Local Action of Increased Pressure Induces Hyperpolarization Electrical Signals and Influences Photosynthetic Light Reactions in Wheat Plants
Abstract
1. Introduction
2. Results
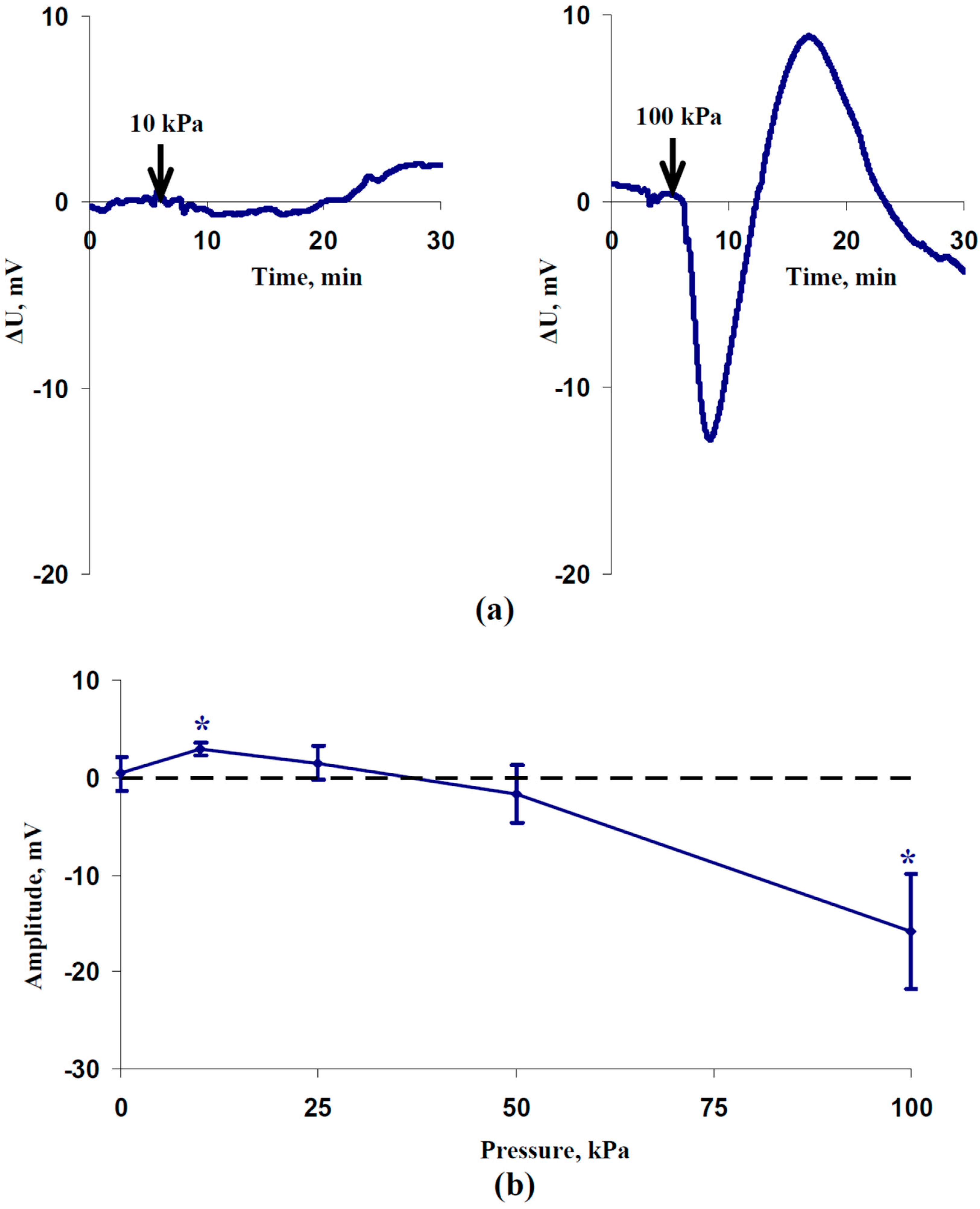
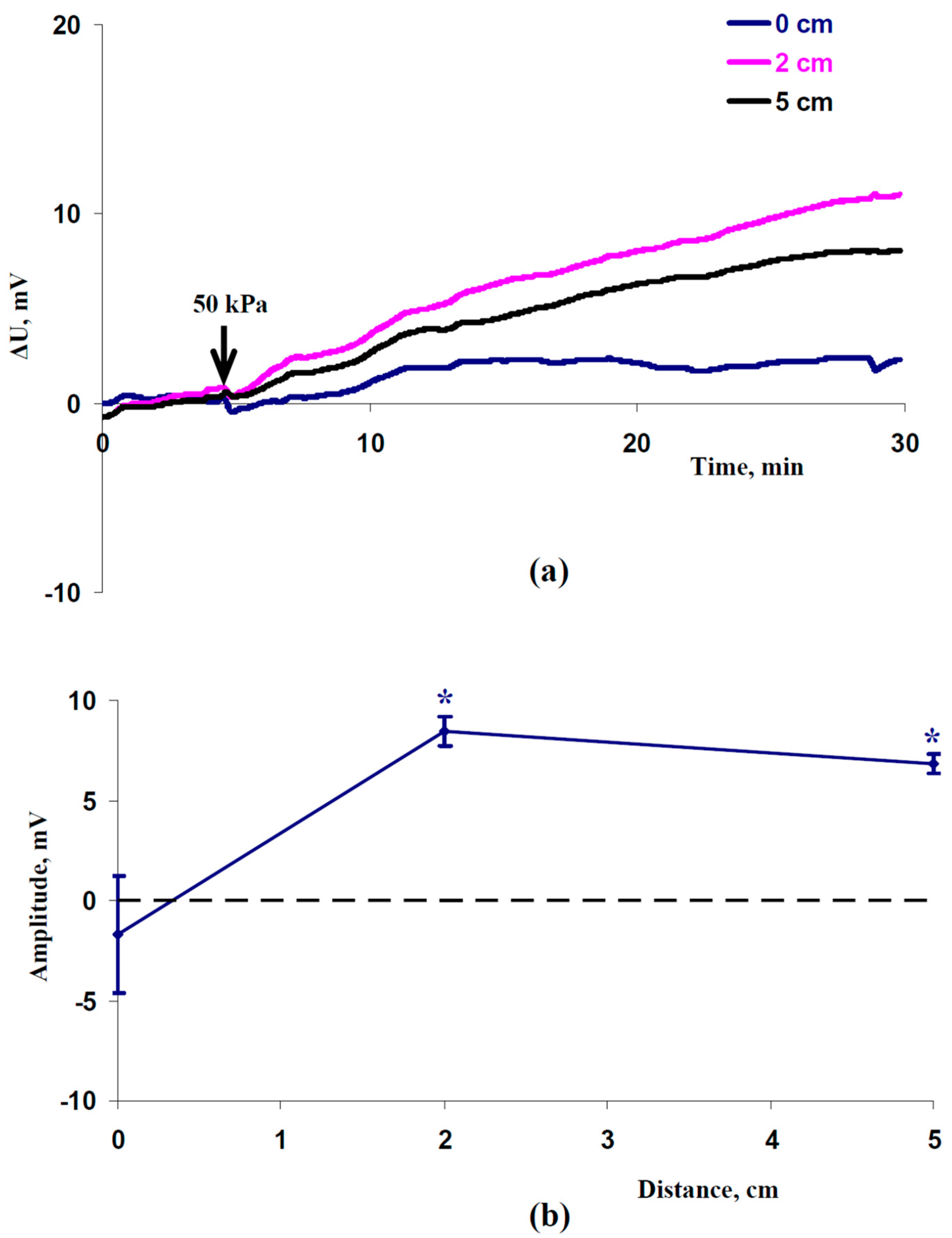
2.1. Induction of Electrical Signals by the Local Action of Increased Pressure
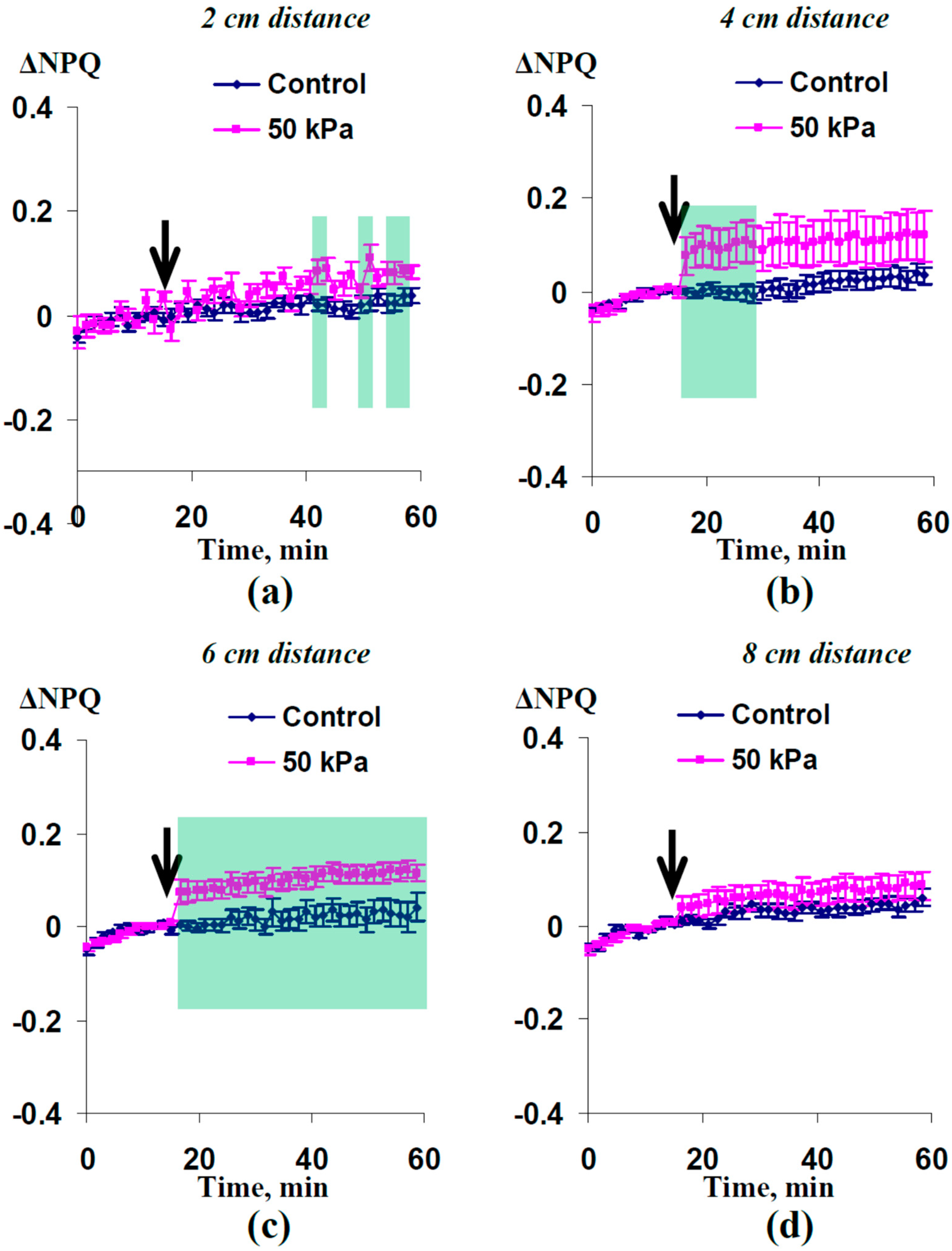
2.2. Induction of Photosynthetic Changes by the Local Action of Increased Pressure
3. Discussion
4. Materials and Methods
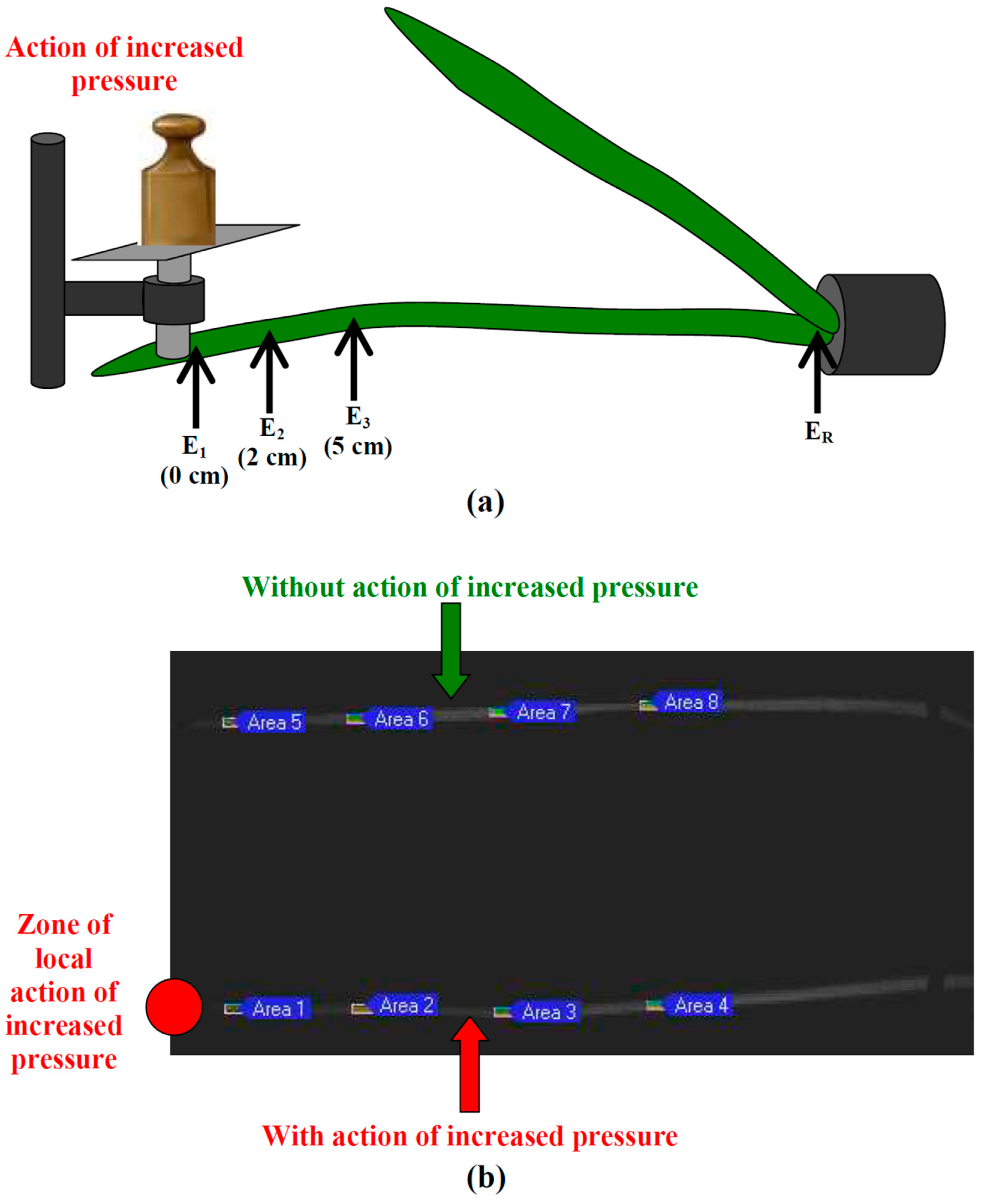
4.1. Plant Material and Local Action of Increased Pressure
4.2. Electrophysiological Measurements
4.3. Measurements of Parameters of Photosynthetic Light Reactions
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sukhov, V.; Sukhova, E.; Vodeneev, V. Long-distance electrical signals as a link between the local action of stressors and the systemic physiological responses in higher plants. Progr. Biophys. Mol. Biol. 2019, 146, 63–84. [Google Scholar] [CrossRef] [PubMed]
- Sukhova, E.; Sukhov, V. Electrical signals in systemic adaptive response of higher plants: Integration through separation. Bioelectricity 2023, 5, 126–131. [Google Scholar] [CrossRef]
- Fromm, J.; Lautner, S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 2007, 30, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Gallé, A.; Lautner, S.; Flexas, J.; Fromm, J. Environmental stimuli and physiological responses: The current view on electrical signaling. Environ. Exp. Bot. 2015, 114, 15–21. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Lewandowska, M.; Karpiński, S. Electrical signaling, photosynthesis and systemic acquired acclimation. Front. Physiol. 2017, 8, 684. [Google Scholar] [CrossRef]
- Zimmermann, M.R.; Maischak, H.; Mithöfer, A.; Boland, W.; Felle, H.H. System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant. Physiol. 2009, 149, 1593–1600. [Google Scholar] [CrossRef]
- Zimmermann, M.R.; Mithöfer, A.; Will, T.; Felle, H.H.; Furch, A.C. Herbivore-triggered electrophysiological reactions: Candidates for systemic signals in higher plants and the challenge of their identification. Plant Physiol. 2016, 170, 2407–2419. [Google Scholar] [CrossRef]
- Yudina, L.; Sukhova, E.; Popova, A.; Zolin, Y.; Abasheva, K.; Grebneva, K.; Sukhov, V. Local action of moderate heating and illumination induces propagation of hyperpolarization electrical signals in wheat plants. Front. Sustain. Food Syst. 2023, 6, 1062449. [Google Scholar]
- Sukhova, E.; Sukhov, V. Electrical signals, plant tolerance to actions of stressors, and programmed cell death: Is interaction possible? Plants 2021, 10, 1704. [Google Scholar] [CrossRef]
- Wildon, D.C.; Thain, J.F.; Minchin, P.E.H.; Gubb, I.R.; Reilly, A.J.; Skipper, Y.D.; Doherty, H.M.; O’Donnell, P.J.; Bowles, D. Electrical signalling and systemic proteinase inhibitor Induction in the wounded plant. Nature 1992, 360, 62–65. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Chauvin, A.; Pascaud, F.; Kellenberger, S.; Farmer, E.E. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 2013, 500, 422–426. [Google Scholar] [CrossRef]
- Grams, T.E.; Lautner, S.; Felle, H.H.; Matyssek, R.; Fromm, J. Heat-induced electrical signals affect cytoplasmic and apoplastic pH as well as photosynthesis during propagation through the maize leaf. Plant Cell Environ. 2009, 32, 319–326. [Google Scholar] [CrossRef]
- Gallé, A.; Lautner, S.; Flexas, J.; Ribas-Carbo, M.; Hanson, D.; Roesgen, J.; Fromm, J. Photosynthetic responses of soybean (Glycine max L.) to heat-induced electrical signalling are predominantly governed by modifications of mesophyll conductance for CO2. Plant Cell Environ. 2013, 36, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Białasek, M.; Górecka, M.; Mittler, R.; Karpiński, S. Evidence for the Involvement of electrical, calcium and ROS signaling in the systemic regulation of non-photochemical quenching and photosynthesis. Plant Cell Physiol. 2017, 58, 207–215. [Google Scholar] [CrossRef]
- Filek, M.; Kościelniak, J. The effect of wounding the roots by high temperature on the respiration rate of the shoot and propagation of electric signal in horse bean seedlings (Vicia faba L. minor). Plant Sci. 1997, 123, 39–46. [Google Scholar] [CrossRef]
- Pavlovič, A.; Slováková, L.; Pandolfi, C.; Mancuso, S. On the mechanism underlying photosynthetic limitation upon trigger hair irritation in the carnivorous plant Venus flytrap (Dionaea muscipula Ellis). J. Exp. Bot. 2011, 62, 1991–2000. [Google Scholar] [CrossRef]
- Lautner, S.; Stummer, M.; Matyssek, R.; Fromm, J.; Grams, T.E.E. Involvement of respiratory processes in the transient knockout of net CO2 uptake in Mimosa pudica upon heat stimulation. Plant Cell Environ. 2014, 37, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Hlavácková, V.; Krchnák, P.; Naus, J.; Novák, O.; Spundová, M.; Strnad, M. Electrical and chemical signals involved in short-term systemic photosynthetic responses of tobacco plants to local burning. Planta 2006, 225, 235–244. [Google Scholar] [CrossRef]
- Hlavinka, J.; Nožková-Hlaváčková, V.; Floková, K.; Novák, O.; Nauš, J. Jasmonic acid accumulation and systemic photo-synthetic and electrical changes in locally burned wild type tomato, ABA-deficient sitiens mutants and sitiens pre-treated by ABA. Plant Physiol. Biochem. 2012, 54, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Gao, Y.Q.; Lenzoni, G.; Wolfender, J.L.; Wu, Q. Wound- and mechanostimulated electrical signals control hormone responses. New Phytol. 2020, 227, 1037–1050. [Google Scholar] [CrossRef]
- Kaiser, H.; Grams, T.E. Rapid hydropassive opening and subsequent active stomatal closure follow heat-induced electrical signals in Mimosa pudica. J. Exp. Bot. 2006, 57, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Grams, T.E.; Koziolek, C.; Lautner, S.; Matyssek, R.; Fromm, J. Distinct roles of electric and hydraulic signals on the reaction of leaf gas exchange upon re-irrigation in Zea mays L. Plant Cell Environ. 2007, 30, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Vuralhan-Eckert, J.; Lautner, S.; Fromm, J. Effect of simultaneously induced environmental stimuli on electrical signalling and gas exchange in maize plants. J. Plant Physiol. 2018, 223, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Furch, A.C.; van Bel, A.J.; Fricker, M.D.; Felle, H.H.; Fuchs, M.; Hafke, J.B. Sieve element Ca2+ channels as relay stations between remote stimuli and sieve tube occlusion in Vicia faba. Plant Cell 2009, 21, 2118–2132. [Google Scholar] [CrossRef]
- Furch, A.C.; Zimmermann, M.R.; Will, T.; Hafke, J.B.; van Bel, A.J. Remote-controlled stop of phloem mass flow by biphasic occlusion in Cucurbita maxima. J. Exp. Bot. 2010, 61, 3697–3708. [Google Scholar] [CrossRef]
- van Bel, A.J.; Furch, A.C.; Will, T.; Buxa, S.V.; Musetti, R.; Hafke, J.B. Spread the news: Systemic dissemination and local impact of Ca2+ signals along the phloem pathway. J. Exp. Bot. 2014, 65, 1761–1787. [Google Scholar] [CrossRef]
- Trebacz, K.; Dziubinska, H.; Krol, E. Electrical signals in long-distance communication in plants. In Communication in Plants. Neuronal Aspects of Plant Life; Baluška, F., Mancuso, S., Volkmann, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 277–290. [Google Scholar]
- Felle, H.H.; Zimmermann, M.R. Systemic signaling in barley through action potentials. Planta 2007, 226, 203–214. [Google Scholar] [CrossRef]
- Sukhova, E.; Akinchits, E.; Sukhov, V. Mathematical models of electrical activity in plants. J. Membr. Biol. 2017, 250, 407–423. [Google Scholar] [CrossRef]
- Stahlberg, R.; Cleland, R.E.; van Volkenburgh, E. Slow wave potentials—A propagating electrical signal unique to higher plants. In Communication in Plants. Neuronal Aspects of Plant Life; Baluška, F., Mancuso, S., Volkmann, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 291–308. [Google Scholar]
- Yudina, L.; Gromova, E.; Grinberg, M.; Popova, A.; Sukhova, E.; Sukhov, V. Influence of burning-induced electrical signals on photosynthesis in pea can be modified by soil water shortage. Plants 2022, 11, 534. [Google Scholar] [CrossRef]
- Yudina, L.; Sukhova, E.; Popova, A.; Zolin, Y.; Abasheva, K.; Grebneva, K.; Sukhov, V. Hyperpolarization electrical signals induced by local action of moderate heating influence photosynthetic light reactions in wheat plants. Front. Plant Sci. 2023, 14, 1153731. [Google Scholar] [CrossRef]
- Stahlberg, R.; Cosgrove, D.J. Induction and ionic basis of slow wave potentials in seedlings of Pisum sativum L. Planta 1996, 200, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Stahlberg, R.; Cosgrove, D.J. The propagation of slow wave potentials in pea epicotyls. Plant Physiol. 1997, 113, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, S. Hydraulic and electrical transmission of wound-induced signals in Vitis vinifera. Aust. J. Plant Physiol. 1999, 26, 55–61. [Google Scholar] [CrossRef]
- Sukhova, E.; Akinchits, E.; Gudkov, S.V.; Pishchalnikov, R.Y.; Vodeneev, V.; Sukhov, V. A theoretical analysis of relations between pressure changes along xylem vessels and propagation of variation potential in higher plants. Plants 2021, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Kitamura, S.; Masaki, N. Activation of the root xylem proton pump by hydraulic signals from leaves under suppressed transpiration. J. Plant Res. 2022, 135, 211–322. [Google Scholar] [CrossRef]
- Lew, R.R. Calcium activates an electrogenic proton pump in neurospora plasma membrane. Plant Physiol. 1989, 91, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Y.; Ma, L.; Yang, Z.; Dong, Q.; Li, Q.; Ni, X.; Kudla, J.; Song, C.; Guo, Y. The Ca2+ sensor SCaBP3/CBL7 modulates plasma membrane H+-ATPase activity and promotes alkali tolerance in Arabidopsis. Plant Cell 2019, 31, 1367–1384. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, C.; Xue, Y.; Liu, X.; Chen, S.; Song, C.; Yang, Y.; Guo, Y. Calcium-activated 14-3-3 proteins as a molecular switch in salt stress tolerance. Nat. Commun. 2019, 10, 1199. [Google Scholar] [CrossRef]
- Grabov, A.; Blatt, M.R. A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol. 1999, 119, 277–288. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Wu, W.H.; Wang, Y. Electrophysiological identification and activity analyses of plasma membrane K+ channels in maize guard cells. Plant Cell Physiol. 2019, 60, 765–777. [Google Scholar] [CrossRef]
- Lautner, S.; Grams, T.E.E.; Matyssek, R.; Fromm, J. Characteristics of electrical signals in poplar and responses in photosynthesis. Plant Physiol. 2005, 138, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Sukhov, V. Electrical signals as mechanism of photosynthesis regulation in plants. Photosynth. Res. 2016, 130, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yudina, L.; Popova, A.; Zolin, Y.; Sukhova, E.; Sukhov, V. Local Action of Increased Pressure Induces Hyperpolarization Electrical Signals and Influences Photosynthetic Light Reactions in Wheat Plants. Plants 2023, 12, 2570. https://doi.org/10.3390/plants12132570
Yudina L, Popova A, Zolin Y, Sukhova E, Sukhov V. Local Action of Increased Pressure Induces Hyperpolarization Electrical Signals and Influences Photosynthetic Light Reactions in Wheat Plants. Plants. 2023; 12(13):2570. https://doi.org/10.3390/plants12132570
Chicago/Turabian StyleYudina, Lyubov, Alyona Popova, Yuriy Zolin, Ekaterina Sukhova, and Vladimir Sukhov. 2023. "Local Action of Increased Pressure Induces Hyperpolarization Electrical Signals and Influences Photosynthetic Light Reactions in Wheat Plants" Plants 12, no. 13: 2570. https://doi.org/10.3390/plants12132570
APA StyleYudina, L., Popova, A., Zolin, Y., Sukhova, E., & Sukhov, V. (2023). Local Action of Increased Pressure Induces Hyperpolarization Electrical Signals and Influences Photosynthetic Light Reactions in Wheat Plants. Plants, 12(13), 2570. https://doi.org/10.3390/plants12132570





