Concepts in Alpine Plant Ecology
Abstract
Why Care for Concepts?
1. ‘Small Is Beautiful’—The Life Form Concept
2. Habitat Mosaics That Matter—The Role of Topography and Relief
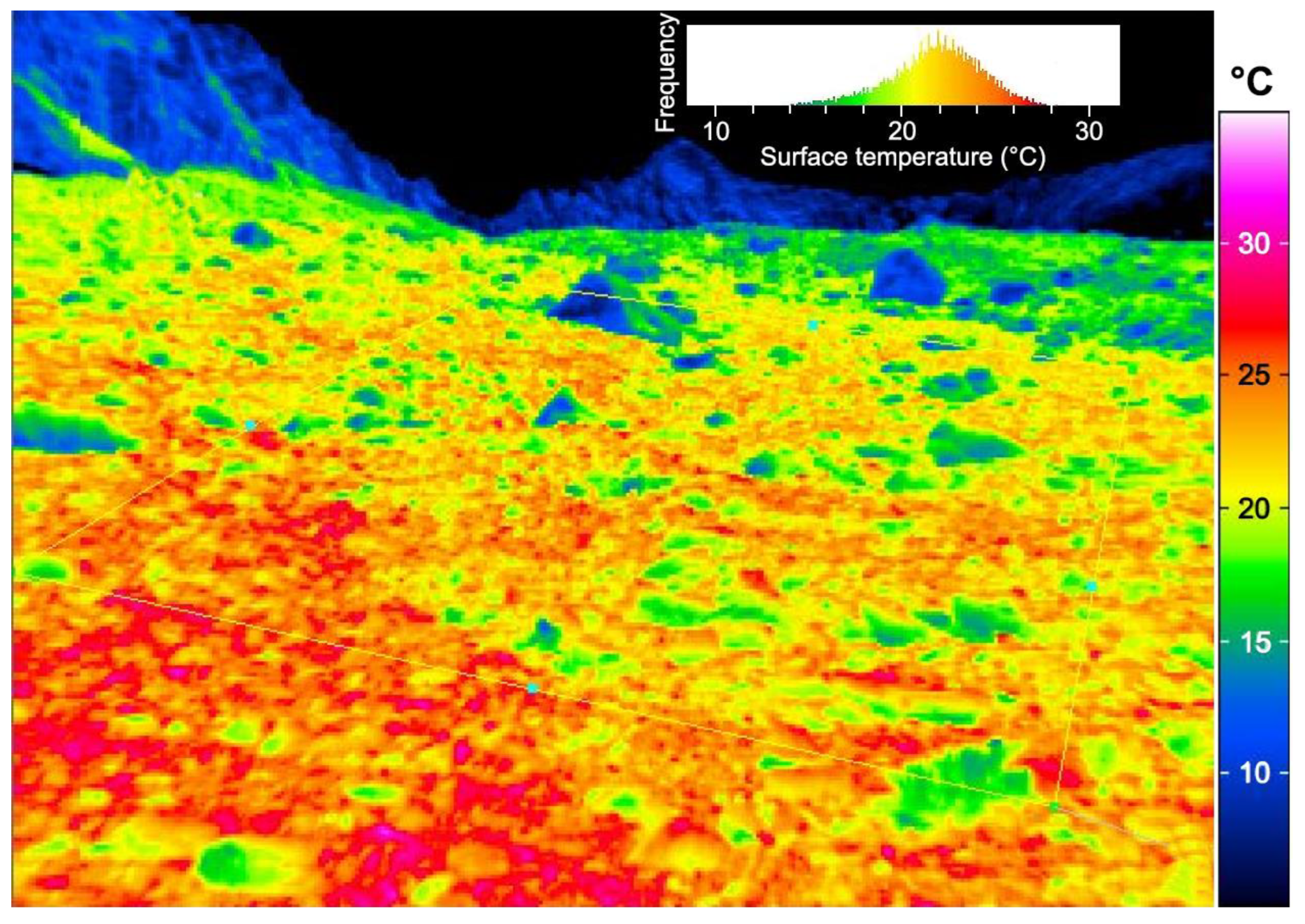
3. A Decoupled World—The Overarching Role of Microclimate
4. From Opportunism to Internal Clock—The Key Role of Developmental Controls
5. Persistence Is More Important Than Vigor—A Life Insurance Principle
6. The Many Solutions to The Same Problem—Plant Functional Diversity
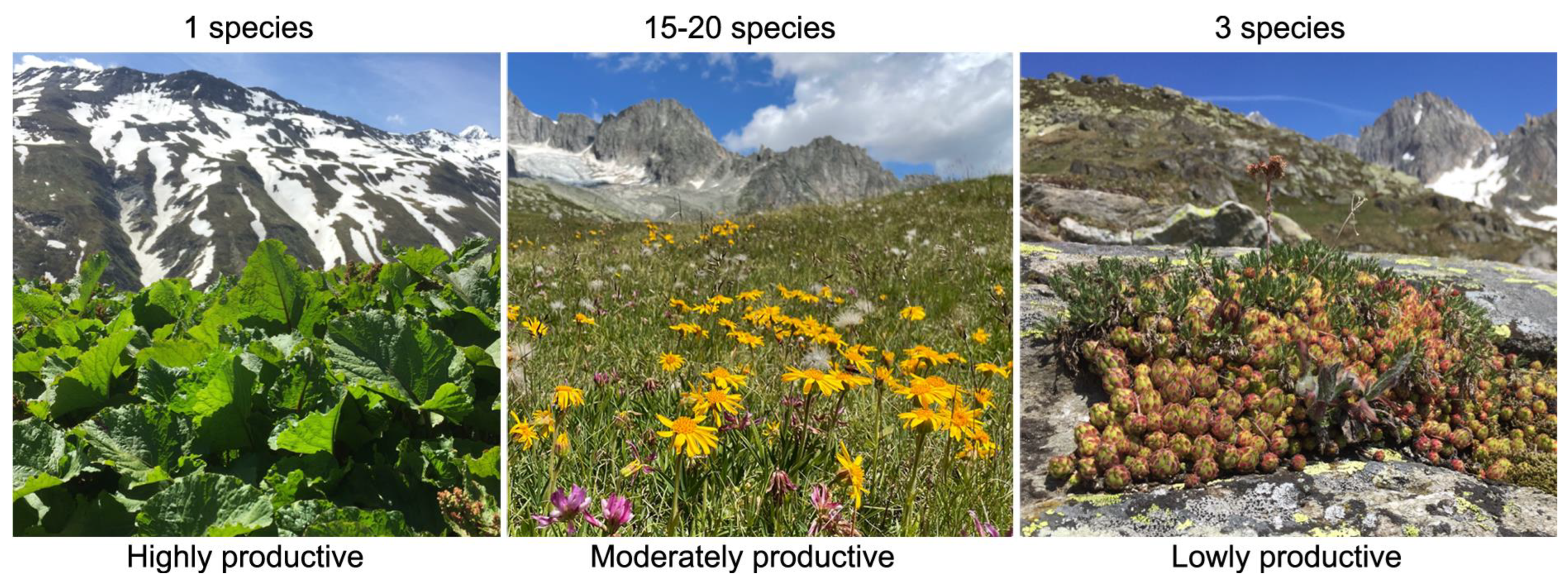
7. The Species Diversity–Productivity Relationship Is Confounded
8. The Concept of Limitation and Stress—Utmost Confusion
9. Alpine Productivity—A Matter of Timing and Ground Cover
10. The Cradle Is the Bottleneck—Alpine Plant Reproduction
11. To Be or Not to Be—The Edge of the Fundamental Niche
12. Environmental Change Is ‘Normal’ in Alpine Life
Concluding Comments
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Körner, C. Tools Shape Paradigms of Plant-Environment Interactions. Prog. Bot. 2021, 82, 1–42. [Google Scholar]
- Körner, C.; Hoch, G. Not every high-latitude or high-elevation forest edge is a treeline. J. Biogeogr. 2023, in press. [Google Scholar] [CrossRef]
- Paulsen, J.; Körner, C. A climate-based model to predict potential treeline position around the globe. Alp. Bot. 2014, 124, 1–12. [Google Scholar] [CrossRef]
- Körner, C.; Paulsen, J.; Spehn, E.M. A definition of mountains and their bioclimatic belts for global comparison of biodiversity data. Alp. Bot. 2011, 121, 73–78. [Google Scholar] [CrossRef]
- Körner, C.; Jetz, W.; Paulsen, J.; Payne, D.; Rudmann-Maurer, K.; Spehn, E.M. A global inventory of mountains for bio-geographical applications. Alp. Bot. 2017, 127, 1–15. [Google Scholar] [CrossRef]
- Körner, C. Concepts in empirical plant ecology. Plant. Ecol. Div. 2018, 11, 405–428. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life, 3rd ed.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Billings, W.D. Arctic and alpine vegetations: Similarities, differences, and susceptibility to disturbance. BioSci 1973, 23, 697–704. [Google Scholar] [CrossRef]
- Körner, C. Alpine plants: Stressed or adapted? In Physiological Plant Ecology; Press, M.C., Scholes, J.D., Barker, M.G., Eds.; Blackwell: Oxford, UK, 1999; pp. 297–311. [Google Scholar]
- Crivellaro, A.; Büntgen, U. New evidence of thermally constrained plant cell wall lignification. Trends Plant. Sci. 2020, 25, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Körner, C.; Riedl, S.; Keplinger, T.; Richter, A.; Wiesenbauer, J.; Schweingruber, F.; Hiltbrunner, E. Life at 0 °C: The biology of the alpine snowbed plant Soldanella pusilla. Alp. Bot. 2019, 129, 63–80. [Google Scholar] [CrossRef]
- Verrall, B.; Green, K.; Pickering, C.M. Temporal dynamics in alpine snowpatch plants along a snowmelt gradient explained by functional traits and strategies. Oecologia 2023, 201, 155–171. [Google Scholar] [CrossRef]
- Billings, W.D.; Bliss, L.C. An alpine snowbank environment and its effects on vegetation, plant development, and productivity. Ecology 1959, 2, 388–397. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Grabherr, G. Vascular plant distribution patterns at the low-temperature limits of plant life—The alpine-nival ecotone of Mount Schrankogel (Tyrol, Austria). Phytocoenologia 1999, 29, 297–325. [Google Scholar] [CrossRef]
- Körner, C.; Hiltbrunner, E. Why is the alpine flora comparatively robust against climatic warming? Diversity 2021, 13, 383. [Google Scholar] [CrossRef]
- Scherrer, D.; Körner, C. Infra-red thermometry of alpine landscapes challenges climatic warming projections. Glob. Chang. Biol. 2009, 16, 2602–2613. [Google Scholar] [CrossRef]
- Billings, W.D. Physiological ecology. Ann. Rev. Plant. Physiol. 1957, 8, 375–391. [Google Scholar] [CrossRef]
- Larcher, W. Bioclimatic temperatures in the high Alps. In Plants in Alpine Regions; Lütz, C., Ed.; Springer Wien: New York, NY, USA, 2012; pp. 21–27. [Google Scholar]
- Oldfather, M.F.; Ackerly, D.D. Microclimate and demography interact to shape stable population dynamics across the range of an alpine plant. New Phytol. 2019, 222, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Gauslaa, Y. Heat resistance and energy budget in different Scandinavian plants. Holarct. Ecol. 1984, 7, 1–78. [Google Scholar] [CrossRef]
- Larcher, W.; Kainmüller, C.; Wagner, J. Survival types of high mountain plants under extreme temperatures. Flora 2010, 205, 3–18. [Google Scholar] [CrossRef]
- Marcante, S.; Erschbamer, B.; Buchner, O.; Neuner, G. Heat tolerance of early developmental stages of glacier foreland species in the growth chamber and in the field. Plant. Ecol. 2014, 215, 747–758. [Google Scholar] [CrossRef]
- Körner, C.; Diemer, M. In situ photosynthetic responses to light, temperature and carbon dioxide in herbaceous plants from low and high altitude. Funct. Ecol. 1987, 1, 179–194. [Google Scholar] [CrossRef]
- Dietrich, L.; Körner, C. Thermal imaging reveals massive heat accumulation in flowers across a broad spectrum of alpine taxa. Alp. Bot. 2014, 124, 27–35. [Google Scholar] [CrossRef]
- Körner, C.; Hiltbrunner, E. The 90 ways to describe plant temperature. Perspect. Plant. Ecol. Evol. Syst. 2018, 30, 16–21. [Google Scholar]
- Klimes, L.; Dolezal, J. An experimental assessment of the upper elevational limit of flowering plants in the western Himalayas. Ecography 2010, 33, 590–596. [Google Scholar] [CrossRef]
- Körner, C. Coldest places on earth with angiosperm plant life. Alp. Bot. 2011, 121, 11–22. [Google Scholar] [CrossRef]
- Monteiro, J.A.F.; Hiltbrunner, E.; Körner, C. Functional morphology and microclimate of Festuca orthophylla, the dominant tall tussock grass in the Andean Altiplanto. Flora 2011, 206, 387–396. [Google Scholar] [CrossRef]
- Cernusca, A. Energie- und Wasserhaushalt eines alpinen Zwergstrauchbestandes während einer Föhnperiode. Arch. Met. Geoph Biokl Ser. B 1976, 24, 219–241. [Google Scholar] [CrossRef]
- Callaway, R.M.; Brooker, R.W.; Choler, P.; Kikvidze, Z.; Lortie, C.J.; Michalet, R.; Paolini, L.; Pugnaire, F.L.; Newingham, B.; Aschehoug, E.T.; et al. Positive interactions among alpine plants increase with stress. Nature 2002, 417, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Cavieres, L.A.; Badano, E.I.; Sierra-Almeida, A.; Gomez-Gonzalez, S.; Molina-Montenegro, M.A. Positive interactions between alpine plant species and the nurse cushion plant Laretia acaulis do not increase with elevation in the Andes of central Chile. New Phytol. 2006, 169, 59–69. [Google Scholar] [CrossRef]
- Möhl, P.; Von Büren, R.S.; Hiltbrunner, E. Growth of alpine grassland will start and stop earlier under climate warming. Nat. Commun. 2022, 13, 7398. [Google Scholar] [CrossRef]
- Steinger, T.; Körner, C.; Schmid, B. Long-term persistence in a changing climate: DNA analysis suggests very old ages of clones of alpine Carex curvula. Oecologia 1996, 105, 94–99. [Google Scholar] [CrossRef]
- De Witte, L.C.; Armbruster, G.F.J.; Gielly, L.; Taberlet, P.; Stöcklin, J.A.F.L.P. Markers reveal high clonal diversity and extreme longevity in four key arctic-alpine species. Mol. Ecol. 2012, 21, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Loehle, C. Height growth rate tradeoffs determine northern and southern range limits for trees. J. Biogeogr. 1998, 25, 735–742. [Google Scholar] [CrossRef]
- Hiltbrunner, E.; Arnaiz, J.; Körner, C. Biomass allocation and seasonal non-structural carbohydrate dynamics do not explain the success of tall forbs in short alpine grassland. Oecologia 2021, 197, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, J.; Weber, U.M.; Körner, C. Tree growth near treeline: Abrupt or gradual reduction with altitude? Arct. Antarct. Alp. Res. 2000, 32, 14–20. [Google Scholar] [CrossRef]
- Vittoz, P.; Randin, C.; Dutoit, A.; Bonnet, F.; Hegg, O. Low impact of climate change on subalpine grasslands in the Swiss Northern Alps. Glob. Chang. Biol. 2009, 15, 209–220. [Google Scholar] [CrossRef]
- Scott, D.; Billings, W.D. Effects of environmental factors on standing crop and productivity of an alpine tundra. Ecol. Monogr. 1964, 34, 243–270. [Google Scholar] [CrossRef]
- Körner, C.; Renhardt, U. Dry matter partitioning and root length/leaf area ratios in herbaceous perennial plants with diverse altitudinal distribution. Oecologia 1987, 74, 411–418. [Google Scholar] [CrossRef]
- Körner, C. Some often overlooked plant characteristics as determinants of plant growth: A reconsideration. Funct. Ecol. 1991, 5, 162–173. [Google Scholar] [CrossRef]
- Patty, L.; Halloy, S.R.P.; Hiltbrunner, E.; Körner, C. Biomass allocation in herbaceous plants under grazing impact in the high semi-arid Andes. Flora 2010, 205, 695–703. [Google Scholar] [CrossRef]
- Yang, Y.; Siegwolf, R.T.W.; Körner, C. Species specific and environment induced variation of delta-13C and delta-15N in alpine plants. Front. Plant. Sci. 2015, 6, 423. [Google Scholar] [CrossRef]
- Körner, C.; Leuzinger, S.; Riedl, S.; Siegwolf, R.T.; Streule, L. Carbon and nitrogen stable isotope signals for an entire alpine flora, based on herbarium samples. Alp. Bot. 2016, 126, 153–166. [Google Scholar] [CrossRef]
- Körner, C.; Neumayer, M.; Pelaez Menendez-Riedl, S. Smeets-Scheel A Functional morphology of mountain plants. Flora 1989, 182, 353–383. [Google Scholar] [CrossRef]
- Kerner, A. Das Pflanzenleben der Donauländer; Wagner: Innsbruck, Austria, 1863. [Google Scholar]
- Kerner, A. Die Abhängigkeit der Pflanzengestalt von Klima und Boden. Festschr 43; Jahresvers dt Naturf Ärzte 29–45; Wagner: Innsbruck, Austria, 1869. [Google Scholar]
- Friedel, H. Schneedeckendauer und Vegetationsverteilungen im Gelände. In: Ökologische Untersuchungen in der subalpinen Stufe zum Zwecke der Hochlagenaufforstung. Mitt. Bundes-Vers. Mariabrunn 1961, 59, 317–369. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie. In Grundzüge der Vegetationskunde, 3rd ed.; Springer: Wien, Austria, 1964. [Google Scholar]
- Onipchenko, V.G.; Semenova, G.V.; van der Maarel, E. Population strategies in severe environments: Alpine plants in the northwestern Caucasus. J. Veg. Sci. 1998, 9, 27–40. [Google Scholar] [CrossRef]
- Von Büren, R.S.; Hiltbrunner, E. Low winter temperatures and divergent freezing resistance set the cold range limit of widespread alpine graminoids. J. Biogeogr. 2022, 49, 1562–1575. [Google Scholar] [CrossRef]
- Körner, C.; Berninger, U.G.; Daim, A.; Eberl, T.; Fernandez Mendoza, F.; Fureder, L.; Grube, M.; Hainzer, E.; Kaiser, R.; Meyer, E.; et al. Long-term monitoring of high-elevation terrestrial and aquatic ecosystems in the Alps—A five-year synthesis. Eco. Mont. 2022, 14, 48–69. [Google Scholar] [CrossRef]
- Körner, C. Limitation or stress—Always or never? J. Veg. Sci. 2003, 14, 141–143. [Google Scholar]
- Alexander, J.M.; Diez, J.M.; Levine, J.M. Novel competitors shape species’ responses to climate change. Nature 2015, 525, 515–518. [Google Scholar] [CrossRef]
- Oehl, F.; Körner, C. Multiple mycorrhization at the coldest place known for Angiosperm plant life. Alp. Bot. 2014, 124, 193–198. [Google Scholar] [CrossRef]
- Spehn, E.M.; Joshi, J.; Schmid, B.; Diemer, M.; Körner, C. Above-ground resource use increases with plant species richness in experimental grassland ecosystems. Funct. Ecol. 2000, 14, 326–337. [Google Scholar]
- Weisser, W.W.; Roscher, C.; Meyer, S.T.; Ebeling, A.; Luo, G.; Allan, E.; Beßler, H.; Barnard, R.L.; Buchmann, N.; Buscot, F.; et al. Biodiversity effects on ecosystem functioning in a 15-year grassland experiment: Patterns, mechanisms, and open questions. Basic. Appl. Ecol. 2017, 23, 1–73. [Google Scholar]
- Kahmen, A.; Perner, J.; Audorff, V.; Weisser, W.; Buchmann, N. Effects of plant diversity, community composition and environmental parameters on productivity in montane European grasslands. Oecologia 2005, 142, 606–615. [Google Scholar] [CrossRef]
- Von Riedmatten, L.; Körner, C.; Hiltbrunner, E.; Hefel, C.; Stöcklin, J. Produktivität und Biodiversität auf alpinen Rasen. Master’s Thesis, University of Basel, Basel, Switzerland, 2007, unpublished. [Google Scholar]
- Wu, J.S.; Shen, Z.X.; Zhang, X.Z. Precipitation and species composition primarily determine the diversity-productivity relationship of alpine grasslands on the Northern Tibetan Plateau. Alp. Bot. 2014, 124, 13–25. [Google Scholar] [CrossRef]
- Caprez, R.; Spehn, E.; Nakhutsrishvili, G.; Körner, C. Drought at erosion edges selects for a ‘hidden’ keystone species. Plant. Ecol. Divers. 2012, 4, 303–311. [Google Scholar] [CrossRef]
- Huck, C.; Körner, C.; Hiltbrunner, E. Plant species dominance shifts across erosion edge-meadow transects in the Swiss Alps. Oecologia 2013, 171, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Kahmen, A.; Perner, J.; Buchmann, N. Diversity-dependent productivity in semi-natural grasslands following climate perturbations. Funct. Ecol. 2005, 19, 594–601. [Google Scholar] [CrossRef]
- Vorkauf, M.; Kahmen, A.; Körner, C.; Hiltbrunner, E. Flowering phenology in alpine grassland strongly responds to shifts in snowmelt but weakly to summer drought. Alp. Bot. 2021, 131, 73–88. [Google Scholar]
- Körner, C.; Möhl, P.; Hiltbrunner, E. Four ways to define growing season. Ecol. Lett. 2023, in press. [Google Scholar] [CrossRef]
- Scholz, K.; Hammerle, A.; Hiltbrunner, E.; Wohlfahrt, G. Analyzing the effects of growing season length on the net ecosystem production of an Aapine Ggassland using model–data fusion. Ecosystems 2017, 21, 982–999. [Google Scholar] [CrossRef]
- Monteiro, J.A.F.; Körner, C. Leaf turnover and herbivory in the tall tussock grass Festuca orthophylla in the Andean Altiplano. Alp. Bot. 2013, 123, 13–20. [Google Scholar] [CrossRef]
- McNaughton, S.J. On plants and herbivores. Am. Nat. 1986, 128, 765–770. [Google Scholar] [CrossRef]
- Briske, D.D. Developmental morphology and physiology of grasses. In Grazing Management. An Ecological Perspective; Heitschmidt, R.K., Stuth, J.W., Eds.; Timber Press: Portland, OR, USA, 1991; pp. 85–108. [Google Scholar]
- Skarpe, C.; Hester, A. Plant traits, browsing and grazing herbivores, and vegetation dynamics. In The Ecology of Browsing and Grazing; Gordon, I.J., Prins, H.H.T., Eds.; Springer: Berlin, Germany, 2008; pp. 217–261. [Google Scholar]
- Frank, D.A.; McNaughton, S.J. Evidence for the promotion of aboveground grassland production by native large herbivore in Yellowstone National Park. Oecologia 1993, 96, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Weppler, T.; Stoll, P.; Stocklin, J. The relative importance of sexual and clonal reproduction for population growth in the long-lived alpine plant Geum reptans. J. Ecol. 2006, 94, 869–879. [Google Scholar] [CrossRef]
- Wagner, J.; Lechleitner, M.; Hosp, D. Pollen limitation is not the rule in nival plants: A study from the European Central Alps. Am. J. Bot. 2016, 103, 375–387. [Google Scholar] [CrossRef]
- Steinacher, G.; Wagner, J. The progamic phase in high-mountain plants: From pollination to fertilization in the cold. Plants 2013, 2, 354–370. [Google Scholar] [CrossRef]
- Bliss, L.C. Arctic and alpine plant life cycles. Ann. Rev. Ecol. Syst. 1971, 2, 405–438. [Google Scholar] [CrossRef]
- Billings, D.W. Arctic and alpine vegetation: Plant adaptations to cold summer climates. In Arctic and Alpine Environments; Ives, J.D., Barry, R.G., Eds.; Routledge: Methuen, MA, USA, 1974; pp. 403–443. [Google Scholar]
- Urbanska, C.M.; Schutz, M. Reproduction by seed in alpine plants and revegetation research above timberline. Bot. Helv. 1986, 96, 43–61. [Google Scholar]
- Diemer, M. Population dynamics and spatial arrangement of Ranunculus glacialis L., an alpine perennial herb, in permanent plots. Vegetatio 1992, 103, 159–166. [Google Scholar] [CrossRef]
- Stöcklin, J.; Bäumler, E. Seed rain, seedling establishment and clonal growth strategies on a glacier foreland. J. Veg. Sci. 1996, 7, 45–56. [Google Scholar] [CrossRef]
- Noroozi, J.; Pauli, H.; Grabherr, G.; Breckle, S.W. The subnival-nival vascular plant species of Iran: A unique high-mountain flora and its threat from climate warming. Biodivers. Conserv. 2011, 20, 1319–1338. [Google Scholar] [CrossRef]
- Winkler, M.; Lamprecht, A.; Steinbauer, K.; Hülber, K.; Theurillat, J.P.; Breiner, F.; Choler, P.; Ertl, S.; Gutiérrez Girón, A.; Rossi, G.; et al. The rich sides of mountain summits—A pan-European view on aspect preferences of alpine plants. J. Biogeogr. 2016, 43, 2261–2273. [Google Scholar] [CrossRef]
- Bürli, S.; Theurillat, J.P.; Winkler, M.; Lamprecht, A.; Pauli, H.; Rixen, C.; Steinbauer, K.; Wipf, S.; Abdaladze, O.; Andrews, C.; et al. A common soil temperature threshold for the upper limit of alpine grasslands in European mountains. Alp. Bot. 2021, 131, 41–52. [Google Scholar] [CrossRef]
- Körner, C.; Basler, D.; Hoch, G.; Kollas, C.; Lenz, A.; Randin, C.F.; Vitasse, Y.; Zimmermann, N.E. Where, why and how? Explaining the low-temperature range limits of temperate tree species. J. Ecol. 2016, 104, 1076–1088. [Google Scholar] [CrossRef]
- Vetaas, O.R. Realized and potential climate niches: A comparison of four Rhododendron tree species. J. Biogeogr. 2002, 29, 545–554. [Google Scholar] [CrossRef]
- Randin, C.F.; Dirnbock, T.; Dullinger, S.; Zimmermann, N.E.; Zappa, M.; Guisan, A. Are niche-based species distribution models transferable in space? J. Biogeogr. 2006, 33, 1689–1703. [Google Scholar] [CrossRef]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Daehler, C.; Kueffer, C. Unifying niche shift studies: Insights from biological invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef]
- Scherrer, D.; Schmid, S.; Körner, C. Elevational species shifts in a warmer climate are overestimated when based on weather station data. Int. J. Biometeorol. 2011, 55, 645–654. [Google Scholar] [CrossRef]
- Randin, C.F.; Engler, R.; Normand, S.; Zappa, M.; Zimmermann, N.E.; Pearman, P.B.; Vittoz, P.; Thuiller, W.; Guisan, A. Climate change and plant distribution: Local models predict high-elevation persistence. Glob. Chang. Biol. 2009, 15, 1557–1569. [Google Scholar] [CrossRef]
- Patsiou, T.S.; Conti, E.; Zimmermann, N.E.; Theodoridis, S.; Randin, C.F. Topo-climatic microrefugia explain the persistence of a rare endemic plant in the Alps during the last 21 millennia. Glob. Change Biol. 2014, 20, 2286–2300. [Google Scholar] [CrossRef]
- Körner, C.; Ohsawa, M. Mountain Systems. In Ecosystems and Human Well-Being: Current State and Trends; Hassan, R., Scholes, R., Ash, N., Eds.; Island Press: Washington, DC, USA, 2005; Volume 1, pp. 681–716. [Google Scholar]
- Körner, C. The alpine life zone under global change. Gayana Bot. 2000, 57, 1–17. [Google Scholar] [CrossRef]
- Verrall, B.; Pickering, C.M. Alpine vegetation in the context of climate change: A global review of past research and future directions. Sci. Total. Environ 2020, 748, 141344. [Google Scholar] [CrossRef]
- Körner, C.; Diemer, M. Evidence that plants from high altitudes retain their greater photosynthetic efficiency under elevated CO2. Funct. Ecol. 1994, 8, 58–68. [Google Scholar] [CrossRef]
- Körner, C.; Diemer, M.; Schäppi, B.; Niklaus, P.; Arnone, J. The responses of alpine grassland to four seasons of CO2 enrichment: A synthesis. Acta Oecologica 1997, 18, 165–175. [Google Scholar] [CrossRef]
- Inauen, N.; Körner, C.; Hiltbrunner, E. No growth stimulation by CO2 enrichment in alpine glacier forefield plants. Glob. Change Biol. 2012, 18, 985–999. [Google Scholar] [CrossRef]
- Möhl, P.; Hiltbrunner, E.; Körner, C. Halving sunlight reveals no carbon limitation of aboveground biomass production in alpine grassland. Glob. Change Biol. 2020, 26, 1857–1872. [Google Scholar] [CrossRef] [PubMed]
- Onipchenko, V.G.; Blinnikov, M.S.; Aksenova, A.A. Experimental evaluation of shading effects in seasonal dynamics of four alpine communities in northwestern Caucasus, Russia. Arct. Antarct. Alp. Res. 2001, 33, 330–339. [Google Scholar] [CrossRef]
- Nagelmüller, S.; Hiltbrunner, E.; Körner, C. Critically low soil temperatures for root growth and root morphology in three alpine plant species. Alp. Bot. 2016, 126, 11–21. [Google Scholar]
- Nagelmüller, S.; Hiltbrunner, E.; Körner, C. Low temperature limits for root growth in alpine species are set by cell differentiation. AoB Plants 2017, 2, plx054. [Google Scholar] [CrossRef]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Fernández Calzado, M.A.R.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Change 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Matteodo, M.; Wipf, S.; Stöckli, V.; Rixen, C.; Vittoz, P. Elevation gradient of successful plant traits for colonizing alpine summits under climate change. Environ. Res. Lett. 2013, 8, 024043. [Google Scholar] [CrossRef]
- Kosonen, Z.; Schnyder, E.; Hiltbrunner, E.; Thimonier, A.; Schmitt, M.; Seitler, E.; Thöni, L. Current atmospheric nitrogen deposition still exceeds critical loads for sensitive, semi-natural ecosystems in Switzerland. Atmos. Environ. 2019, 211, 214–225. [Google Scholar] [CrossRef]
- Roth, T.; Kohli, L.; Rihm, B.; Achermann, B. Nitrogen deposition is negatively related to species richness and species composition of vascular plants and bryophytes in Swiss mountain grassland. Agric. Ecosyst. Environ. 2013, 178, 121–126. [Google Scholar] [CrossRef]
- Dobzhansky, T. Nothing in biology makes sense except in the light of evolution. Am. Biol. Teach. 1973, 35, 125–129. [Google Scholar] [CrossRef]
- Diekmann, M. Species indicator values as an important tool in applied plant ecology—A review. Basic. Appl. Ecol. 2003, 4, 493–506. [Google Scholar] [CrossRef]
- Landolt, E.; Bäumler, B.; Erhardt, A.; Hegg, O.; Klötzli, F.; Lämmler, W.; Nobis, M.; Rudmann-Maurer, K.; Schweingruber, F.H.; Theurillat, J.-P.; et al. Flora Indicative; Haupt: Bern, Switzerland, 2010. [Google Scholar]
- Bartelheimer, M.; Poschlod, P. Ellenberg-type indicator values for European vascular plant species. Funct. Ecol. 2016, 30, 506–516. [Google Scholar] [CrossRef]
- Nakhutsrishvili, G.; Batsatsashvili, K.; Rudmann-Maurer, K.; Körner, C.; Spehn, E. New indicator values for Central Caucasus flora. In Plant Diversity in the Central Great Caucasus: A Quantitative Assessment; Nakhutsrishvili, G., Abdaladze, O., Batsatsashvili, K., Spehn, E., Körner, C., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 145–170. [Google Scholar]
- Rumpf, S.B.; Hülber, K.; Klonner, G.; Moser, D.; Schütz, M.; Wessely, J.; Willner, W.; Zimmermann, N.E.; Dullinger, S. Range dynamics of mountain plants decrease with elevation. Proc. Natl. Acad. Sci. USA 2018, 115, 18481853. [Google Scholar] [CrossRef]
- Dalle Fratte, M.; Brusa, G.; Pierce, S.; Zanzottera, M.; Cerabolini, B.E.L. Plant trait variation along environmental indicators to infer global change impacts. Flora 2019, 254, 113–121. [Google Scholar] [CrossRef]
- Birks, H.J.B. Reflections on the Use of Ecological Attributes and Traits in Quaternary Botany. Front. Ecol. Evol. 2020, 40, 166. [Google Scholar] [CrossRef]
- Tichý, L.; Axmanová, I.; Dengler, J.; Guarino, R.; Jansen, F.; Midolo, G.; Nobis, M.P.; Van Meerbeek, K.; Aćić, S.; Attorre, F.; et al. Ellenberg-type indicator values for European vascular plant species. J. Veg. Sci. 2023, 34, e13168. [Google Scholar] [CrossRef]
- Stöcklin, J.; Armbruster, G.F.J. Environmental filtering, not local adaptation of established plants, determines the occurrence of seed- and bulbil-producing Poa alpina in a local flora. Basic. Appl. Ecol. 2016, 17, 586–595. [Google Scholar] [CrossRef]
- Stöcklin, J.; Kuss, P.; Pluess, A.R. Genetic diversity, phenotypic variation and local adaption in the alpine landscape: Case studies with alpine plant species. Bot. Helv. 2009, 119, 125–133. [Google Scholar] [CrossRef]
- Hautier, Y.; Randin, C.F.; Stöcklin, J.; Guisan, A. Changes in reproductive investment with altitude in an alpine plant. J. Plant. Ecol. 2009, 2, 125–134. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Körner, C. Concepts in Alpine Plant Ecology. Plants 2023, 12, 2666. https://doi.org/10.3390/plants12142666
Körner C. Concepts in Alpine Plant Ecology. Plants. 2023; 12(14):2666. https://doi.org/10.3390/plants12142666
Chicago/Turabian StyleKörner, Christian. 2023. "Concepts in Alpine Plant Ecology" Plants 12, no. 14: 2666. https://doi.org/10.3390/plants12142666
APA StyleKörner, C. (2023). Concepts in Alpine Plant Ecology. Plants, 12(14), 2666. https://doi.org/10.3390/plants12142666





