Anatomy and Histochemistry of the Vegetative System of Brachystele guayanensis (Lindl.) Schltr. (Orchidaceae), a Potential Medicinal Species
Abstract
1. Introduction
2. Results and Discussion
2.1. Anatomical Data
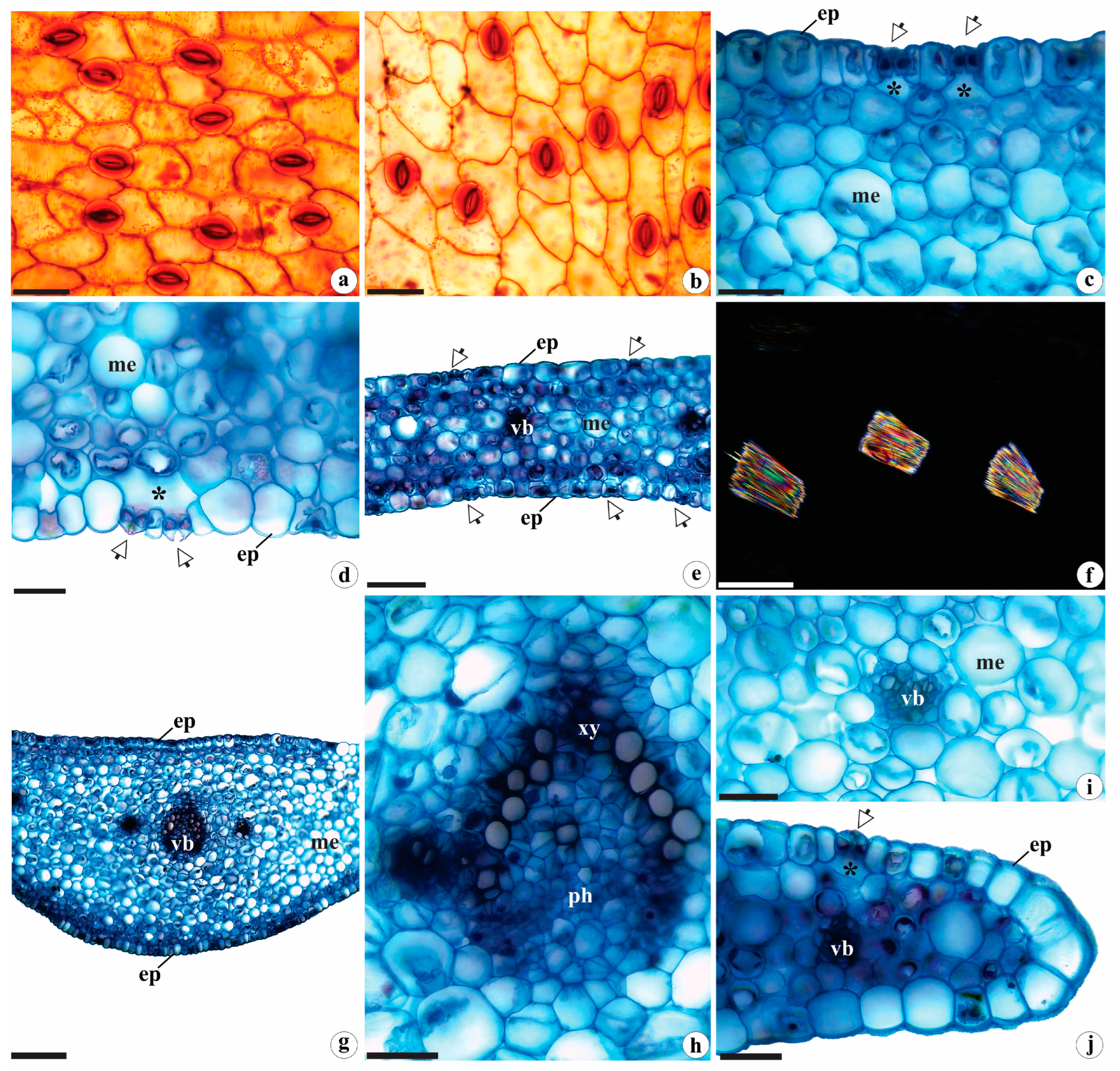
2.1.1. Leaf Anatomy
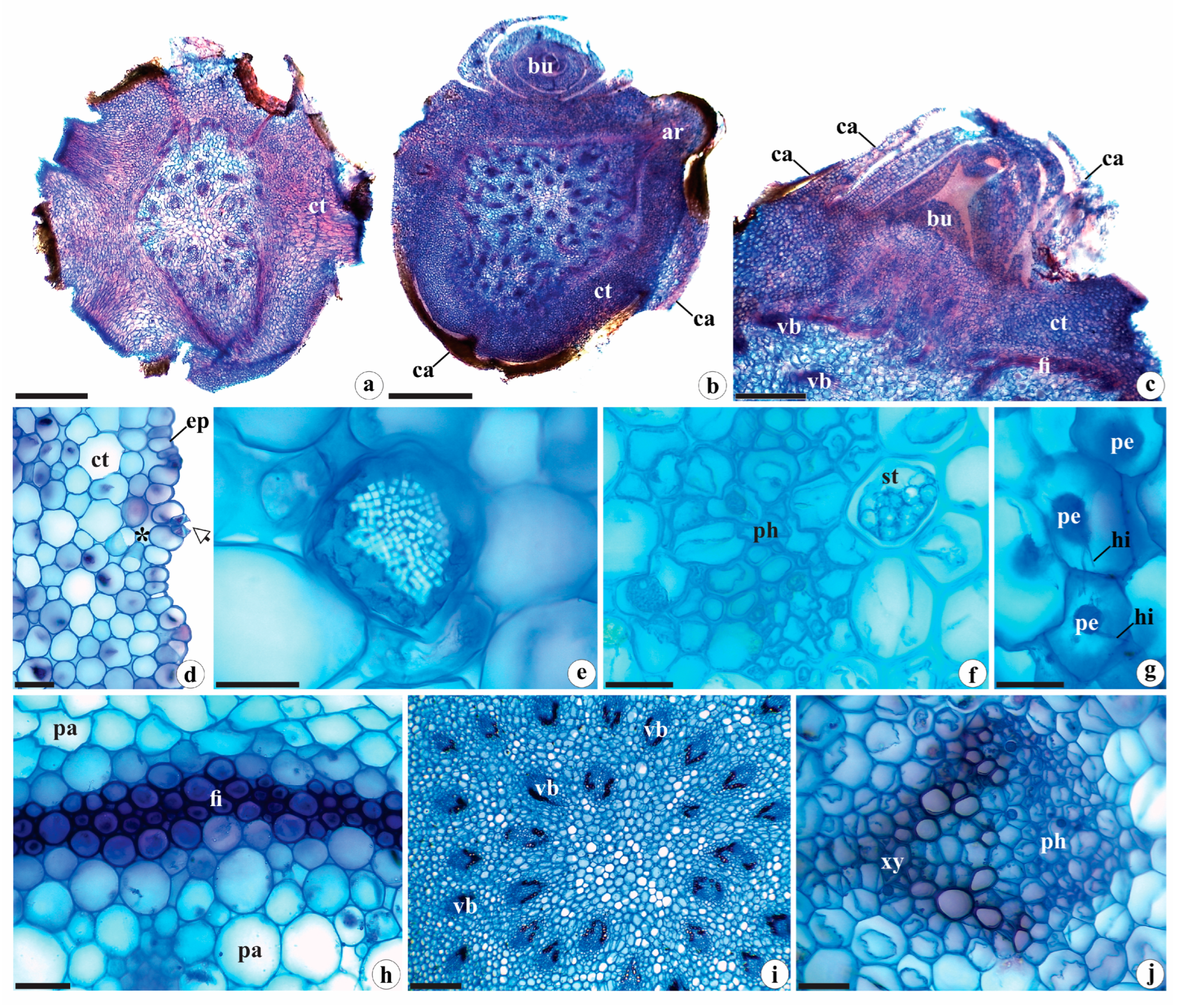
2.1.2. Rhizome Anatomy
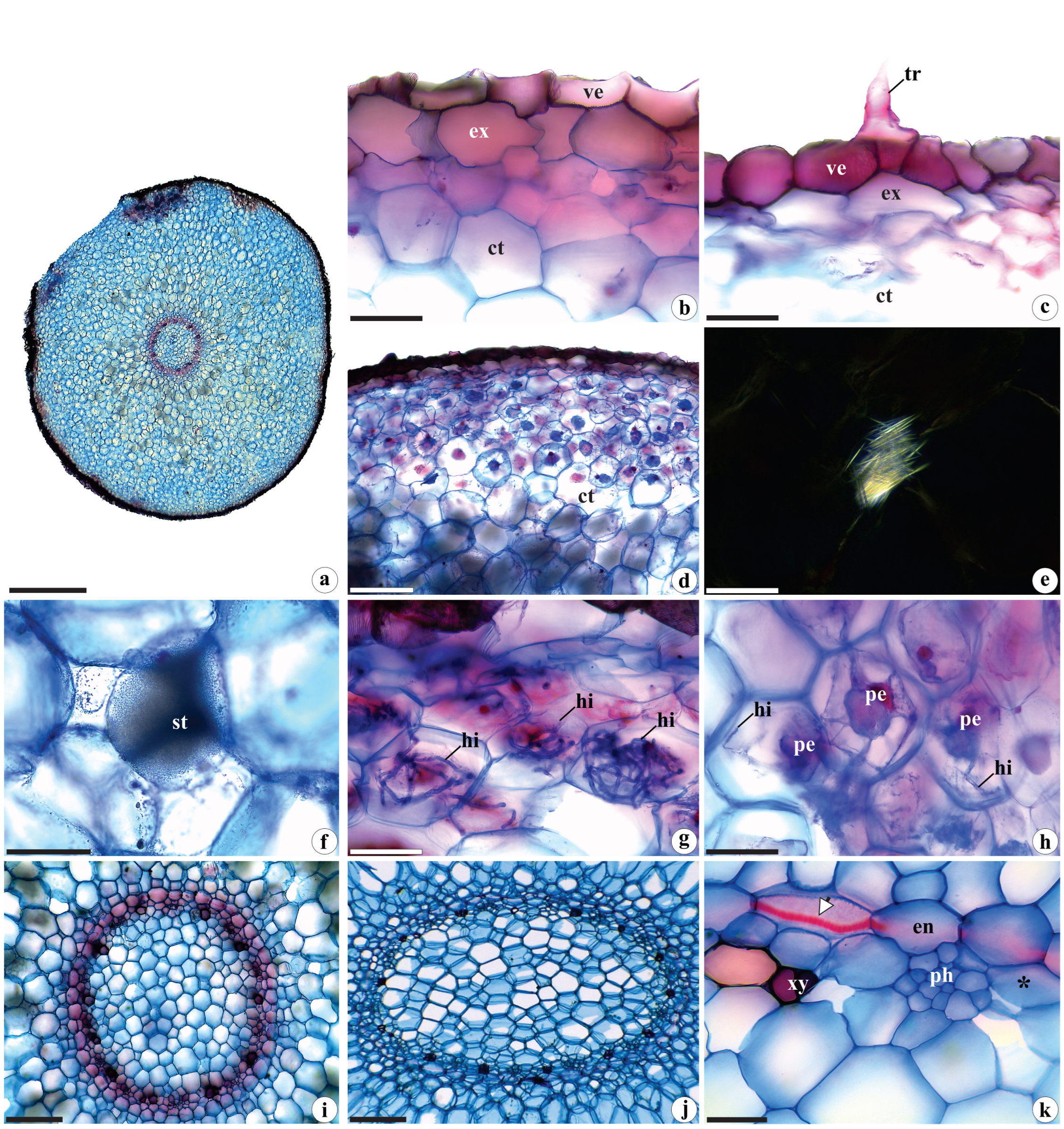
2.1.3. Root Anatomy
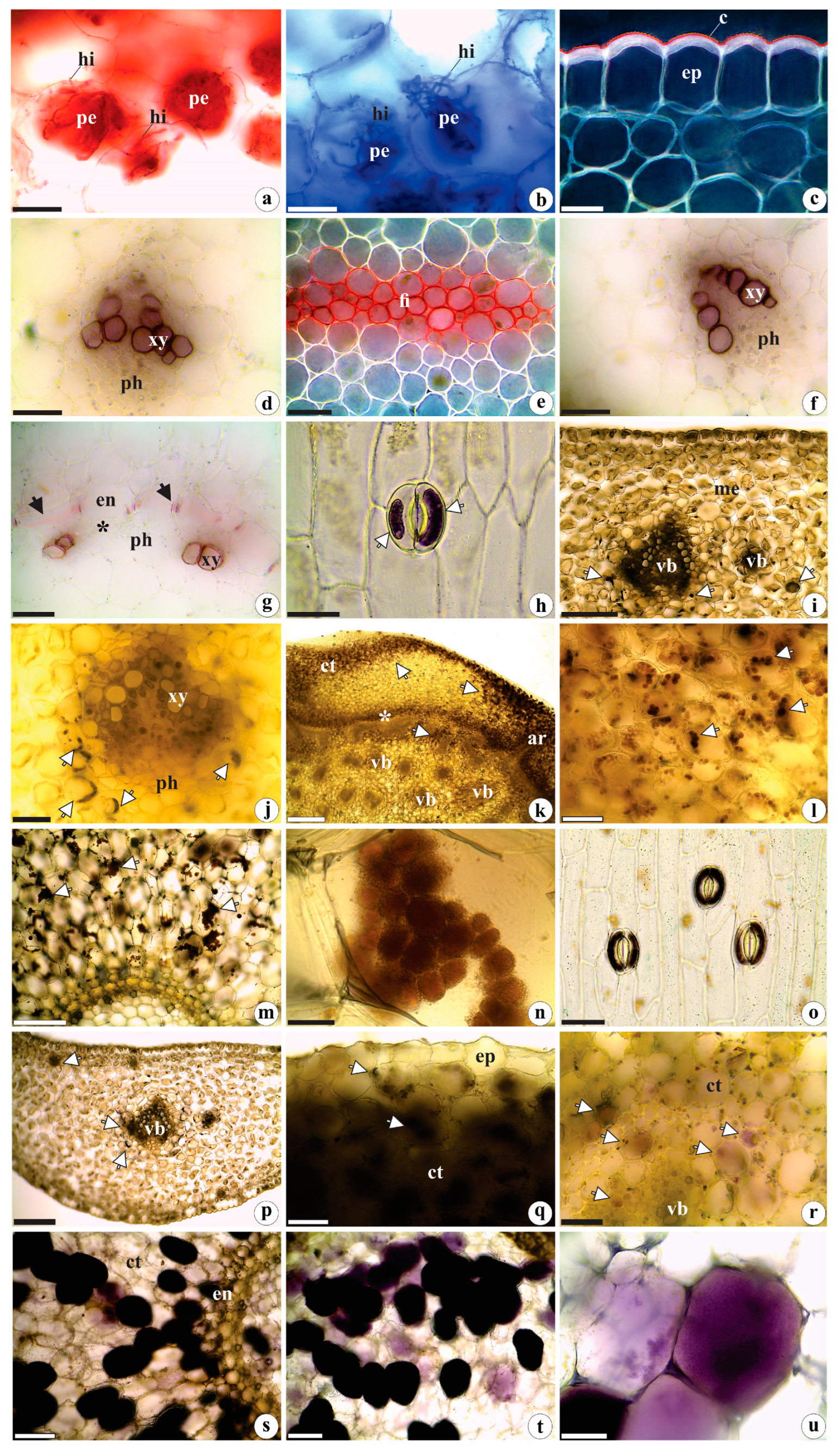
2.2. Histochemistry Data
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pridgeon, A.M.; Cribb, P.J.; Chase, M.W.; Rasmussen, F.N. Genera Orchidacearum—Orchidoideae (Part 1), 1st ed.; Oxford University Press: Oxford, UK, 2001; Volume 2, pp. 1–416. [Google Scholar]
- Pridgeon, A.M.; Cribb, P.J.; Chase, M.W.; Rasmussen, F.N. Genera Orchidacearum—Orchidoideae—Vanilloideae (Part 2), 1st ed.; Oxford University Press: Oxford, UK, 2003; Volume 3, pp. 1–360. [Google Scholar]
- Flora e Funga do Brasil—Orchidaceae Juss. and Brachystele Schltr. Jardim Botânico do Rio de Janeiro. 2015. Available online: https://servicos.jbrj.gov.br/flora/#/CheckList (accessed on 10 June 2023).
- Dressler, R. Phylogeny and Classifications of the Orchid Family, 1st ed.; Dioscorides press: Portland, OR, USA, 1993; p. 314. [Google Scholar]
- Hossain, M.M. Therapeutic orchids: Traditional uses and recent advances—An overview. Fitoterapia 2011, 82, 102–140. [Google Scholar] [CrossRef] [PubMed]
- Teoh, E.S. Medicinal Orchids of Asia, 1st ed.; Springer International Publishing: Heidelberg/Berlin, Germany, 2016; pp. 1–752. [Google Scholar] [CrossRef]
- Teoh, E.S. Orchids as Aphrodisiac, Medicine or Food, 1st ed.; Springer International Publishing: Heidelberg/Berlin, Germany, 2019; pp. 1–376. [Google Scholar] [CrossRef]
- Stern, W.L. Anatomy of the Monocotyledons: X. Orchidaceae, 1st ed.; Oxford University Press: Oxford, UK, 2014; pp. 1–288. [Google Scholar]
- Tremblay, R.L. Trends in the pollination ecology of the Orchidaceae: Evolution and systematics. Can. J. Bot. 1992, 70, 642–650. [Google Scholar] [CrossRef]
- Kurzweil, H.; Linder, H.P.; Stern, W.L.; Pridgeon, A.M. Comparative vegetative anatomy and classification of Diseae (Orchidaceae). Bot. J. Linn. Soc. 1995, 117, 171–220. [Google Scholar] [CrossRef]
- Sut, S.; Maggi, F.; Dall’Acqua, S. Bioactive secondary metabolites from orchids (Orchidaceae). Chem. Biodivers. 2017, 14, e1700172. [Google Scholar] [CrossRef]
- Cota, B.B.; Magalhães, A.; Pimenta, A.; Siqueira, E.P.; Alves, T.; Zani, C.L. Chemical constituents of Habenaria petalodes Lindl. (Orchidaceae). J. Braz. Chem. Soc. 2008, 19, 1098–1104. [Google Scholar] [CrossRef]
- Godinho, C.C. Estudos Metabolômicos de Espécies Brasileiras de Orchidaceae. Ph.D. Thesis, Universidade de São Paulo, Ribeirão Preto, SP, Brazil, 2022. [Google Scholar]
- Silva, I.V.; Meira, R.M.S.A.; Azevedo, A.A.; Euclydes, R.M.A. Estratégias anatômicas foliares de treze espécies de Orchidaceae ocorrentes em um campo de altitude no Parque Estadual da Serra do Brigadeiro (PESB)—MG, Brasil. Acta Bot. Bras. 2006, 20, 741–750. [Google Scholar] [CrossRef]
- Bernal, A.A.; Smidt, E.C.; Bona, C. Spiral root hairs in Spiranthinae (Cranichideae: Orchidaceae). Rev. Bras. Bot. 2015, 38, 411–415. [Google Scholar] [CrossRef]
- Bona, C.; Engels, M.E.; Pieczak, F.S.; Smidt, E.C. Comparative vegetative anatomy of Neotropical Goodyerinae Klotzsch (Orchidaceae Juss.: Orchidoideae Lindl.). Acta Bot. Bras. 2020, 34, 530–539. [Google Scholar] [CrossRef]
- Rutkowski, P.; Mytnik, J.; Szlachetko, D.L. New taxa and new combinations in Mesoamerican Spiranthinae (Orchidaceae, Spirantheae). Ann. Bot. Fenn. 2004, 41, 471–477. [Google Scholar]
- Verettoni, H.N. Contribución al Conocimiesão Bioativas de las Plantas Medicinales de la Región de Bahía Blanca, 1st ed.; Harris y Cia: Bahía Blanca, Argentina, 1985; pp. 1–374. [Google Scholar]
- Neumann, C. Orchideen als Arzneipflanzen: Ein Querschnitt durch ausgewählte medizinische und botanisch-pharmazeutische Literatur des 19. Jahrhunderts. Z. Phytother. 2009, 30, 1–29. [Google Scholar] [CrossRef]
- Andreota, R.C.; Barros, F.; Sajo, M.G. Root and leaf anatomy of some terrestrial representatives of the Cranichideae tribe (Orchidaceae). Rev. Bras. Bot. 2015, 38, 367–378. [Google Scholar] [CrossRef]
- Corredor, B.A.D.; Arias, R.L. Morfoanatomía en Cranichideae (Orchidaceae) de la Estación Loma Redonda del Parque Nacional “Sierra Nevada”, Mérida, Venezuela. Lankesteriana 2012, 12, 61–75. [Google Scholar] [CrossRef]
- Aybeke, M. Comparative anatomy of selected rhizomatous and tuberous taxa of subfamilies Orchidoideae and Epidendroideae (Orchidaceae) as an aid to identification. Plant Syst. Evol. 2012, 298, 1643–1658. [Google Scholar] [CrossRef]
- Şenel, G.; Akbulut, M.K.; Şeker, Ş.S. Comparative anatomical properties of some Epidendroideae and Orchidoideae species distributed in NE Turkey. Protoplasma 2019, 256, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Stern, W.L.; Morris, M.W.; Judd, W.S.; Pridgeon, A.M.; Dressler, R.L. Comparative vegetative anatomy and systematics of Spiranthoideae (Orchidaceae). Bot. J. Linn. Soc. 1993, 113, 161–197. [Google Scholar] [CrossRef]
- Stern, W.L. Vegetative anatomy of subtribe Orchidinae (Orchidaceae). Bot. J. Linn. Soc. 1997, 124, 121–136. [Google Scholar] [CrossRef]
- Stern, W.L. Vegetative anatomy of subtribe Habenariinae (Orchidaceae). Bot. J. Linn. Soc. 1997, 125, 211–227. [Google Scholar] [CrossRef]
- Aybeke, M.; Sezik, E.; Olgun, G. Vegetative anatomy of some Ophrys, Orchis and Dactylorhiza (Orchidaceae) taxa in Trakya region of Turkey. Flora 2010, 205, 73–89. [Google Scholar] [CrossRef]
- Oliveira, V.C.; Sajo, M.G. Leaf anatomy of epiphyte species of Orchidaceae. Rev. Bras. Bot. 1999, 22, 365–374. [Google Scholar] [CrossRef]
- Silva, C.I.; Milaneze-Gutierre, M.A. Caracterização morfoanatômica dos órgãos vegetativos de Cattleya walkeriana Gardner (Orchidaceae). Acta Sci. 2004, 26, 91–100. [Google Scholar] [CrossRef]
- Fahn, A. Plant Anatomy, 4th ed.; Pergamon Press: Oxford, UK, 1990; pp. 1–530. [Google Scholar]
- Smith, W.K.; Vogelmann, T.C.; DeLucia, E.H.; Bell, D.T.; Shepherd, K.A. Leaf form and photosynthesis. Bioscience 1997, 47, 785–793. [Google Scholar] [CrossRef]
- Stern, W.L.; Aldrich, H.C.; McDowell, L.M.; Morris, M.W.; Pridgeon, A.M. Amyloplasts from cortical root cells of Spiranthoideae (Orchidaceae). Protoplasma 1993, 172, 49–55. [Google Scholar] [CrossRef]
- Sachs, J. Lectures on the Physiology of Plants, 1st ed.; Clarendon Press: London, UK, 1887; pp. 1–836. [Google Scholar]
- Haberlandt, G. Physiological Plant Anatomy, 4th ed.; Macmillan and Co., Ltd.: London, UK, 1928; pp. 381–382. [Google Scholar]
- Şeker, Ş.S. What does the quantitative morphological diversity of starch grains in terrestrial orchids indicate? Microsc. Res. Tech. 2022, 85, 2931–2942. [Google Scholar] [CrossRef] [PubMed]
- Stern, W.L.; Judd, W.S. Comparative vegetative anatomy and systematics of Vanilla (Orchidaceae). Bot. J. Linn. Soc. 1999, 131, 353–382. [Google Scholar] [CrossRef]
- Stern, W.L.; Judd, W.S. Comparative anatomy and systematics of the orchid tribe Vanilleae excluding Vanilla. Bot. J. Linn. Soc. 2000, 134, 179–202. [Google Scholar] [CrossRef]
- Carlsward, B.S.; Stern, W.L. Vegetative anatomy and systematics of Triphorinae (Orchidaceae). Bot. J. Linn. Soc. 2009, 159, 203–210. [Google Scholar] [CrossRef]
- Menezes, N.L.; Elbl, P.M.; Cury, G.; Appezzato-da-Glória, B.; Sasaki, K.L.; Silva, C.G.; Costa, G.R.; Lima, V.G. The meristematic activity of the endodermis and the pericycle and its role in the primary thickening of stems in monocotyledonous plants. Plant Ecol. Divers. 2012, 5, 153–165. [Google Scholar] [CrossRef]
- Pereira, O.L.; Kasuya, M.C.M.; Rollemberg, C.L.; Chaer, G.M. Isolamento e identificação de fungos micorrízicos rizoctonióides associados a três espécies de orquídeas epífitas neotropicais no Brasil. Rev. Bras. Cienc. Solo 2005, 29, 191–197. [Google Scholar] [CrossRef]
- Pridgeon, A.M. Multicellular trichomes in tribe Diurideae (Orchidaceae): Systematic and biological significance. Kew Bull. 1994, 49, 569–579. [Google Scholar] [CrossRef]
- Bougoure, J.; Ludwig, M.; Brundrett, M.; Grierson, P. Identity and specificity of the fungi forming mycorrhizas with the rare mycoheterotrophic orchid Rhizanthella gardneri. Mycol. Res. 2009, 113, 1097–1106. [Google Scholar] [CrossRef]
- Uma, E.; Rajendran, R.; Muthukumar, T. Morphology, anatomy and mycotrophy of pseudobulb and subterranean organs in Eulophia epidendraea and Malaxis acuminata (Epidendroideae, Orchidaceae). Flora 2015, 217, 14–23. [Google Scholar] [CrossRef]
- Suetsugu, K.; Haraguchi, T.F.; Tanabe, A.S.; Tayasu, I. Specialized mycorrhizal association between a partially mycoheterotrophic orchid Oreorchis indica and a Tomentella taxon. Mycorrhiza 2021, 31, 243–250. [Google Scholar] [CrossRef]
- Pedroso-de-Moraes, C.; Souza-Leal, T.; Barros, F.; Sajo, M.G. Vegetative anatomy of some Brazilian Zygopetalinae (Orchidaceae). Iheringia 2018, 73, 159–175. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 1–787. [Google Scholar]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Hardan, A.N. Importance of mycorrhizae in crop productivity. In Mitigating Environmental Stresses for Agricultural Sustainability in Egypt, 1st ed.; Awaad, H., Abu-hashim, M., Negm, A., Eds.; Springer Water: Gewerbestrasse, Switzerland, 2021; Volume 1, pp. 471–484. [Google Scholar] [CrossRef]
- Li, X.L.; Marschner, H.; George, E. Acquisition of phosphorus and copper by VA-mycorrhizal hyphae and root-to-shoot transport in white clover. Plant Soil 1991, 136, 49–57. [Google Scholar] [CrossRef]
- Azaizeh, H.A.; Marschner, H.; Römheld, V.; Wittenmayer, L. Effects of a vesicular-arbuscular mycorrhizal fungus and other soil microorganisms on growth, mineral nutrient acquisition and root exudation of soil grown maize plants. Mycorrhiza 1995, 5, 321–327. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 3rd ed.; Artmed: Porto Alegre, RS, Brazil, 2006; pp. 1–719. [Google Scholar]
- Bedini, S.; Pellegrino, E.; Avio, L.; Pellegrini, S.; Bazzoffi, P.; Argese, E.; Giovannetti, M. Changes in soil aggregation and glomalinrelated soil protein content as affected by the arbuscular mycorrhizal fungal species Glomus mosseae and Glomus intraradices. Soil Biol. Biochem. 2009, 41, 1491–1496. [Google Scholar] [CrossRef]
- Metcalfe, C.R. Comparative anatomy as a modern Botanical discipline. In Advances in Botanical Research, 1st ed.; Metcalfe, C.R., Ed.; Academic Press: New York, NY, USA, 1963; Volume 6, pp. 101–147. [Google Scholar]
- Appezzato-da-Glória, B.; Carmello-Guerreiro, S.M. Anatomia Vegetal, 2nd ed.; Editora UFV: Viçosa, BA, Brazil, 2006; pp. 1–438. [Google Scholar]
- Andreota, R.C. Anatomia dos Órgãos Vegetativos de Representantes da Tribo Cranichideae (Orchidoideae: Orchidaceae). Masters’ Thesis, Universidade Estadual Paulista, Rio Claro, SP, Brazil, 2013. [Google Scholar]
- Figueroa, C.; Salazar, G.A.; Zavaleta, H.A.; Engleman, E.M. Root character evolution and systematics in Cranichidinae, Prescottiinae and Spiranthinae (Orchidaceae, Cranichideae). Ann. Bot. 2008, 101, 509–520. [Google Scholar] [CrossRef]
- Porembski, S.; Barthlott, W. Velamen radicum micromorphology and classification of Orchidaceae. Nord. J. Bot. 1988, 8, 117–137. [Google Scholar] [CrossRef]
- Moreira, A.S.F.P.; Isaias, R.M.S. Comparative anatomy of the absorption roots of terrestrial and epiphytic orchids. Braz. Arch. Biol. Technol. 2008, 51, 83–93. [Google Scholar] [CrossRef]
- Pridgeon, A.M. The velamen and exodermis of orchid roots. In Orchid Biology, Reviews and Perspectives, 1st ed.; Arditti, J., Ed.; Cornell University Press: Ithaca, NY, USA, 1987; Volume 4, pp. 139–192. [Google Scholar]
- Chomicki, G.; Bidel, L.P.R.; Ming, F.; Coiro, M.; Zhang, X.; Wang, Y.; Baissac, Y.; Jay-Allemand, C.; Renner, S.S. The velamen protects photosynthetic orchid roots against UV-B damage, and a large dated phylogeny implies multiple gains and losses of this function during the Cenozoic. New Phytol. 2015, 205, 1330–1341. [Google Scholar] [CrossRef]
- Cameron, D.D.; Johnson, I.; Leake, J.R.; Read, D.J. Mycorrhizal acquisition of inorganic phosphorus by the green-leaved terrestrial orchid Goodyera repens. Ann. Bot. 2007, 99, 831–834. [Google Scholar] [CrossRef]
- Juniper, B.E.; Jeffree, C.E. Plant Surfaces, 1st ed.; Edward Arnold Publishers: London, UK, 1983; pp. 1–93. [Google Scholar]
- Dickison, W.C. Integrative Plant Anatomy, 1st ed.; Academic Press: San Diego, CA, USA, 2000; pp. 1–533. [Google Scholar]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells and Tissues of the Plant Body: Their Structure, Function and Development, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 1–601. [Google Scholar]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef]
- Haridasan, M. Alumínio é um elemento tóxico para as plantas nativas do cerrado? In Fisiologia Vegetal: Práticas em Relações Hídricas, Fotossíntese e Nutrição Mineral, 1st ed.; Prado, C.H.B.A., Casali, C.A., Eds.; Editora Manole: Barueri, SP, Brazil, 2006; Volume 1, pp. 1–10. [Google Scholar]
- Peterson, C.A.; Murrmann, M.; Steudle, E. Location of the major barriers to water and ion movement in young roots of Zea mays L. Planta 1993, 190, 127–136. [Google Scholar] [CrossRef]
- Enstone, D.E.; Peterson, C.A.; Ma, F. Root endodermis and exodermis: Structure, function, and responses to the environment. J. Plant Growth Regul. 2003, 21, 335–351. [Google Scholar] [CrossRef]
- Lux, A.; Luxová, M. Growth and differentiation of root endodermis in Primula acaulis Jacq. Biol. Plant. 2003, 47, 91–97. [Google Scholar] [CrossRef]
- D’Amelio, E.D.; Zeiger, E. Diversity of guard cell plastids of the Orchidaceae: A structural and functional study. Canad. J. Bot. 1988, 66, 257–271. [Google Scholar] [CrossRef]
- Nunes, E.; Scopel, M.; Vignoli-Silva, M.; Vendruscolo, G.S.; Henriques, A.T.; Mentz, L.A. Caracterização farmacobotânica das espécies de Sambucus (Caprifoliaceae) utilizadas como medicinais no Brasil. Parte II. Sambucus australis Cham. Schltdl. Braz. J. Pharmacog. 2007, 17, 414–425. [Google Scholar] [CrossRef]
- Kikuchi, T.Y.S.; Braga, Z.V.; Potiguara, R.C.V. Anatomia foliar de Socratea exorrhiza (Mart.) H. Wendl. (Arecaceae). Biota Amazôn. 2016, 6, 73–79. [Google Scholar] [CrossRef]
- Reis, R.E.; Alvim, M.N. Anatomia foliar comparada de três espécies do gênero Oxalis L. (Oxalidaceae). NBC 2013, 3, 59–72. [Google Scholar] [CrossRef]
- Vavasseur, A.; Raghavendra, A.S. Guard cell metabolism and CO2 sensing. New Phytol. 2005, 165, 665–682. [Google Scholar] [CrossRef]
- Araújo, W.L.; Nunes-Nesi, A.; Osorio, S.; Usadel, B.; Fuentes, D.; Nagy, R.; Balbo, I.; Lehmann, M.; Studart-Witkowski, C.; Tohge, T.; et al. Antisense inhibition of the iron-sulphur subunit of succinate dehydrogenase enhances photosynthesis and growth in tomato via an organic acid mediated effect on stomatal aperture. Plant Cell. 2011, 23, 600–627. [Google Scholar] [CrossRef]
- Bulpitt, C.J.; Li, Y.; Bulpitt, P.F.; Wang, J. The use of orchids in Chinese medicine. J. R. Soc. Med. 2007, 100, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, T. Chemical ecology of pyrrolizidine alkaloids. Planta 1999, 207, 483–495. [Google Scholar] [CrossRef]
- Sepúlveda-Jiménez, G.; Porta-Ducoing, H.; Rocha-Sosa, M. La participación de los metabolitos secundarios en la defensa de las plantas. Rev. Mex. Fitopatol. 2003, 21, 355–363. [Google Scholar]
- Vizzotto, M.; Krolow, A.C.R.; Weber, G.E.B. Metabólitos Secundários Encontrados em Plantas e sua Importância, 1st ed.; Embrapa Clima Temperado: Pelotas, RS, Brazil, 2010; pp. 1–17. [Google Scholar]
- Li, J.W.; Zhang, Z.B.; Zhang, S.B. Widely targeted metabolic, physical and anatomical analyses reveal diverse defensive strategies for pseudobulbs and succulent roots of orchids with industrial value. Ind. Crops Prod. 2022, 177, 114510. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P. Orchids: A review of uses in traditional medicine, its phytochemistry and pharmacology. J. Med. Plant Res. 2010, 4, 592–638. [Google Scholar] [CrossRef]
- Brito, H.O.; Noronha, E.P.; França, L.M. Phytochemical analysis composition from Annona squamosa (ATA) ethanolic extract leaves. Rev. Bras. Farm. 2008, 89, 180–184. [Google Scholar]
- Ng, T.B.; Liu, J.; Wong, J.H.; Ye, X.; Sze, S.C.W.; Tong, Y.; Zhang, K.Y. Review of research on Dendrobium, a prized folk medicine. Appl. Microbiol. Biotechnol. 2012, 93, 1795–1803. [Google Scholar] [CrossRef]
- Li, R.; Liu, T.; Liu, M.; Chen, F.; Liu, S.; Yang, J. Anti-influenza a virus activity of dendrobine and its mechanism of action. J. Agric. Food Chem. 2017, 65, 3665–3674. [Google Scholar] [CrossRef]
- Mori, S.A.; Silva, L.A.; Lisboa, G.; Coradin, L. Manual de Manejo do Herbário Fanerogâmico, 2nd ed.; CEPLAC: Ilhéus, BA, Brazil, 1989; pp. 1–104. [Google Scholar]
- Johansen, D.A. Plant Microtechnique, 1st ed.; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1940; pp. 1–523. [Google Scholar]
- Kraus, J.E.; Arduin, M. Manual Básico de Métodos em Morfologia Vegetal, 1st ed.; EDUR: Rio de Janeiro, Brazil, 1997; pp. 1–198. [Google Scholar]
- Bukatsch, F. Bermerkungen zur Doppelfrbung Astrablau-Safranin. Mikrokosmos 1972, 61, 255. [Google Scholar]
- Fisher, D.B. Protein staining of ribboned epon sections for light microscopy. Histochemie 1968, 16, 92–96. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.P.; McCully, M.E. The Study of Plant Structure Principles and Selected Methods, 1st ed.; Termarcarphi: Melbourne, Australia, 1981; pp. 1–344. [Google Scholar]
- Furr, M.; Mahlberg, P.G. Histochemical analyses of laticifers and glandular trichomes in Cannabis sativa. J. Nat. Prod. 1981, 44, 153–159. [Google Scholar] [CrossRef]
- Jensen, W.A. Botanical Histochemistry: Principles and Practice, 1st ed.; W.H. Freeman: San Francisco, CA, USA, 1962; pp. 1–408. [Google Scholar]
- Mace, M.E.; Howell, C.R. Histological and identification of condensed tannin precursor in roots of cotton seedlings. Can. J. Bot. 1974, 52, 2423–2426. [Google Scholar] [CrossRef]
- Chamberlain, C.J. Methods in Plant Histology, 5th ed.; University of Chicago Press: Chicago, CA, USA, 1932; pp. 1–416. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, I.S.d.; Silva, M.J.d. Anatomy and Histochemistry of the Vegetative System of Brachystele guayanensis (Lindl.) Schltr. (Orchidaceae), a Potential Medicinal Species. Plants 2023, 12, 2635. https://doi.org/10.3390/plants12142635
Santos ISd, Silva MJd. Anatomy and Histochemistry of the Vegetative System of Brachystele guayanensis (Lindl.) Schltr. (Orchidaceae), a Potential Medicinal Species. Plants. 2023; 12(14):2635. https://doi.org/10.3390/plants12142635
Chicago/Turabian StyleSantos, Igor Soares dos, and Marcos José da Silva. 2023. "Anatomy and Histochemistry of the Vegetative System of Brachystele guayanensis (Lindl.) Schltr. (Orchidaceae), a Potential Medicinal Species" Plants 12, no. 14: 2635. https://doi.org/10.3390/plants12142635
APA StyleSantos, I. S. d., & Silva, M. J. d. (2023). Anatomy and Histochemistry of the Vegetative System of Brachystele guayanensis (Lindl.) Schltr. (Orchidaceae), a Potential Medicinal Species. Plants, 12(14), 2635. https://doi.org/10.3390/plants12142635






