The Comparative Root System Architecture of Declining and Non-Declining Trees in Two Apple Orchards in New York
Abstract
1. Introduction
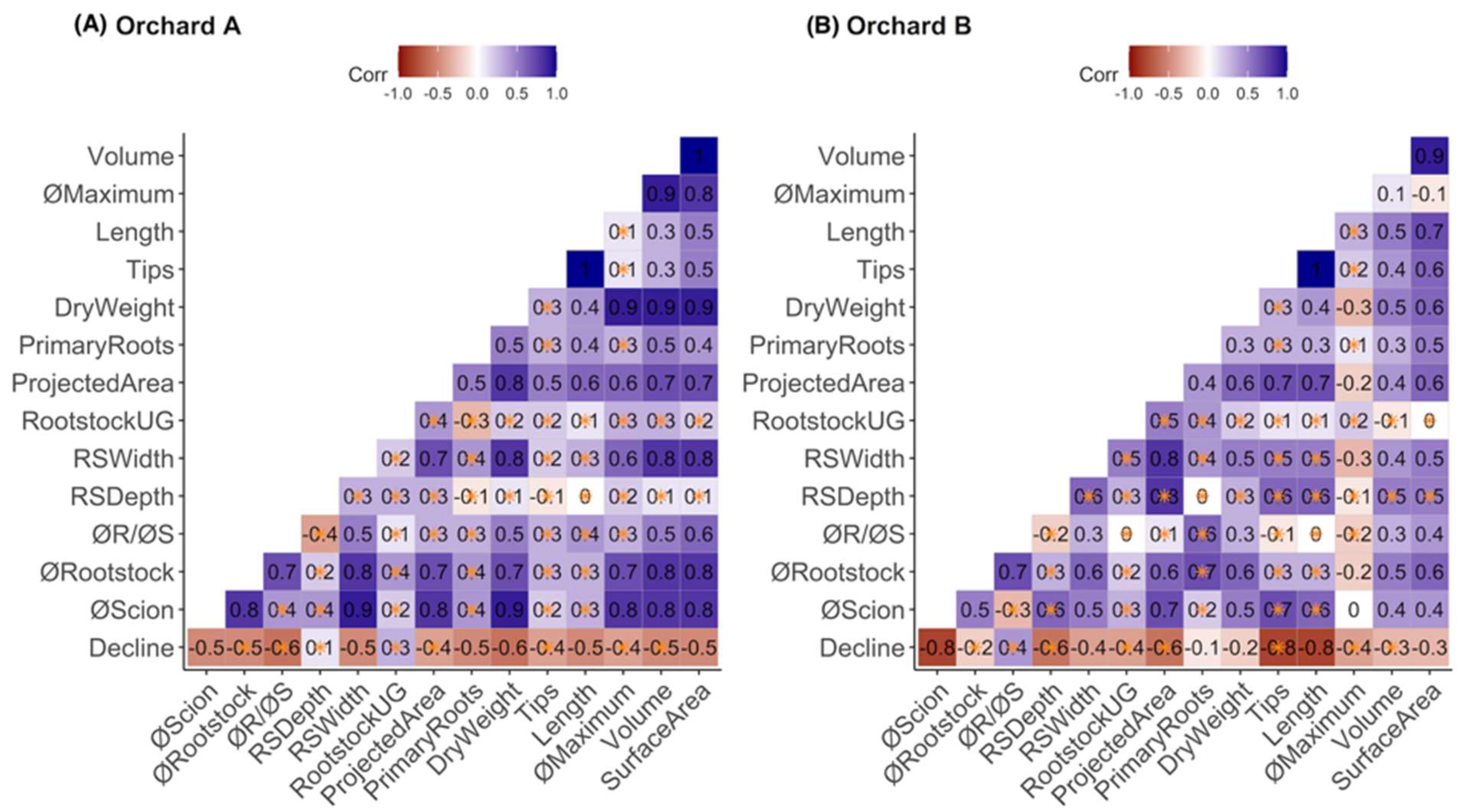
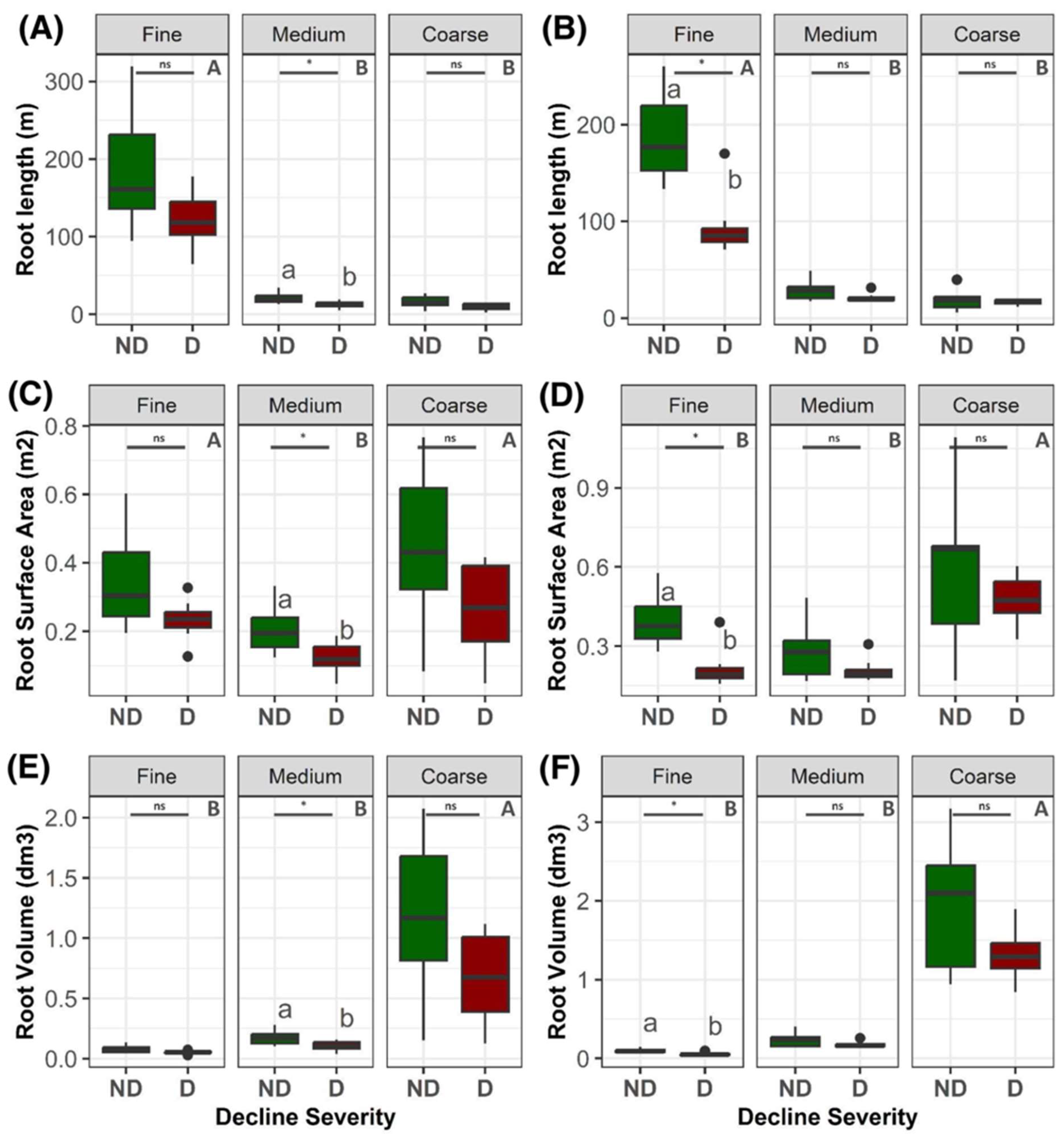
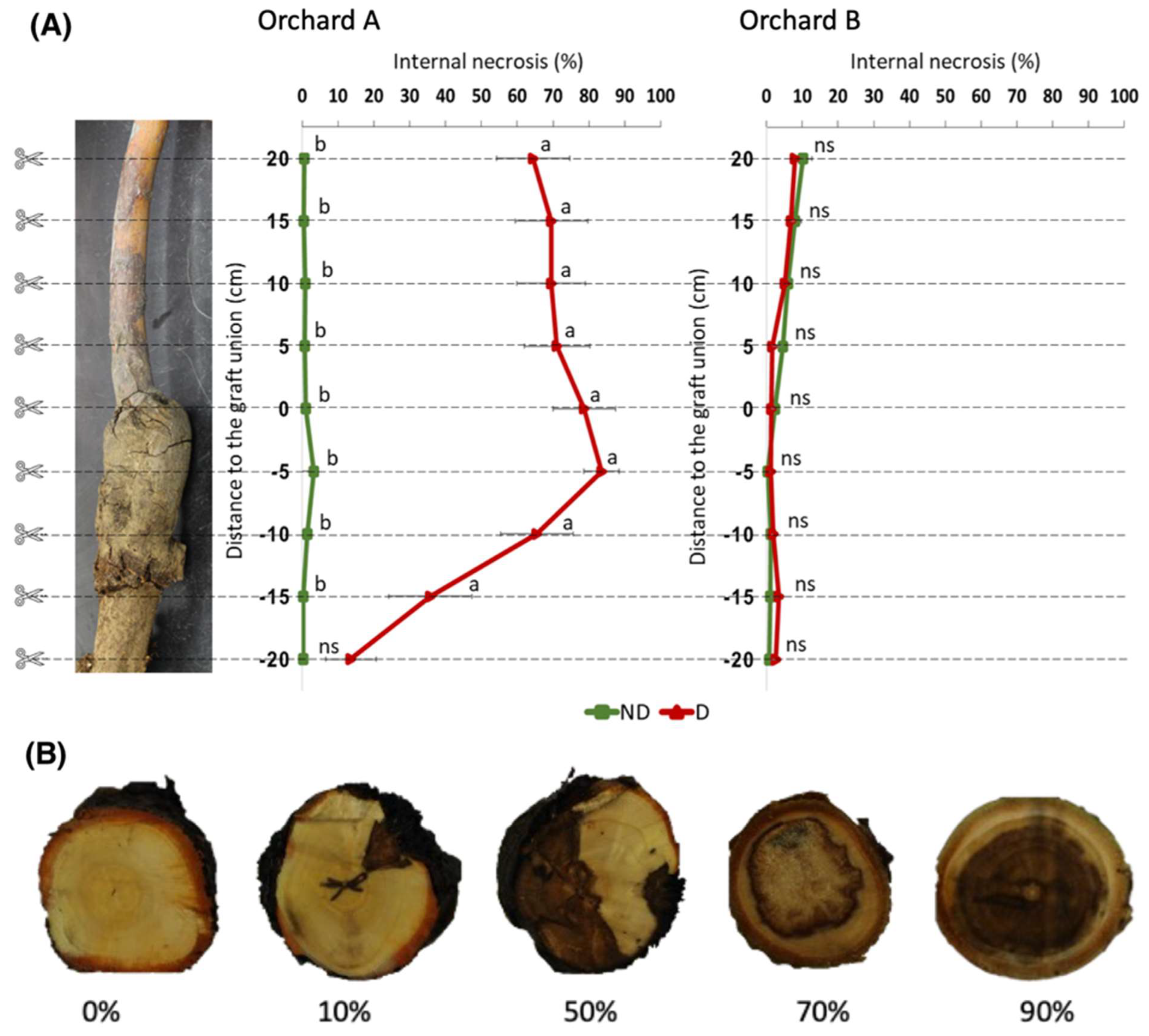
2. Results
3. Discussion
4. Materials and Methods
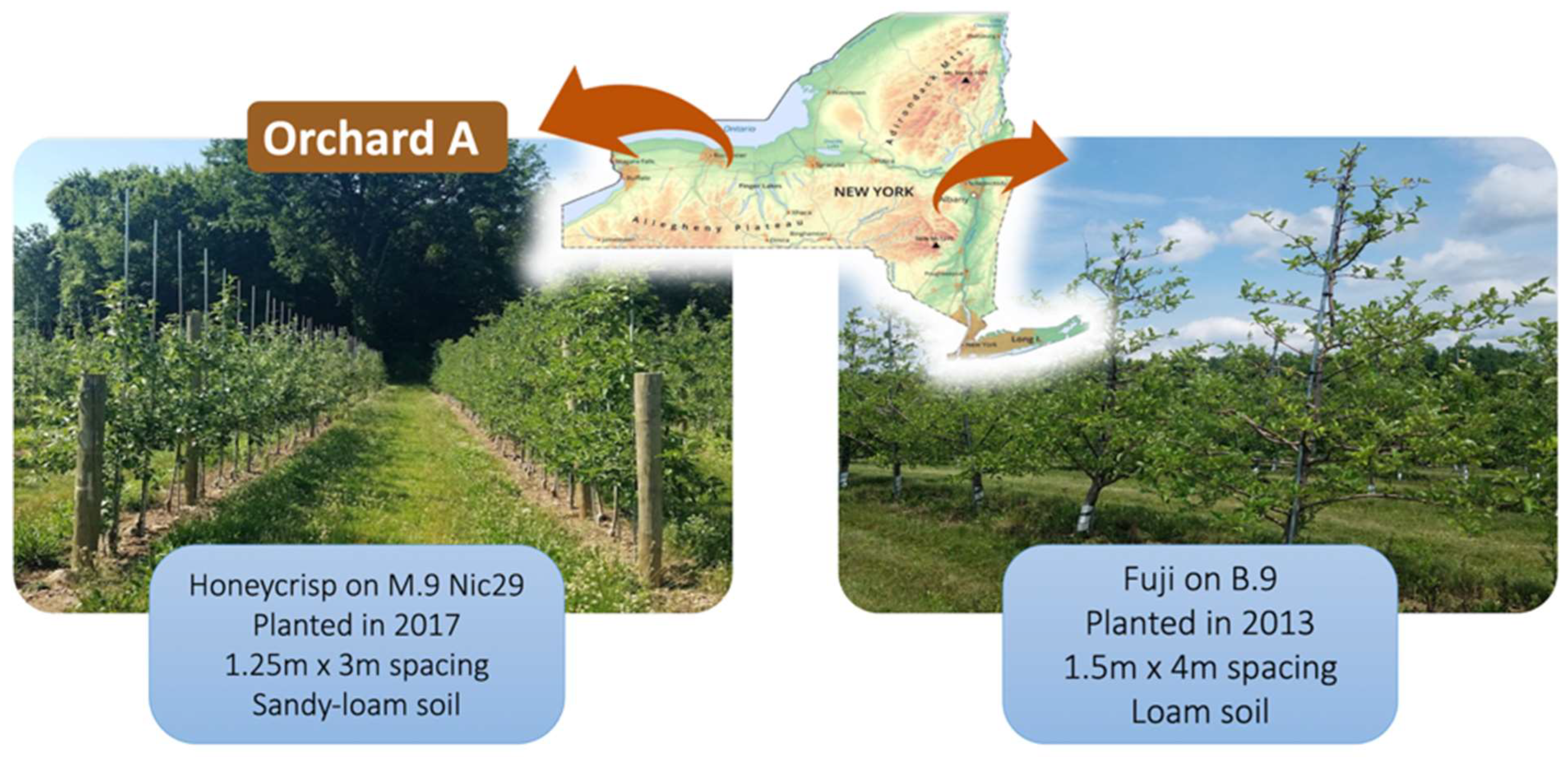
4.1. Study Apple Orchards
4.2. Assessment of Apple Tree Decline Severity
4.3. Apple Tree Root System Sampling
4.4. Processing and Evaluation of RSA and Root Traits
4.5. Apple Orchard Soil Sampling
4.6. Virus Detection
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Food and Agriculture Organization of the United Nations. Agricultural Production Statistics (2000–2021); FAO: Rome, Italy, 2021. [Google Scholar]
- Zhou, Z.; Zhang, L.; Shu, J.; Wang, M.; Li, H.; Shu, H.; Wang, X.; Sun, Q.; Zhang, S. Root Breeding in the Post-Genomics Era: From Concept to Practice in Apple. Plants 2022, 11, 1408. [Google Scholar] [CrossRef]
- Liang, B.; Shi, Y.; Yin, B.; Zhou, S.; Li, Z.; Zhang, X.; Xu, J. Effect of Different Dwarfing Interstocks on the Vegetative Growth and Nitrogen Utilization Efficiency of Apple Trees under Low-Nitrate and Drought Stress. Sci. Hortic. 2022, 305, 111369. [Google Scholar] [CrossRef]
- Fazio, G. Genetics, Breeding, and Genomics of Apple Rootstocks. In The Apple Genome; Korban, S.S., Ed.; Compendium of Plant Genomes; Springer International Publishing: Cham, Switzerland, 2021; pp. 105–130. ISBN 978-3-030-74682-7. [Google Scholar]
- Singh, J.; Fabrizio, J.; Desnoues, E.; Silva, J.P.; Busch, W.; Khan, A. Root System Traits Impact Early Fire Blight Susceptibility in Apple (Malus × Domestica). BMC Plant Biol. 2019, 19, 579. [Google Scholar] [CrossRef]
- Lordan, J.; Fazio, G.; Francescatto, P.; Robinson, T. Effects of Apple (Malus×domestica) Rootstocks on Scion Performance and Hormone Concentration. Sci. Hortic. 2017, 225, 96–105. [Google Scholar] [CrossRef]
- Gregory, P.J.; Atkinson, C.J.; Bengough, A.G.; Else, M.A.; Fernández-Fernández, F.; Harrison, R.J.; Schmidt, S. Contributions of Roots and Rootstocks to Sustainable, Intensified Crop Production. J. Exp. Bot. 2013, 64, 1209–1222. [Google Scholar] [CrossRef]
- Adams, S.; Lordan, J.; Fazio, G.; Bugbee, B.; Francescatto, P.; Robinson, T.L.; Black, B. Effect of Scion and Graft Type on Transpiration, Hydraulic Resistance and Xylem Hormone Profile of Apples Grafted on Geneva (R) 41 and M.9-NIC (TM) 29 Rootstocks. Sci. Hortic. 2018, 227, 213–222. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Else, M.A.; Taylor, L.; Dover, C.J. Root and Stem Hydraulic Conductivity as Determinants of Growth Potential in Grafted Trees of Apple (Malus pumila Mill.). J. Exp. Bot. 2003, 54, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Tworkoski, T.; Fazio, G. Effects of Size-Controlling Apple Rootstocks on Growth, Abscisic Acid, and Hydraulic Conductivity of Scion of Different Vigor. Int. J. Fruit Sci. 2015, 15, 369–381. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, M.; Yao, J.; Zhou, Y.; Wang, Y.; Zhang, X.; Li, W.; Wu, T.; Han, Z.; Xu, X.; et al. Root Architecture Characteristics of Differing Size-Controlling Rootstocks and the Influence on the Growth of ‘Red Fuji’ Apple Trees. Sci. Hortic. 2021, 281, 109959. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, X.; Yao, J.; Zhang, Z.; Wang, Y.; Zhang, X.; Li, W.; Wu, T.; Han, Z.; Xu, X.; et al. Morphological and Photosynthetic Responses Differ among Eight Apple Scion-Rootstock Combinations. Sci. Hortic. 2020, 261, 108981. [Google Scholar] [CrossRef]
- Valverdi, N.A.; Cheng, L.; Kalcsits, L. Apple Scion and Rootstock Contribute to Nutrient Uptake and Partitioning under Different Belowground Environments. Agronomy 2019, 9, 415. [Google Scholar] [CrossRef]
- Polverigiani, S.; Franzina, M.; Neri, D. Effect of Soil Condition on Apple Root Development and Plant Resilience in Intensive Orchards. Appl. Soil Ecol. 2018, 123, 787–792. [Google Scholar] [CrossRef]
- Sokalska, D.I.; Haman, D.Z.; Szewczuk, A.; Sobota, J.; Dereń, D. Spatial Root Distribution of Mature Apple Trees under Drip Irrigation System. Agric. Water Manag. 2009, 96, 917–924. [Google Scholar] [CrossRef]
- Harper, S.J. Apple Decline [WSU Tree Fruit|Washington State University]. 2020. Available online: https://treefruit.wsu.edu/article/apple-decline/ (accessed on 10 January 2022).
- Liu, H.; Wu, L.; Nikolaeva, E.; Peter, K.; Liu, Z.; Mollov, D.; Cao, M.; Li, R. Characterization of a New Apple Luteovirus Identified by High-Throughput Sequencing. Virol. J. 2018, 15, 85. [Google Scholar] [CrossRef]
- Peter, K.A. Apple Disease—Rapid Apple Decline. 2020. Available online: https://extension.psu.edu/apple-disease-rapid-apple-decline (accessed on 10 January 2022).
- Prengaman, K. What’s Killing These Trees? Good Fruit Grower. 2017. Available online: https://www.goodfruit.com/whats-killing-these-trees/ (accessed on 10 January 2022).
- Singh, J.; Silva, K.J.P.; Fuchs, M.; Khan, A. Potential Role of Weather, Soil and Plant Microbial Communities in Rapid Decline of Apple Trees. PLoS ONE 2019, 14, e0213293. [Google Scholar] [CrossRef]
- Xu, H.; Hannam, K.D.; MacDonald, J.L.; Ediger, D. Field Investigation into Tree Fates from Recent Apple Tree Decline: Abrupt Hydraulic Failure versus Gradual Hydraulic Loss. Stresses 2023, 3, 256–269. [Google Scholar] [CrossRef]
- Wunsch, A.; van Zoeren, J.; Miranda Sazo, M.; Basedow, M.; Khan, A.; Fuchs, M. Decline of Young Trees in High-Density Orchards: A Virologist’s Perspective. N. Y. Fruit Q. 2021, 29, 27–30. [Google Scholar]
- Grigg-McGuffin, K.; Celetti, M. Apple Tree Collapse: What We Know (and Don’t Know). Available online: http://omafra.gov.on.ca/english/crops/hort/news/orchnews/2016/on-1216a6.htm (accessed on 10 January 2022).
- Peter, K.A. Apple Disease—Rapid Apple Decline (RAD) or Sudden Apple Decline (SAD)? 2017. Available online: https://extension.psu.edu/apple-disease-rapid-apple-decline-rad-or-sudden-apple-decline-sad (accessed on 10 February 2022).
- Wright, A.A.; Cross, A.R.; Harper, S.J. A Bushel of Viruses: Identification of Seventeen Novel Putative Viruses by RNA-Seq in Six Apple Trees. PLoS ONE 2020, 15, e0227669. [Google Scholar] [CrossRef]
- Xiao, H.; Hao, W.; Storoschuk, G.; MacDonald, J.L.; Sanfaçon, H. Characterizing the Virome of Apple Orchards Affected by Rapid Decline in the Okanagan and Similkameen Valleys of British Columbia (Canada). Pathogens 2022, 11, 1231. [Google Scholar] [CrossRef]
- Barnard, R.L.; Osborne, C.A.; Firestone, M.K. Responses of Soil Bacterial and Fungal Communities to Extreme Desiccation and Rewetting. ISME J. 2013, 7, 2229–2241. [Google Scholar] [CrossRef]
- Jensen, P.J.; Rytter, J.; Detwiler, E.A.; Travis, J.W.; McNellis, T.W. Rootstock Effects on Gene Expression Patterns in Apple Tree Scions. Plant Mol. Biol. 2003, 53, 493–511. [Google Scholar] [CrossRef]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, Domestication, and Impacts on Shoot Phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef]
- de Dorlodot, S.; Forster, B.; Pagès, L.; Price, A.; Tuberosa, R.; Draye, X. Root System Architecture: Opportunities and Constraints for Genetic Improvement of Crops. Trends Plant Sci. 2007, 12, 474–481. [Google Scholar] [CrossRef]
- Khan, M.A.; Gemenet, D.C.; Villordon, A. Root System Architecture and Abiotic Stress Tolerance: Current Knowledge in Root and Tuber Crops. Front. Plant Sci. 2016, 7, 1584. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Inukai, Y.; Yamauchi, A. Root Development and Nutrient Uptake. Crit. Rev. Plant Sci. 2006, 25, 279–301. [Google Scholar] [CrossRef]
- Rogers, E.D.; Benfey, P.N. Regulation of Plant Root System Architecture: Implications for Crop Advancement. Curr. Opin. Biotechnol. 2015, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hou, C.W.; Zhang, X.Z.; Li, H.L.; Han, D.G.; Wang, Y.; Han, Z.H. Seasonal Growth and Spatial Distribution of Apple Tree Roots on Different Rootstocks or Interstems. J. Am. Soc. Hortic. Sci. 2013, 138, 79–87. [Google Scholar] [CrossRef]
- Lo Bianco, R.; Policarpo, M.; Scariano, L. Effects of Rootstock Vigour and In-Row Spacing on Stem and Root Growth, Conformation and Dry-Matter Distribution of Young Apple Trees. J. Hortic. Sci. Biotechnol. 2003, 78, 828–836. [Google Scholar] [CrossRef]
- Errea, P. Implications of Phenolic Compounds in Graft Incompatibility in Fruit Tree Species. Sci. Hortic. 1998, 74, 195–205. [Google Scholar] [CrossRef]
- Tedesco, S.; Pina, A.; Fevereiro, P.; Kragler, F. A Phenotypic Search on Graft Compatibility in Grapevine. Agronomy 2020, 10, 706. [Google Scholar] [CrossRef]
- Zarrouk, O.; Testillano, P.S.; Risueño, M.C.; Moreno, M.Á.; Gogorcena, Y. Changes in Cell/Tissue Organization and Peroxidase Activity as Markers for Early Detection of Graft Incompatibility in Peach/Plum Combinations. J. Am. Soc. Hortic. Sci. 2010, 135, 9–17. [Google Scholar] [CrossRef]
- Mng’omba, S.A.; du Toit, E.S.; Akinnifesi, F.K. The Relationship between Graft Incompatibility and Phenols in Uapaca Kirkiana Müell Arg. Sci. Hortic. 2008, 117, 212–218. [Google Scholar] [CrossRef]
- Chen, J.; Tang, H.-H.; Li, L.; Qin, S.-J.; Wang, G.-P.; Hong, N. Effects of Virus Infection on Plant Growth, Root Development and Phytohormone Levels in in Vitro-Cultured Pear Plants. Plant Cell Tissue Organ Cult. 2017, 131, 359–368. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Seethepalli, A.; Dhakal, K.; Griffiths, M.; Guo, H.; Freschet, G.T.; York, L.M. RhizoVision Explorer: Open-Source Software for Root Image Analysis and Measurement Standardization. AoB Plants 2021, 13, plab056. [Google Scholar] [CrossRef]
- Menzel, W.; Jelkmann, W.; Maiss, E. Detection of Four Apple Viruses by Multiplex RT-PCR Assays with Coamplification of Plant MRNA as Internal Control. J. Virol. Methods 2002, 99, 81–92. [Google Scholar] [CrossRef]
- de Mendiburu, F.; Simon, R. Agricolae—Ten Years of an Open Source Statistical Tool for Experiments in Breeding, Agriculture and Biology. PeerJ PrePrints 2015, 3, e1404v1. [Google Scholar] [CrossRef]
- Kassambara, A. Practical Guide to Principal Component Methods in R: PCA, M (CA), FAMD, MFA, HCPC, Factoextra. Volume 2. Sthda. 2016. Available online: http://www.sthda.com/english/rpkgs/factoextra~BugReports (accessed on 10 January 2021).


| RSA Parameters a | Orchard A | Orchard B | ||||
|---|---|---|---|---|---|---|
| Non-Declining | Declining | p-Value | Non-Declining | Declining | p-Value | |
| ØScion (mm) | 28.50 ± 1.41 | 23.90 ± 1.14 | 0.025 | 80.50 ± 4.04 | 63.50 ± 2.05 | 0.002 |
| Ørootstock (mm) | 56.10 ± 2.25 | 48.80 ± 3.13 | 0.080 | 98.20 ± 7.48 | 91.60 ± 6.61 | 0.515 |
| ØR/ØS (mm) | 1.21 ± 0.05 | 1.03 ± 0.08 | 0.086 | 1.21 ± 0.05 | 1.44 ± 0.08 | 0.044 |
| RSDepth (cm) | 46.80 ± 2.10 | 47.60 ± 1.89 | 0.773 | 53.00 ± 2.08 | 44.40 ± 2.62 | 0.025 |
| RSWidth (cm) | 70.60 ± 4.24 | 54.90 ± 5.37 | 0.037 | 86.30 ± 7.60 | 69.70 ± 4.00 | 0.065 |
| RootstockUG (cm) | 32.50 ± 0.82 | 34.10 ± 0.97 | 0.229 | 32.70 ±2.01 | 27.70 ± 2.29 | 0.125 |
| Projected Area (m2) | 0.08 ± 0.01 | 0.06 ± 0.07 | 0.078 | 0.13 ± 0.02 | 0.08 ± 0.01 | 0.049 |
| Primary Roots | 30.40 ± 3.44 | 20.60 ± 2.62 | 0.041 | 33.70 ± 4.65 | 29.00 ± 2.74 | 0.384 |
| Dry Weight (g) | 367.00 ± 59.04 | 179.00 ± 28.21 | 0.012 | 805.00 ± 290.65 | 474.00 ± 43.13 | 0.249 |
| Tips | 24,021 ± 3877 | 17,378 ± 2100 | 0.154 | 22,716 ± 2195 | 11,902 ± 1347 | 0.001 |
| ØMaximum (mm) | 27.00 ± 2.41 | 24.00 ± 1.19 | 0.285 | 39.70 ± 4.80 | 31.40 ± 1.06 | 0.093 |
| Length (m) | 219.18 ± 30.79 | 143.06 ± 14.74 | 0.043 | 235.04 ± 25.65 | 131.50 ± 13.13 | 0.003 |
| Surface Area (m2) | 1.00 ± 0.14 | 0.61 ± 0.08 | 0.033 | 1.26 ± 0.19 | 0.90 ± 0.06 | 0.077 |
| Volume (dm3) | 1.43 ± 0.26 | 0.81 ± 0.16 | 0.061 | 2.25 ± 0.35 | 1.55 ± 0.12 | 0.064 |
| Orchard | Status | Total Trees | Number of Positive Samples a | ||
|---|---|---|---|---|---|
| ACLSV | ASGV | ASPV | |||
| A | Non-declining | 8 | 7 | 1 | 1 |
| Declining | 8 | 7 | 0 | 0 | |
| B | Non-declining | 8 | 0 | 0 | 0 |
| Declining | 7 | 0 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, A.; Wunsch, A.; Sabety, J.; van Zoeren, J.; Basedow, M.; Miranda Sazo, M.; Fuchs, M.; Khan, A. The Comparative Root System Architecture of Declining and Non-Declining Trees in Two Apple Orchards in New York. Plants 2023, 12, 2644. https://doi.org/10.3390/plants12142644
Serrano A, Wunsch A, Sabety J, van Zoeren J, Basedow M, Miranda Sazo M, Fuchs M, Khan A. The Comparative Root System Architecture of Declining and Non-Declining Trees in Two Apple Orchards in New York. Plants. 2023; 12(14):2644. https://doi.org/10.3390/plants12142644
Chicago/Turabian StyleSerrano, Alicia, Anna Wunsch, Jean Sabety, Janet van Zoeren, Michael Basedow, Mario Miranda Sazo, Marc Fuchs, and Awais Khan. 2023. "The Comparative Root System Architecture of Declining and Non-Declining Trees in Two Apple Orchards in New York" Plants 12, no. 14: 2644. https://doi.org/10.3390/plants12142644
APA StyleSerrano, A., Wunsch, A., Sabety, J., van Zoeren, J., Basedow, M., Miranda Sazo, M., Fuchs, M., & Khan, A. (2023). The Comparative Root System Architecture of Declining and Non-Declining Trees in Two Apple Orchards in New York. Plants, 12(14), 2644. https://doi.org/10.3390/plants12142644









