Identification and Expression Analysis of DFR Gene Family in Brassica napus L.
Abstract
1. Introduction
2. Results
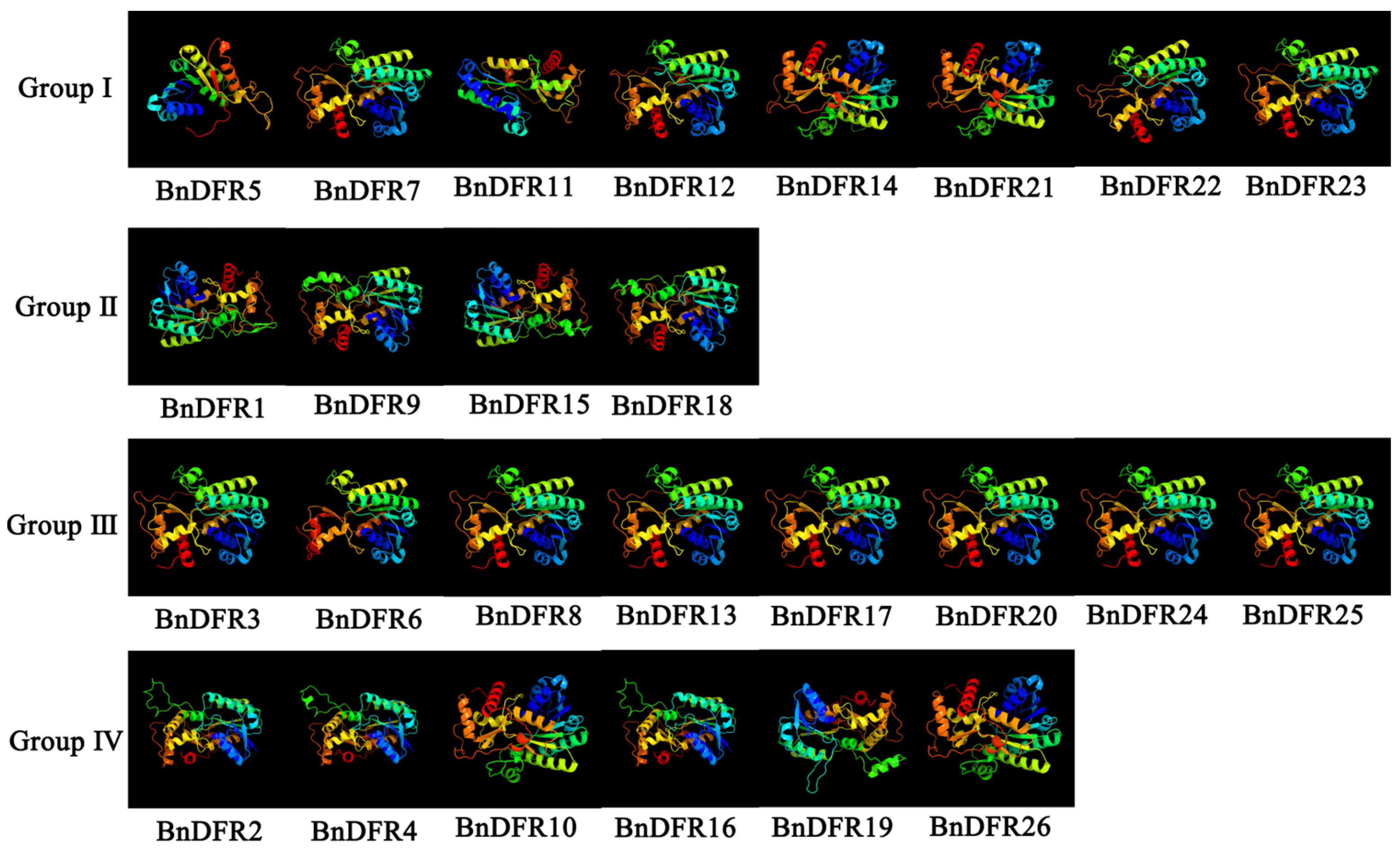
2.1. Analysis of BnDFR Family Proteins
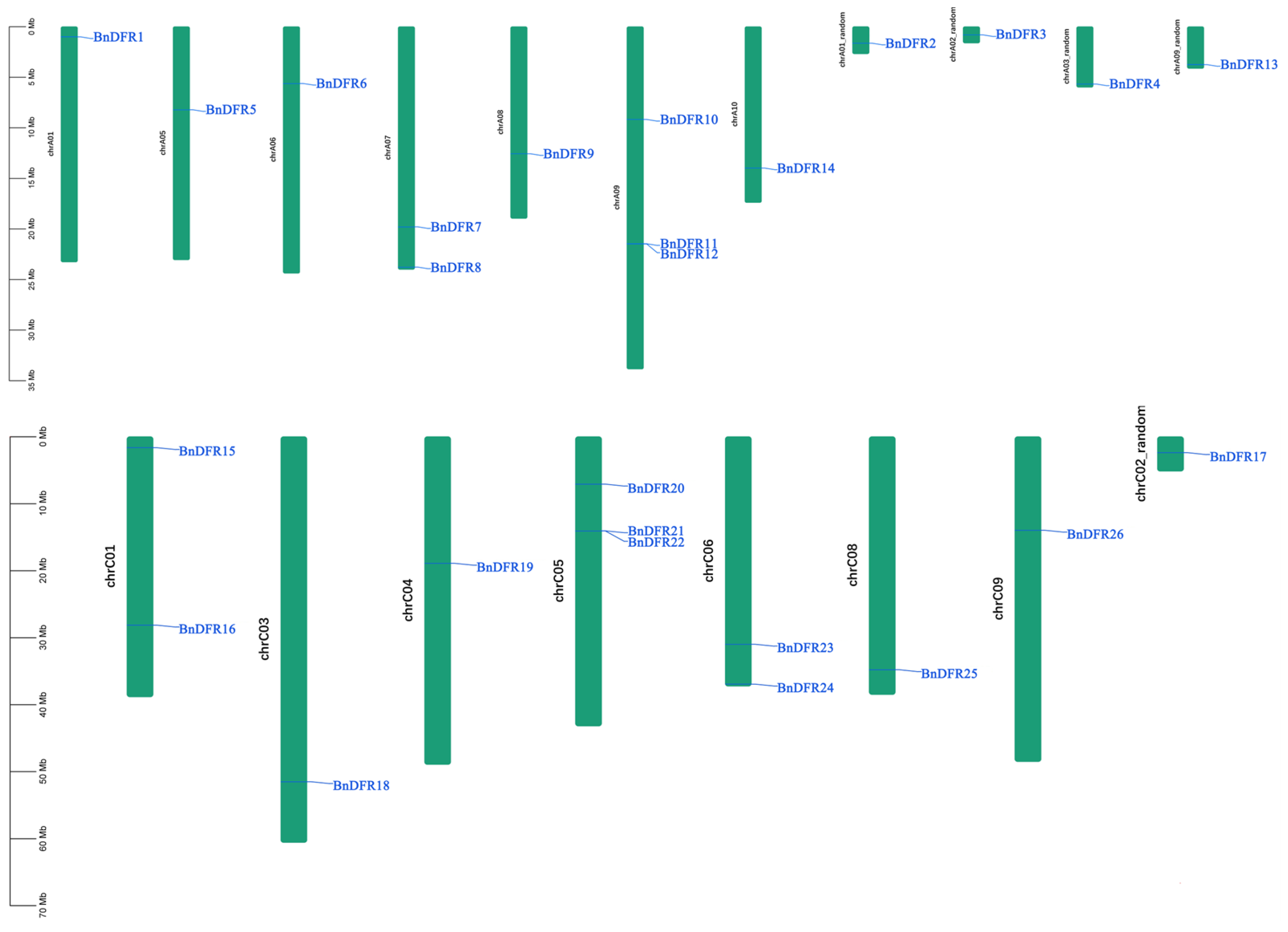
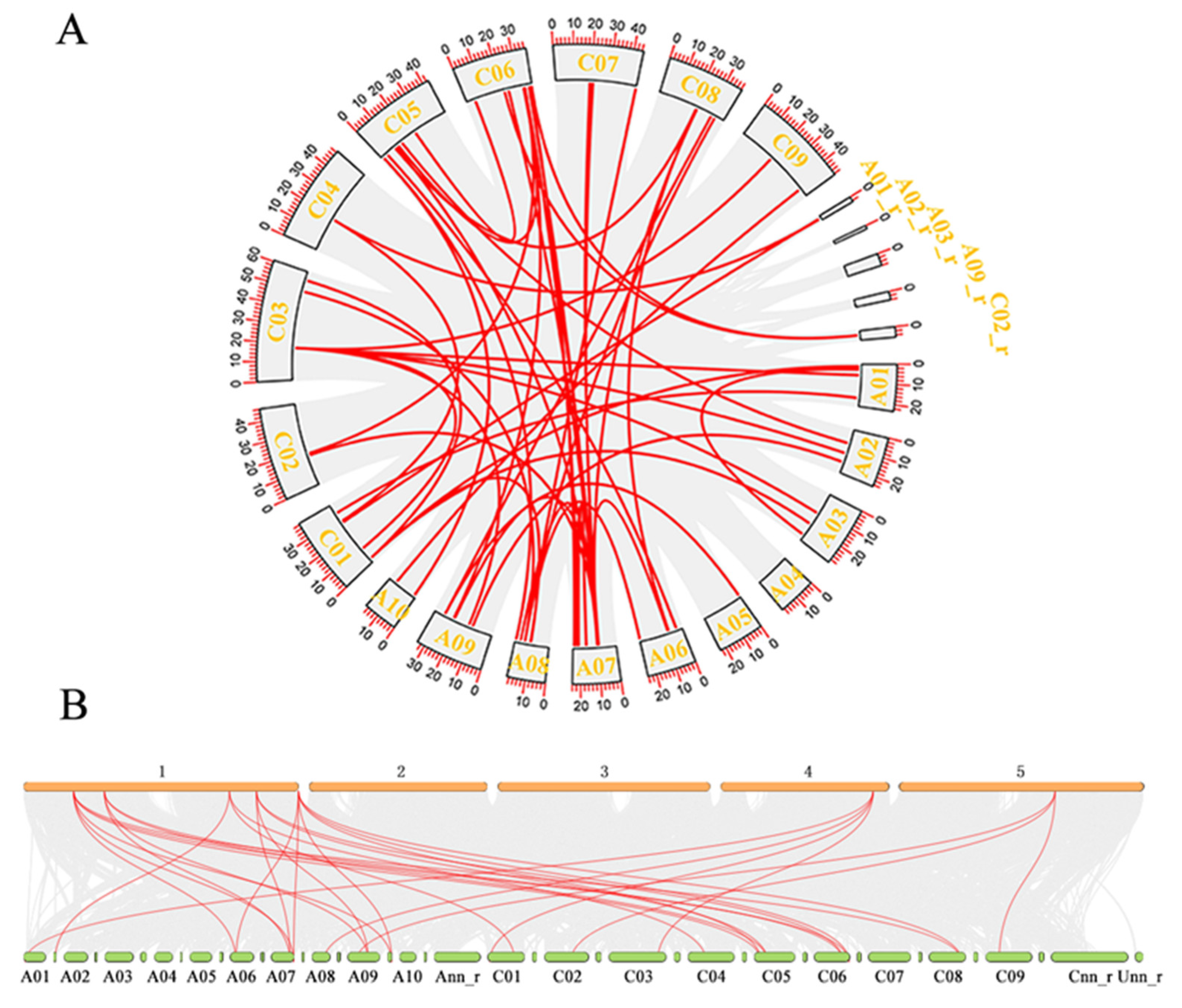
2.2. Chromosome Localization and Collinearity Analysis of DFR Family
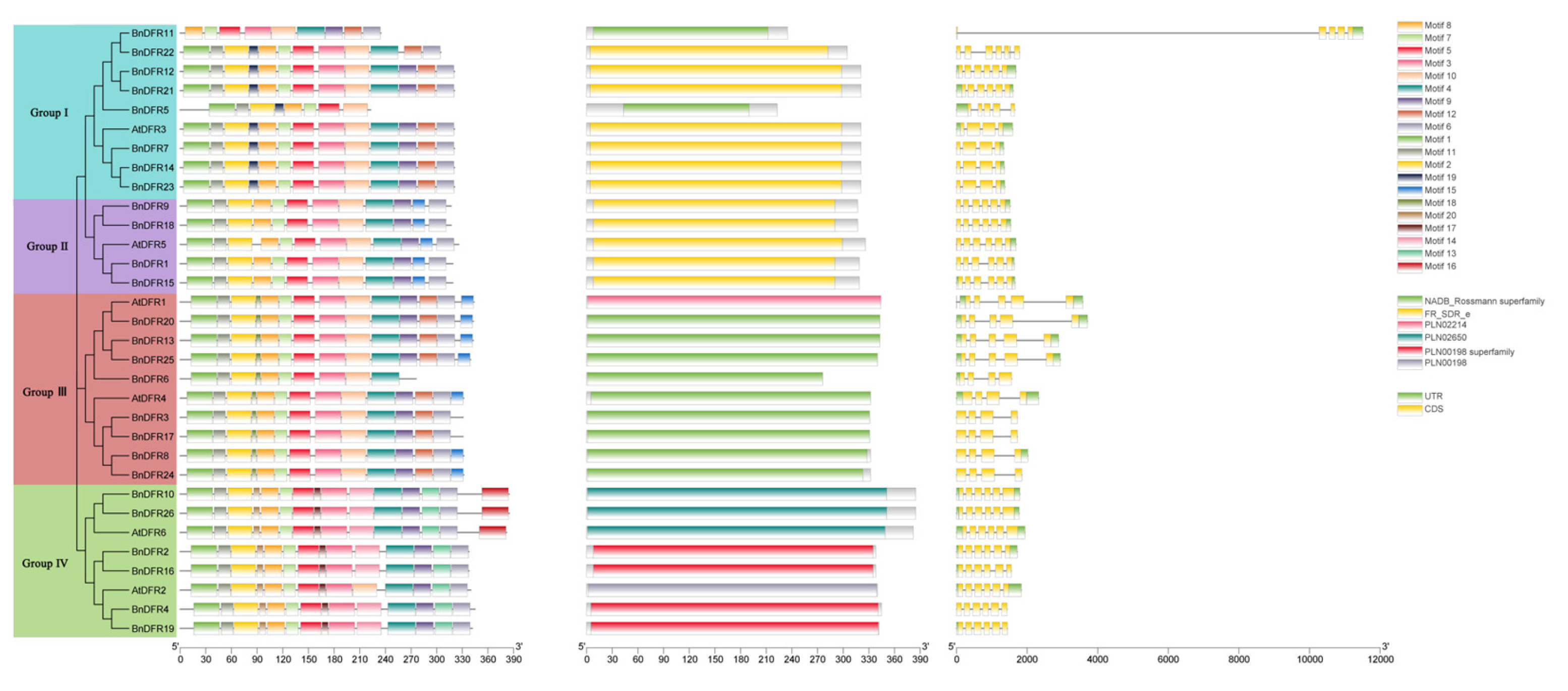
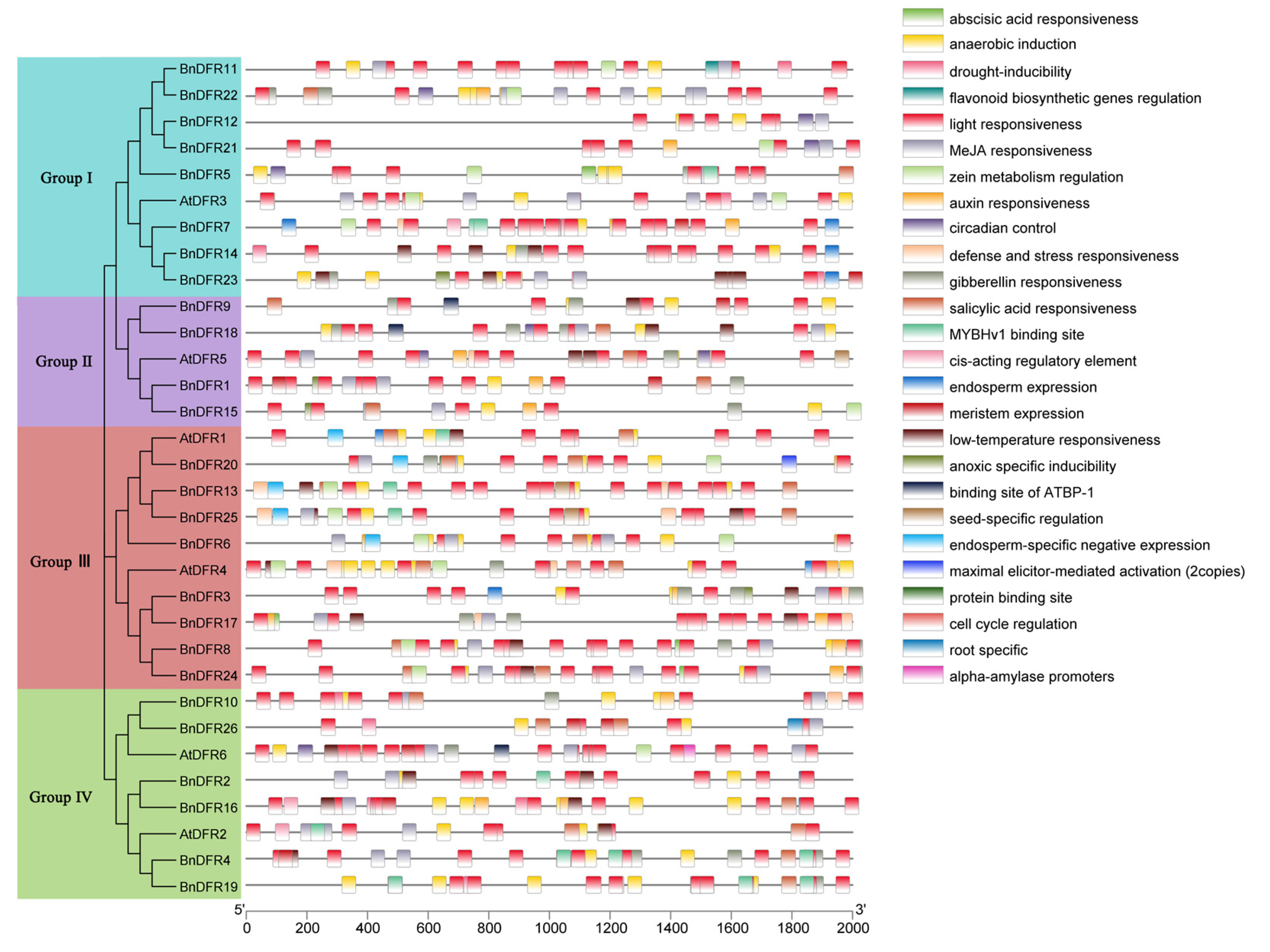
2.3. Evolutionary Analysis of DFR Gene Family
2.4. Analysis of Expression Characteristics of DFR Gene Family
2.5. Determination of Anthocyanin Content and Analysis of BnDFRs Expression in Rape of Different Color
3. Discussion
4. Materials and Methods
4.1. Materials and Data Sources
4.2. Analysis of BnDFR Family Proteins
4.3. BnDFR Gene Location and Collinearity Analysis
4.4. Analysis of Gene Structure, Conserved Motifs, and Conserved Domain of DFR Family Proteins
4.5. Cis-Acting Element Analysis of DFR Gene Promoter Region
4.6. Evolutionary Analysis and Tissue Expression Specificity Analysis of DFR Gene Family
4.7. Anthocyanin Content Determination, Expression Modeling Analysis of the BnDFRs, and qRT-PCR Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, C.; Napier, J.A.; Clemente, T.E.; Cahoon, E.B. New frontiers in oilseed biotechnology: Meeting the global demand for vegetable oils for food, feed, biofuel, and industrial applications. Curr. Opin. Biotechnol. 2011, 22, 252–259. [Google Scholar] [CrossRef]
- Kimber, D.; Mcgregor, D.I. Brassica Oilseeds—Production and Utilization; CAB International: Wallington, UK, 1996; Volume 98, p. 321. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, K.; Zheng, W.; Yu, Y.; Shen, W.; Chen, B.; Xie, G.; Cheng, J. Breeding, promotion and application of New landscape Rapeseed Varieties in International Cultural Tourism Demonstration Zone in southern Anhui. Anhui Agric. Sci. Bull. 2020, 26, 63–65. [Google Scholar]
- Zhu, Z.; Zhou, K.; Zheng, W.; Yu, Y.; Shen, W.; Chen, B.; Xie, G.; Cheng, J. Current situation of construction of creative Rapeseed Flower Sea in Southern Anhui International Cultural Tourism Demonstration Zone: Problems and suggestions. Anhui Agric. Sci. 2021, 49, 129–133,143. [Google Scholar]
- Liu, W.; Zhang, Z.; Li, Y.; Zhu, L.; Jiang, L. Efficient production of d-tagatose via DNA scaffold mediated oxidoreductases assembly in vivo from whey powder. Food Res. Int. 2023, 166, 112637. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zaman, W.; Lu, J.; Niu, Q.; Zhang, X.; Ayaz, A.; Saqib, S.; Yang, B.; Zhang, J.; Zhao, H.; et al. Natural lupeol level variation among castor accessions and the upregulation of lupeol synthesis in response to light. Ind. Crops Prod. 2023, 192, 116090. [Google Scholar] [CrossRef]
- Zhao, D.; Tao, J. Recent advances on the development and regulation of flower color in ornamental plants. Front. Plant Sci. 2015, 6, 261. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, C.; Wang, Y.; Yao, X.; Wang, F.; Wu, J.; King, G.J.; Liu, K. Disruption of a Carotenoid Cleavage Dioxygenase 4 gene converts flower colour from white to yellow in Brassica species. New Phytol. 2015, 206, 1513–1526. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Dharmawansa, K.V.S.; Hoskin, D.W.; Rupasinghe, H.P.V. Chemopreventive Effect of Dietary Anthocyanins against Gastrointestinal Cancers: A Review of Recent Advances and Perspectives. Int. J. Mol. Sci. 2020, 21, 6555. [Google Scholar] [CrossRef]
- Miyagawa, N.; Miyahara, T.; Okamoto, M.; Hirose, Y.; Sakaguchi, K.; Hatano, S.; Ozeki, Y. Dihydroflavonol 4-reductase activity is associated with the intensity of flower colors in delphinium. Plant Biotechnol. 2015, 32, 249–255. [Google Scholar] [CrossRef]
- Liu, X.; Gao, B.; Han, F.; Fang, Z.; Yang, L.; Zhuang, M.; Lv, H.; Liu, Y.; Li, Z.; Cai, C.; et al. Genetics and fine mapping of a purple leaf gene, BoPr, in ornamental kale (Brassica oleracea L. var. acephala). BMC Genom. 2017, 18, 1–9. [Google Scholar] [CrossRef]
- Cheng, H.; Li, L.; Cheng, S.; Cao, F.; Xu, F.; Yuan, H.; Wu, C. Molecular Cloning and Characterization of Three Genes Encoding Dihydroflavonol-4-Reductase from Ginkgo biloba in Anthocyanin Biosynthetic Pathway. PLoS ONE 2013, 8, e72017. [Google Scholar]
- Cheng, C.-Y.; Krishnakumar, V.; Chan, A.P.; Schobel, S.; Town, C.D. Araport11: A complete reannotation of the Arabidopsis thaliana reference genome. Plant J. 2016, 89, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Devic, M.; Guilleminot, J.; Debeaujon, I.; Bechtold, N.; Bensaude, E.; Koornneef, M.; Pelletier, G.; Delseny, M. The BANYULS gene encodes a DFR-like protein and is a marker of early seed coat development. Plant J. 1999, 19, 387–398. [Google Scholar] [CrossRef]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Theologis, A.; Ecker, J.R.; Palm, C.J.; Federspiel, N.A.; Kaul, S.; White, O.; Alonso, J.M.; Altafi, H.; Araujo, R.; Bowman, C.L.; et al. Sequence and analysis of chromosome 1 of the plant Arabidopsis thaliana. Nature 2000, 408, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Koita, H.; Nakatsubo, T.; Hasegawa, K.; Wakabayashi, K.; Takahashi, H.; Umemura, K.; Umezawa, T.; Shimamoto, K. Cinnamoyl-CoA reductase, a key enzyme in lignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proc. Natl. Acad. Sci. USA 2006, 103, 230–235. [Google Scholar] [CrossRef]
- Tanaka, T.; Antonio, B.A.; Kikuchi, S.; Matsumoto, T.; Nagamura, Y.; Numa, H.; Sakai, H.; Wu, J.; Itoh, T.; Sasaki, T.; et al. The Rice Annotation Project Database (RAP-DB): 2008 update. Nucleic Acids Res. 2007, 36, D1028–D1033. [Google Scholar]
- O’Reilly, C.; Shepherd, N.S.; Pereira, A.; Schwarz-Sommer, Z.; Bertram, I.; Robertson, D.S.; Peterson, P.; Saedler, H. Molecular cloning of the a1 locus of Zea mays using the transposable elements En and Mu1. EMBO J. 1985, 4, 877–882. [Google Scholar] [CrossRef]
- Jeong, J.-C.; Jang, S.W.; Kim, T.H.; Kwon, C.H.; Kim, Y.K. Mulberry Fruit (Moris fructus) Extracts Induce Human Glioma Cell Death In Vitro Through ROS-Dependent Mitochondrial Pathway and Inhibits Glioma Tumor Growth In Vivo. Nutr. Cancer 2010, 62, 402–412. [Google Scholar] [CrossRef]
- Johnson, E.T.; Ryu, S.H.; Yi, H.; Shin, B.; Cheong, H.; Choi, G. Alteration of a single amino acid changes the substrate specificity of dihydroflavonol 4-reductase. Plant J. 2001, 25, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Wang, S.; Zhang, F.; Niu, Y.; Wang, D. Cloning and temporal-spatial expression analysis of dfr gene from Scutellaria baicalensis with different colors. Chin. J. Biotechnol. 2021, 37, 1312–1323. [Google Scholar]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Geourjon, C.; Deléage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput. Appl. Biosci. CABIOS 1995, 11, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Investigation on 2008’low temperature and freeze injure on winter rape along Yangtze River. Chin. J. Oil Crop Sci. 2008, 30, 122. [Google Scholar]
- Deng, R.; Zheng, W.; Hua, W.; Ren, R. Rape culture mining in the development of leisure agriculture in China. World Agric. 2016, 6, 64–69. [Google Scholar]
- Yin, N.; Wang, S.; Jia, L.; Zhu, M.; Yang, J.; Zhou, B.; Yin, J.; Lu, K.; Wang, R.; Li, J.; et al. Identification and characterization of major constituents in different-colored rapeseed petals by UPLC-HESI-MS/MS. J. Agric. Food Chem. 2019, 67, 11053–11065. [Google Scholar] [CrossRef]
- Tanaka, Y. Flower colour and cytochromes P450. Phytochem. Rev. 2006, 5, 283–291. [Google Scholar] [CrossRef]
- Yan, F.; Di, S.; Rojas Rodas, F.; Rodriguez Torrico, T.; Murai, Y.; Iwashina, T.; Anai, T.; Takahashi, R. Allelic variation of soybean flower color gene W4 encoding dihydroflavonol 4-reductase 2. BMC Plant Biol. 2014, 14, 58. [Google Scholar] [CrossRef]
- Zhao, D.; Tao, J.; Han, C.; Ge, J. Flower color diversity revealed by differential expression of flavonoid biosynthetic genes and flavonoid accumulation in herbaceous peony (Paeonia lactiflora Pall.). Mol. Biol. Rep. 2012, 39, 11263–11275. [Google Scholar] [CrossRef]
- Nakatsuka, A.; Izumi, Y.; Yamagishi, M. Spatial and temporal expression of chalcone synthase and dihydroflavonol 4-reductase genes in the Asiatic hybrid lily. Plant Sci. 2003, 165, 759–767. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Nishihara, M.; Mishiba, K.-i.; Yamamura, S. Temporal expression of flavonoid biosynthesis-related genes regulates flower pigmentation in gentian plants. Plant Sci. 2005, 168, 1309–1318. [Google Scholar] [CrossRef]
- Parkin, I.A.P.; Gulden, S.; Sharpe, A.G.; Lukens, L.; Trick, M.; Osborn, T.C.; Lydiate, D.J. Segmental Structure of the Brassica napus Genome Based on Comparative Analysis With Arabidopsis thaliana. Genetics 2005, 171, 765–781. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, E.; Gusmaroli, G.; Allegra, D.; Galbiati, M.; Wade, H.K.; Jenkins, G.I.; Tonelli, C. Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J. Plant Physiol. 2008, 165, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.W.; Lewis, D.H.; Zhang, H.; Irving, L.J.; Jameson, P.E.; Davies, K.M. Light-induced vegetative anthocyanin pigmentation in Petunia. J. Exp. Bot. 2009, 60, 2191–2202. [Google Scholar] [CrossRef]
- Quattrocchio, F.M.; Verweij, W.; Kroon, A.I.d.; Spelt, C.E.; Mol, J.N.; Koes, R. PH4 of Petunia Is an R2R3 MYB Protein That Activates Vacuolar Acidification through Interactions with Basic-Helix-Loop-Helix Transcription Factors of the Anthocyanin Pathway. Plant Cell Online 2006, 18, 1274–1291. [Google Scholar] [CrossRef]
- Shao, W.L.; Li, Y.L.; Gao, S.; Li, J.M.; Liang, Z.S. Effects of light intensity on the fruit coloration and anthocyanian biosynthesis in Fragaria × ananassa Duch. Bull. Bot. Res. 2018, 38, 661–668. [Google Scholar]
- Huang, K.L.; Miyajima, I.; Okubo, H. Effects of temperature and shade treatment on flower colors and characteristics in newly established reddish-purple tuberose (Polianthes). J. Fac. Agric. Kyushu Univ. 2000, 45, 57–63. [Google Scholar] [CrossRef]
- Xie, D.; Sharma, S.; Wright, E.C.; Wang, Z.; Dixon, R.A. Metabolic engineering of proanthocyanidins through co-expression of anthocyanidin reductase and the PAP1 MYB transcription factor. Plant J. 2006, 45, 895–907. [Google Scholar] [CrossRef]
- Ramsay, N.A.; Glover, B.J. MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005, 10, 63–70. [Google Scholar] [CrossRef]
- Chanoca, A.; Kovinich, N.; Burkel, B.M.; Stecha, S.; Bohorquez-Restrepo, A.; Ueda, T.; Eliceiri, K.W.; Grotewold, E.; Otegui, M.S. Anthocyanin Vacuolar Inclusions Form by a Microautophagy Mechanism. Plant Cell 2015, 27, 2545–2559. [Google Scholar] [CrossRef]
- Holton, T.A.; Cornish, E.C. Genetics and Biochemistry of Anthocyanin Biosynthesis. Plant Cell 1995, 7, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Mol, J.N.; Grotewold, E.; Koes, R. How genes paint flowers and seeds. Trends Plant Sci. 1998, 3, 212–217. [Google Scholar] [CrossRef]
- Yoshida, K.; Iwasaka, R.; Shimada, N.; Ayabe, S.-i.; Aoki, T.; Sakuta, M. Transcriptional control of the dihydroflavonol 4-reductase multigene family in Lotus japonicus. J. Plant Res. 2010, 123, 801–805. [Google Scholar] [CrossRef]
- Dogan, T. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2018, 47, D506–D515. [Google Scholar]
- Garg, V.K.; Avashthi, H.; Tiwari, A.; Jain, P.A.; Ramkete, P.W.; Kayastha, A.M.; Singh, V.K. MFPPI–Multi FASTA ProtParam Interface. Bioinformation 2016, 12, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Ye, Z.; Yuan, Z.; Xu, H.; Pan, L.; Chen, J.; Gatera, A.; Uzair, M.; Xu, D. Genome-Wide Identification and Expression Analysis of Kinesin Family in Barley (Hordeum vulgare). Genes 2022, 13, 2376. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools-an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Yang, M.; Derbyshire, M.K.; Yamashita, R.A.; Marchler-Bauer, A. NCBI’s Conserved Domain Database and Tools for Protein Domain Analysis. Curr. Protoc. Bioinform. 2019, 69, e90. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, A.; Saqib, S.; Huang, H.-j.; Zaman, W.; Lü, S.; Zhao, H. Genome-wide comparative analysis of long-chain acyl-CoA synthetases (LACSs) gene family: A focus on identification, evolution and expression profiling related to lipid synthesis. Plant Physiol. Biochem. 2021, 161, 1–11. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Peer, Y.V.d.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Letunić, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Li, T.; Luo, C.; Huang, H.; Ruan, Y.; Li, X.; Niu, Y.; Fan, Y.; Sun, W.; Zhang, K.; et al. BrassicaEDB: A Gene Expression Database for Brassica Crops. Int. J. Mol. Sci. 2020, 21, 5831. [Google Scholar] [CrossRef]
- Mehrtens, F.; Kranz, H.D.; Bednarek, P.; Weisshaar, B. The Arabidopsis Transcription Factor MYB12 Is a Flavonol-Specific Regulator of Phenylpropanoid Biosynthesis1. Plant Physiol. 2005, 138, 1083–1096. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Gene | Len (aa) | MW (kD) | pI | II | GR | SL |
|---|---|---|---|---|---|---|
| AtDFR1 | 344 | 37.49 | 6.13 | 32.17 | −0.26 | Cytoplasm |
| AtDFR2 | 340 | 37.91 | 5.89 | 26.2 | −0.176 | Nucleus |
| AtDFR3 | 321 | 35.67 | 6.02 | 39.55 | −0.159 | Nucleus |
| AtDFR4 | 332 | 36.62 | 6.4 | 25.89 | −0.166 | Cytoplasm |
| AtDFR5 | 326 | 36.46 | 5.88 | 38.22 | −0.122 | Chloroplast |
| AtDFR6 | 382 | 42.77 | 5.43 | 36.85 | −0.225 | Chloroplast |
| BnDFR1 | 319 | 35.74 | 5.95 | 36.76 | −0.093 | Chloroplast |
| BnDFR2 | 338 | 37.7 | 5.07 | 37.27 | −0.203 | Cytoplasm |
| BnDFR3 | 331 | 36.51 | 6.71 | 30.04 | −0.193 | Cytoplasm |
| BnDFR4 | 345 | 38.26 | 5.96 | 34.92 | −0.171 | Nucleus |
| BnDFR5 | 223 | 25.08 | 8.76 | 41.34 | −0.526 | Mitochondrion |
| BnDFR6 | 276 | 30.04 | 5.35 | 28.51 | −0.048 | Cytoplasm |
| BnDFR7 | 321 | 35.68 | 6.52 | 36.34 | −0.168 | Nucleus |
| BnDFR8 | 332 | 36.49 | 6.19 | 30.6 | −0.195 | Cytoplasm |
| BnDFR9 | 317 | 35.54 | 6.27 | 35.05 | −0.14 | Chloroplast |
| BnDFR10 | 385 | 42.89 | 5.54 | 37.87 | −0.255 | Chloroplast |
| BnDFR11 | 235 | 26.61 | 8.79 | 38.4 | −0.206 | Nucleus |
| BnDFR12 | 321 | 35.82 | 5.5 | 37.27 | −0.172 | Chloroplast |
| BnDFR13 | 343 | 37.51 | 5.53 | 33.85 | −0.271 | Cytoplasm |
| BnDFR14 | 321 | 35.73 | 6.52 | 37.77 | −0.162 | Nucleus |
| BnDFR15 | 319 | 35.62 | 5.96 | 36.23 | −0.127 | Chloroplast |
| BnDFR16 | 338 | 37.68 | 4.93 | 37.03 | −0.164 | Cytoplasm |
| BnDFR17 | 331 | 36.42 | 6.66 | 32.36 | −0.182 | Cytoplasm |
| BnDFR18 | 317 | 35.46 | 5.55 | 43.06 | −0.138 | Chloroplast |
| BnDFR19 | 342 | 37.87 | 5.8 | 34.06 | −0.156 | Nucleus |
| BnDFR20 | 343 | 37.56 | 5.79 | 34.7 | −0.29 | Cytoplasm |
| BnDFR21 | 321 | 35.85 | 5.5 | 36.64 | −0.171 | Chloroplast |
| BnDFR22 | 305 | 34.1 | 5.47 | 34.04 | −0.125 | Cytoplasm |
| BnDFR23 | 321 | 35.79 | 6.52 | 37.84 | −0.219 | Nucleus |
| BnDFR24 | 332 | 36.57 | 6.26 | 31.35 | −0.202 | Cytoplasm |
| BnDFR25 | 340 | 37.22 | 5.53 | 32.91 | −0.254 | Cytoplasm |
| BnDFR26 | 385 | 42.93 | 5.64 | 37.07 | −0.256 | Chloroplast |
| Secondary Structures | 3D Structures | |||||
|---|---|---|---|---|---|---|
| Gene | α-Helix | Extended Strand | β-Turn | Random Coil | Confidence | Coverage |
| BnDFR1 | 40.44 | 15.32 | 7.52 | 36.68 | 100.0% | 95.0% |
| BnDFR2 | 42.9 | 14.79 | 7.4 | 34.91 | 100.0% | 92.0% |
| BnDFR3 | 44.11 | 15.41 | 6.65 | 33.84 | 100.0% | 96.0% |
| BnDFR4 | 39.71 | 15.94 | 7.25 | 37.1 | 100.0% | 90.0% |
| BnDFR5 | 46.19 | 12.11 | 4.93 | 36.77 | 99.8% | 81.0% |
| BnDFR6 | 48.19 | 17.03 | 5.43 | 29.35 | 100.0% | 97.0% |
| BnDFR7 | 42.06 | 13.08 | 5.92 | 38.94 | 100.0% | 97.0% |
| BnDFR8 | 45.78 | 15.36 | 5.72 | 33.13 | 100.0% | 96.0% |
| BnDFR9 | 43.22 | 15.77 | 7.89 | 33.12 | 100.0% | 96.0% |
| BnDFR10 | 40.52 | 11.95 | 7.53 | 40 | 100.0% | 83.0% |
| BnDFR11 | 40.85 | 12.77 | 5.11 | 41.28 | 99.9% | 97.0% |
| BnDFR12 | 41.74 | 14.95 | 7.17 | 36.14 | 100.0% | 97.0% |
| BnDFR13 | 42.27 | 14.29 | 5.25 | 38.19 | 100.0% | 92.0% |
| BnDFR14 | 39.88 | 14.64 | 6.54 | 38.94 | 100.0% | 95.0% |
| BnDFR15 | 42.63 | 14.73 | 7.84 | 34.8 | 100.0% | 95.0% |
| BnDFR16 | 42.6 | 14.2 | 7.1 | 36.09 | 100.0% | 92.0% |
| BnDFR17 | 45.92 | 15.71 | 5.44 | 32.93 | 100.0% | 96.0% |
| BnDFR18 | 41.01 | 16.72 | 7.89 | 34.38 | 100.0% | 96.0% |
| BnDFR19 | 41.52 | 14.33 | 7.31 | 36.84 | 100.0% | 91.0% |
| BnDFR20 | 43.73 | 13.12 | 5.25 | 37.9 | 100.0% | 92.0% |
| BnDFR21 | 41.74 | 15.26 | 5.92 | 39.07 | 100.0% | 97.0% |
| BnDFR22 | 41.31 | 16.07 | 7.21 | 35.41 | 100.0% | 97.0% |
| BnDFR23 | 43.3 | 14.95 | 6.85 | 34.89 | 100.0% | 97.0% |
| BnDFR24 | 45.78 | 15.96 | 6.02 | 32.23 | 100.0% | 95.0% |
| BnDFR25 | 45 | 12.94 | 5 | 37.06 | 100.0% | 94.0% |
| BnDFR26 | 42.6 | 13.25 | 6.23 | 37.92 | 100.0% | 83.0% |
| Gene Name | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| BnActin7 | AACCTTCTCTCAAGTCTCTGTG | CCAGAATCATCACAAAGCATCC |
| BnDFR3 | GTGCTGATCTTCTCGACTATGA | TCACGGTTAGGGTTCATGTAAA |
| BnDFR6 | ATATACTTTCGACCGCTGGAAA | TCCAAGAAGCTATGTATCCACC |
| BnDFR8 | CGATAAGAATCCAAGGGCAAAG | TGATGGTCGTATCGTGTTCTAG |
| BnDFR12 | CATTTGAGACCAAGTGCAGTAG | GCCAAGTTCACGTATCTTTGTT |
| BnDFR13 | AATTGGTGCAGTTTACATGGAC | GGTGTTTTTGCAGAACTCAAGA |
| BnDFR17 | AGTATCCGCTTCCTATCAAGTG | GCTCTTGACAGATTCGTAGAGA |
| BnDFR20 | GTTAAGAGCTTGCAAGAGAAGG | ATCGCTGAGGATGATACTTCAG |
| BnDFR21 | GCTAAAGATTTTCGAAGCCGAT | TTCTCCAGATCATTGTTGTCCA |
| BnDFR24 | TTTACATGAACCCTAACCGTCA | TAAGGTTAGCATAGGTCTTGGC |
| BnDFR25 | CAGGACTACGATGCTCTTAAGT | AATTACAAACTTGGCTCCGTTC |
| BnDFR26 | CTGCCAAGGGACGTTATATTTG | CAAACGTTGAAGGCACGTTATA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, X.; Zheng, W.; Hu, J.; Ma, J.; Sun, M.; Li, Y.; Liu, N.; Chen, T.; Wang, M.; Wang, L.; et al. Identification and Expression Analysis of DFR Gene Family in Brassica napus L. Plants 2023, 12, 2583. https://doi.org/10.3390/plants12132583
Qian X, Zheng W, Hu J, Ma J, Sun M, Li Y, Liu N, Chen T, Wang M, Wang L, et al. Identification and Expression Analysis of DFR Gene Family in Brassica napus L. Plants. 2023; 12(13):2583. https://doi.org/10.3390/plants12132583
Chicago/Turabian StyleQian, Xingzhi, Wenyin Zheng, Jian Hu, Jinxu Ma, Mengyuan Sun, Yong Li, Nian Liu, Tianhua Chen, Meiqi Wang, Ling Wang, and et al. 2023. "Identification and Expression Analysis of DFR Gene Family in Brassica napus L." Plants 12, no. 13: 2583. https://doi.org/10.3390/plants12132583
APA StyleQian, X., Zheng, W., Hu, J., Ma, J., Sun, M., Li, Y., Liu, N., Chen, T., Wang, M., Wang, L., Hou, X., Cai, Q., Ye, Z., Zhang, F., & Zhu, Z. (2023). Identification and Expression Analysis of DFR Gene Family in Brassica napus L. Plants, 12(13), 2583. https://doi.org/10.3390/plants12132583






