. (2023). Chemical Variability of the Essential Oils from Two Portuguese Apiaceae: Coriandrum sativum L. and Foeniculum vulgare Mill. Plants, 12(14), 2749. https://doi.org/10.3390/plants12142749







