Interference Effects of Commercial Persistent Luminescence Materials on Rice Germination and Seedling Growth
Abstract
1. Introduction
2. Results and Discussion
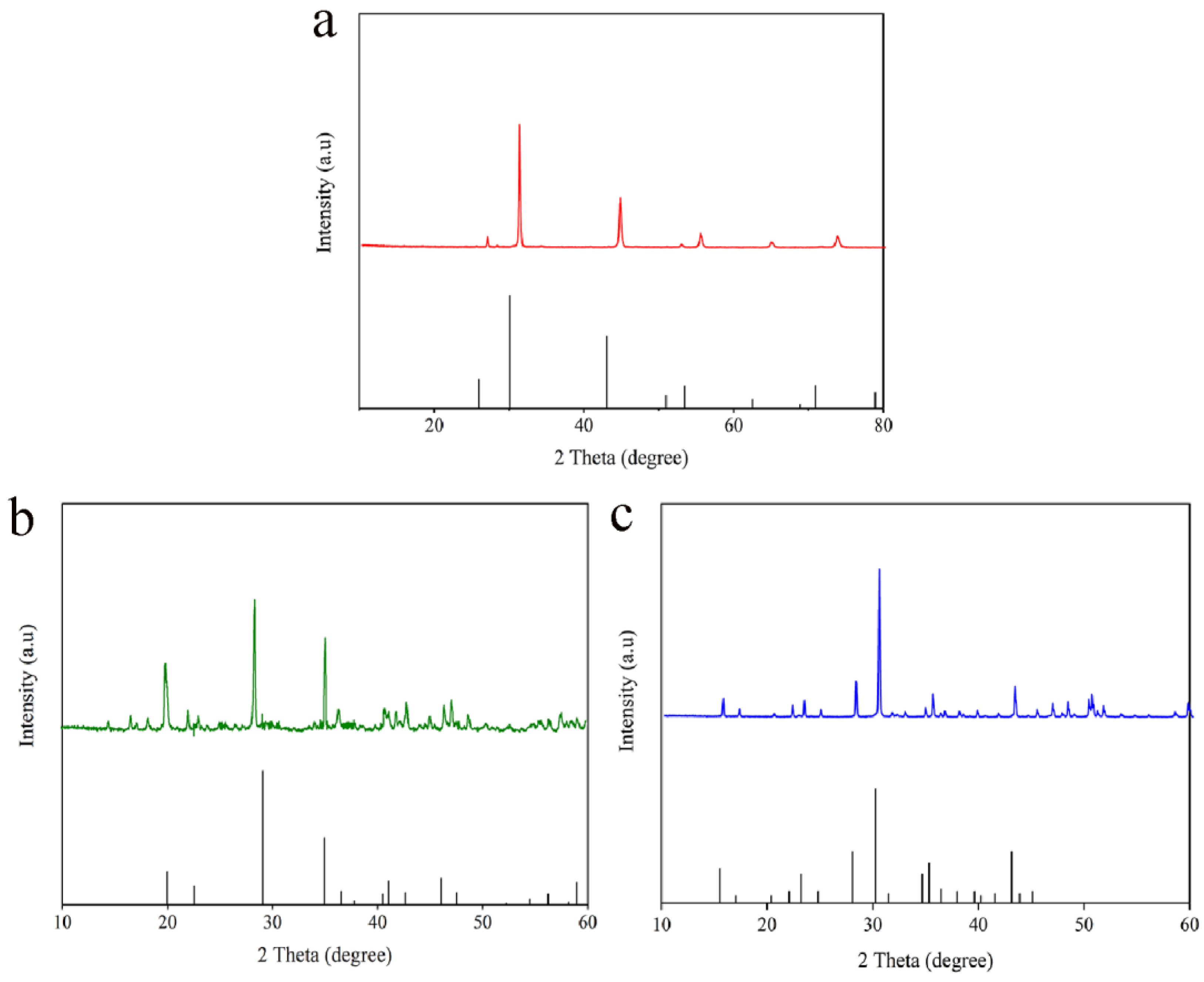
2.1. Analysis of Physical Phase States
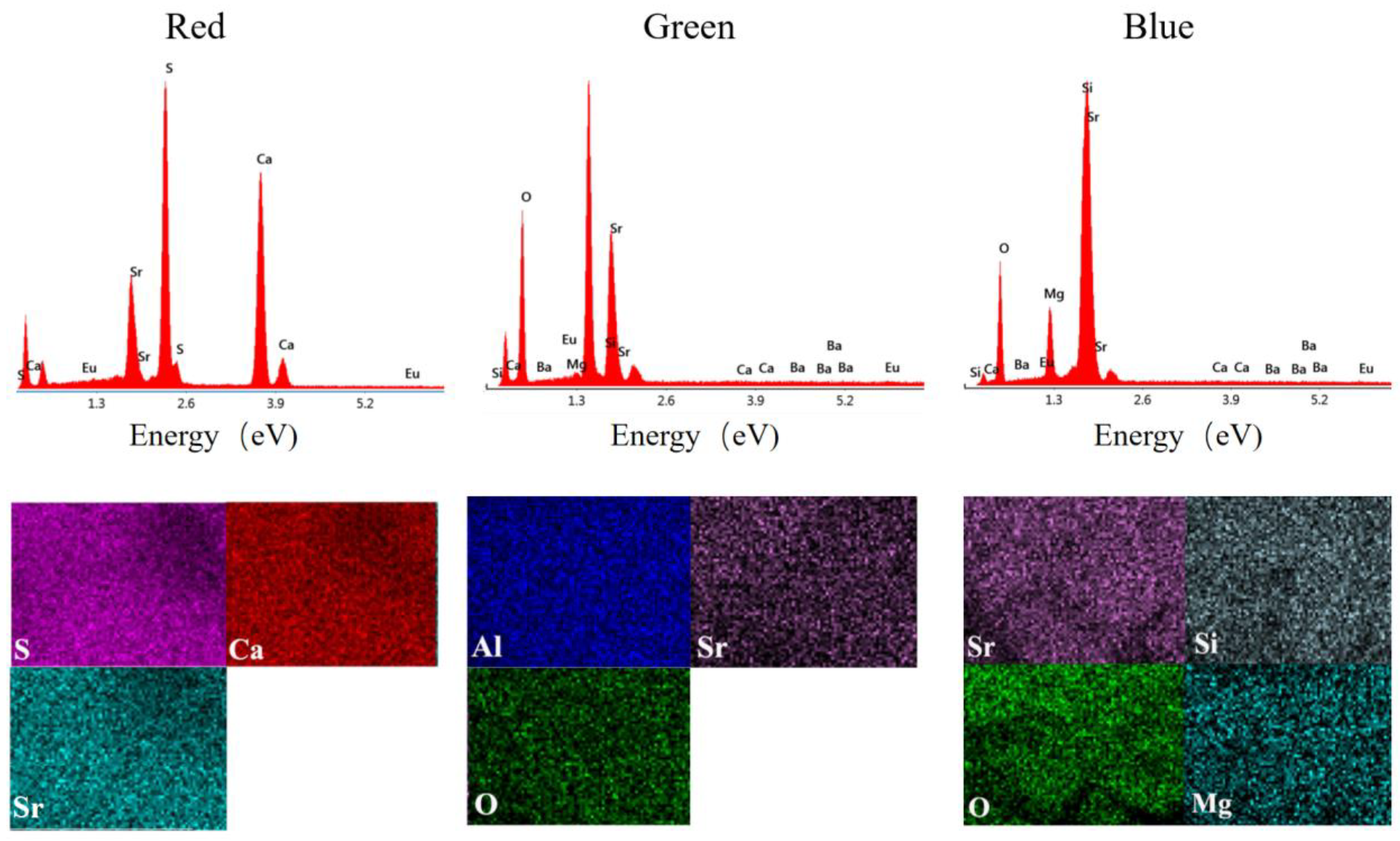
2.2. Morphological and Granulometric Analysis
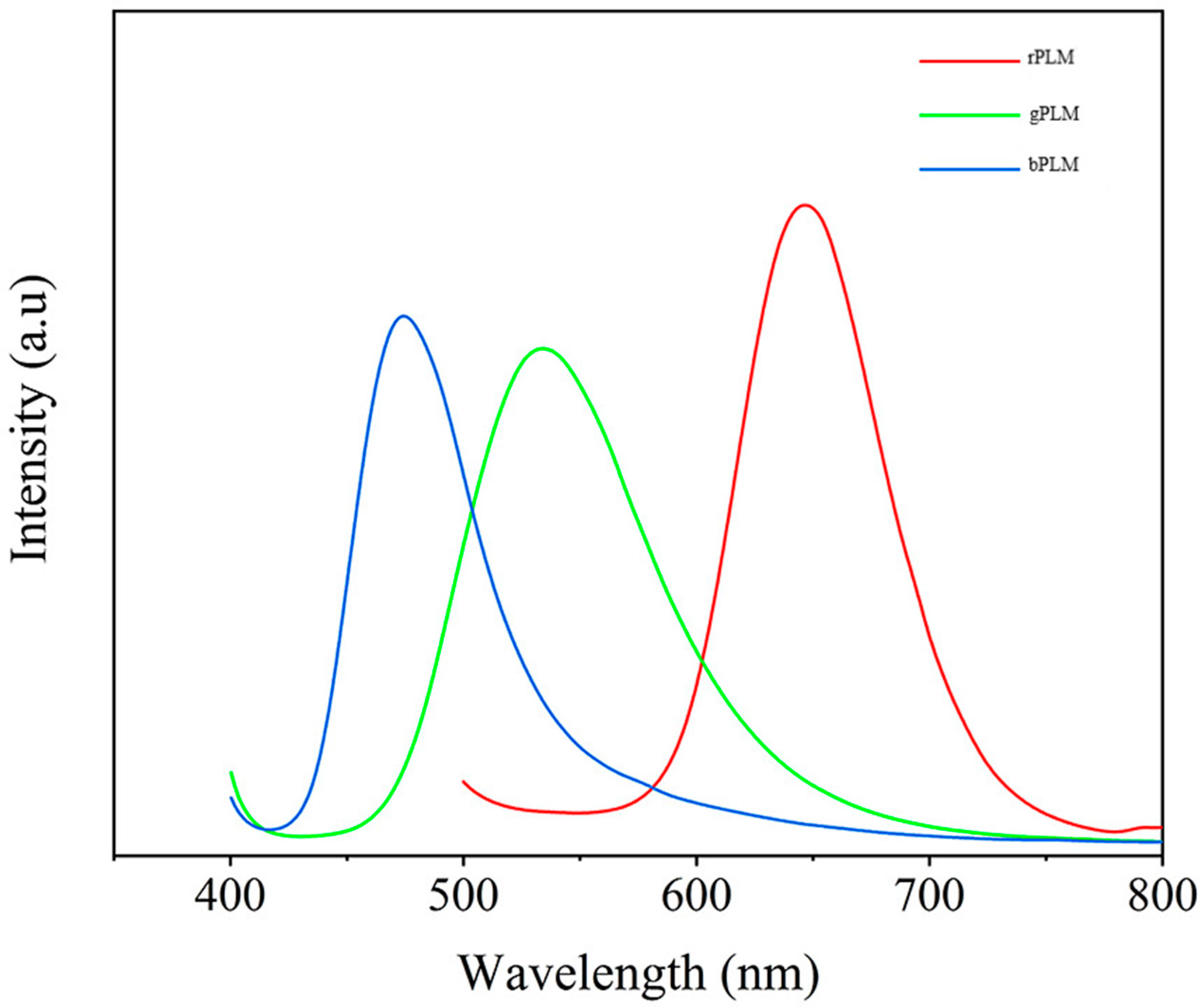
2.3. Spectral Analysis
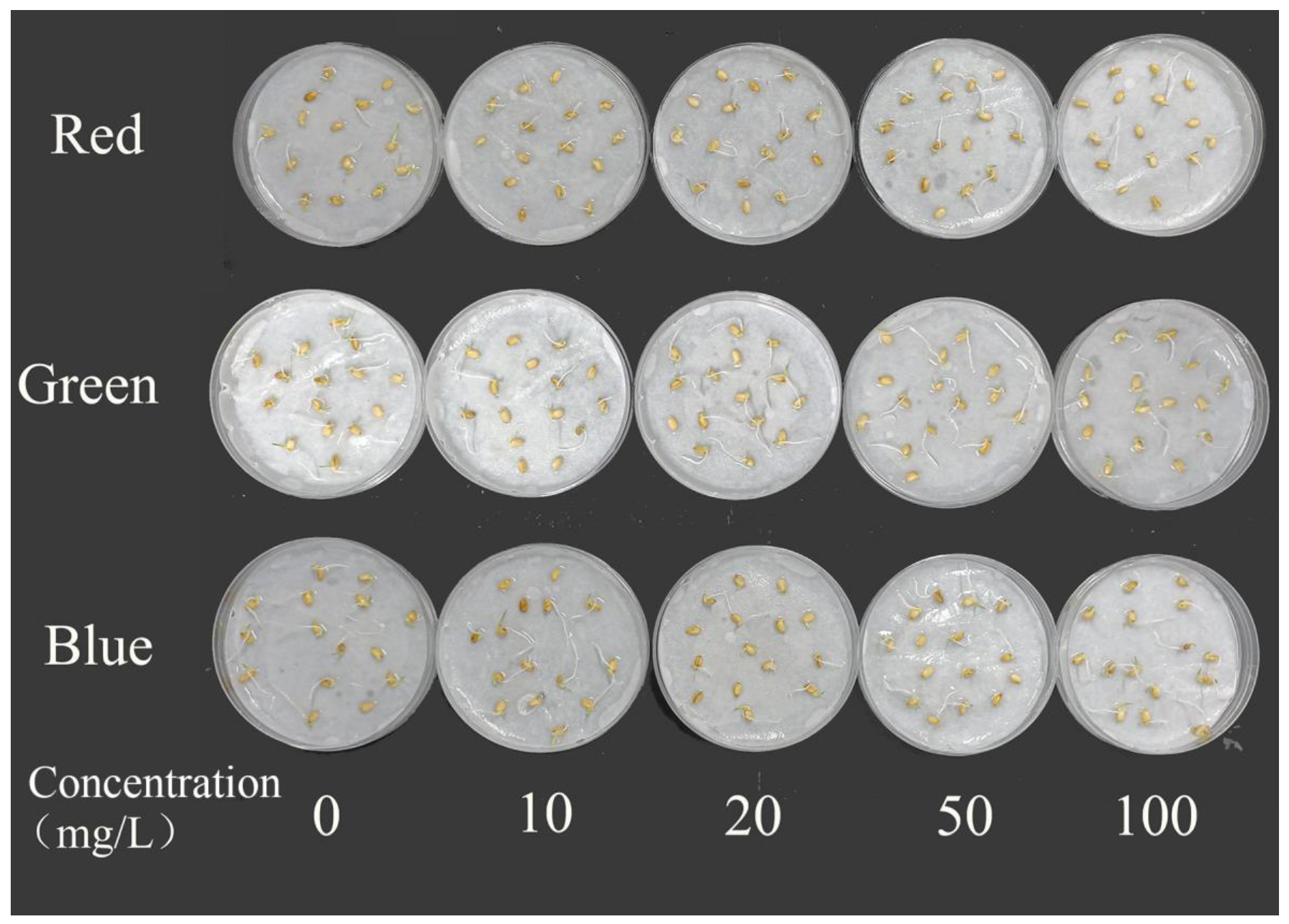
2.4. Effects on Rice Seed Germination
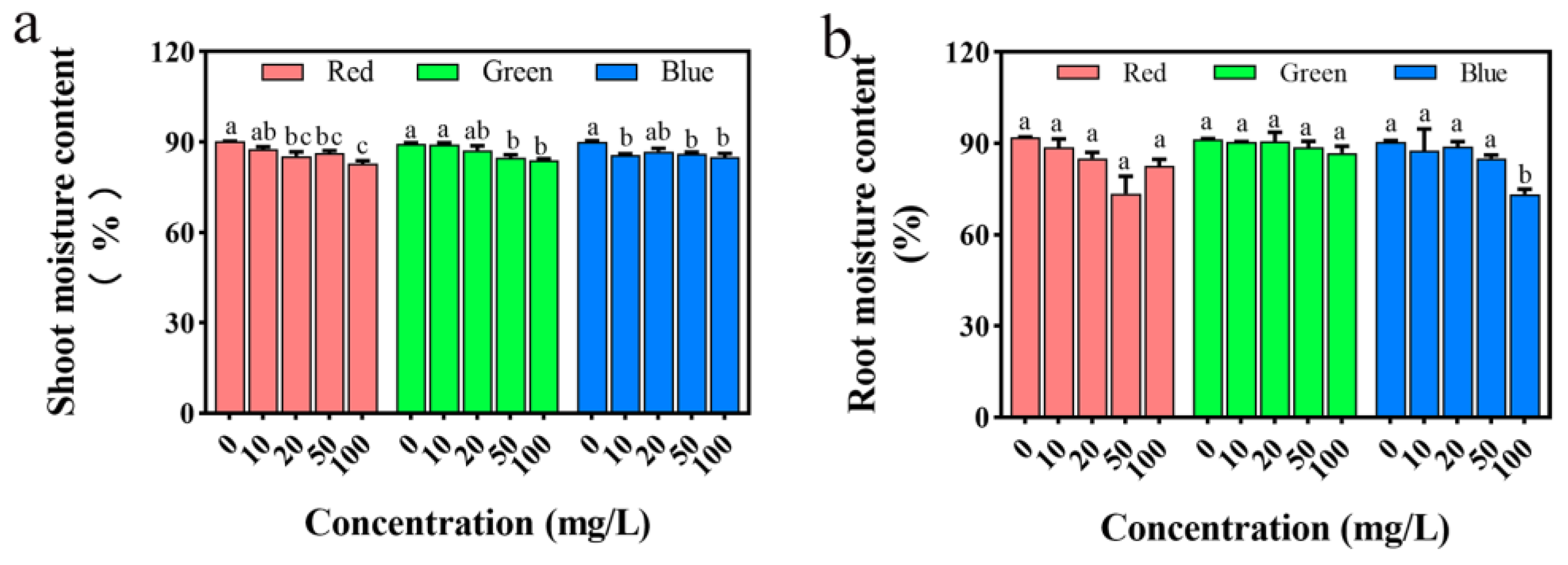
2.5. Effects on Seedling Roots and Stems
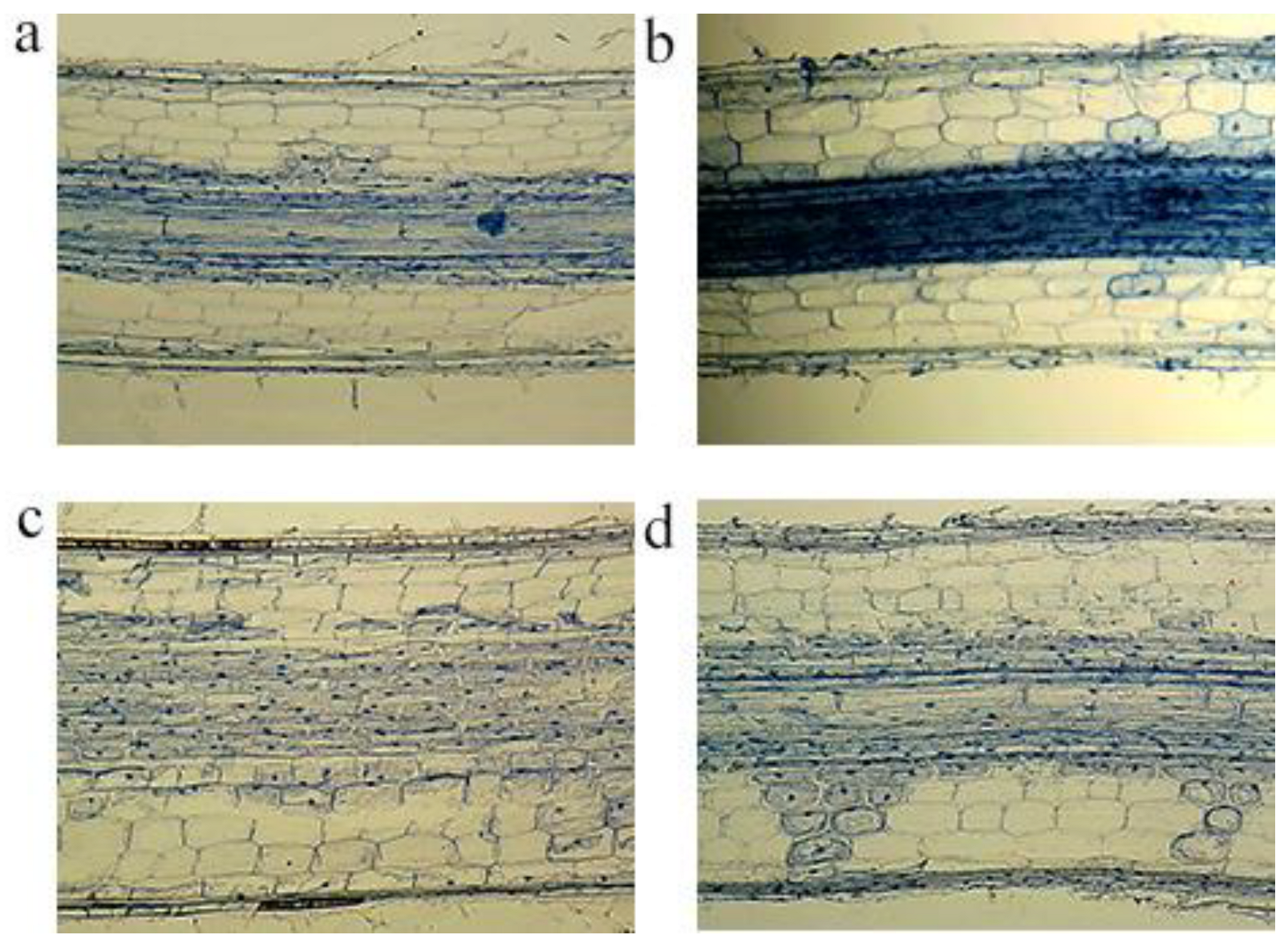
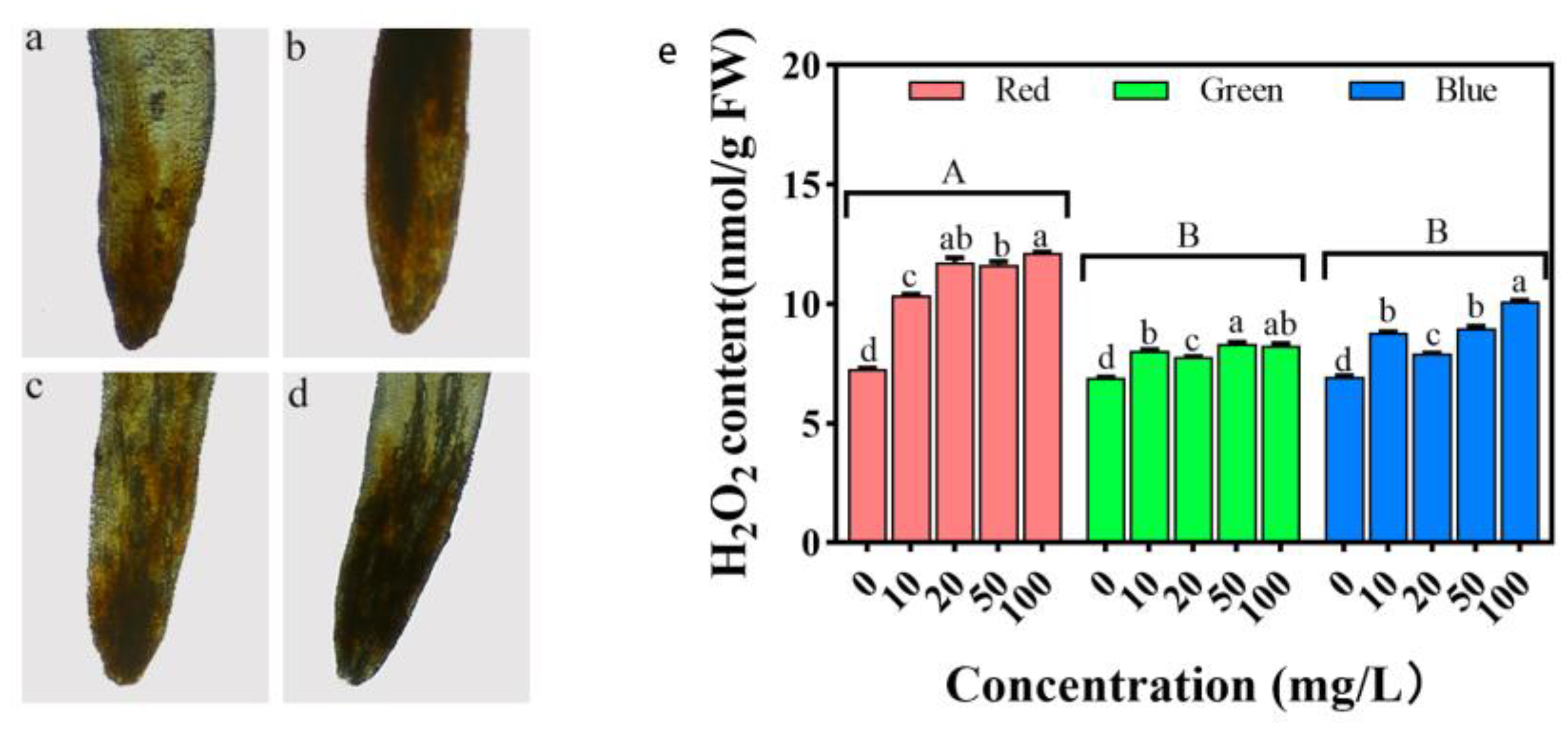
2.6. Impacts on Root Cell Morphology
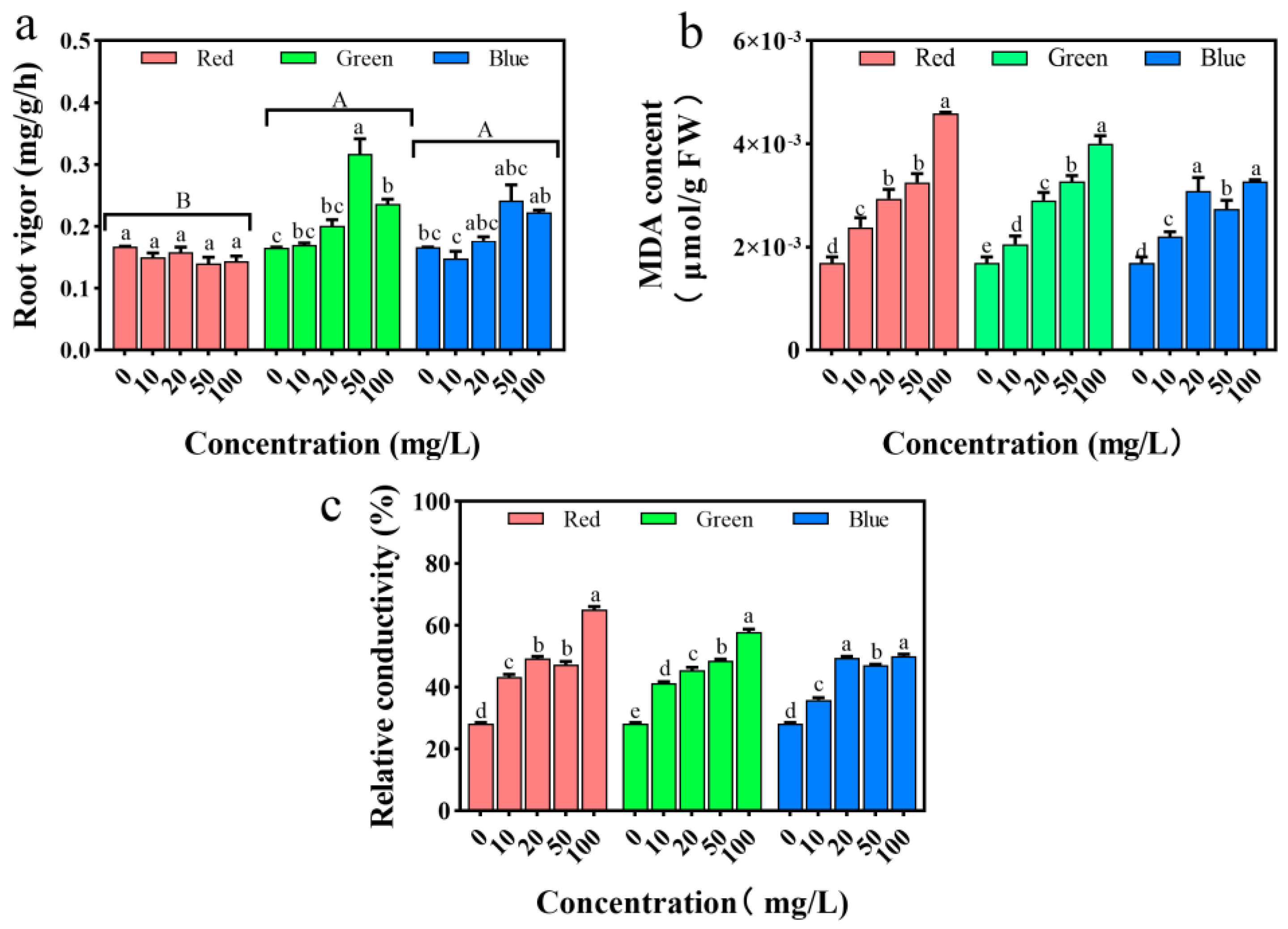
2.7. Impact on Root Vitality and Cell Membrane System
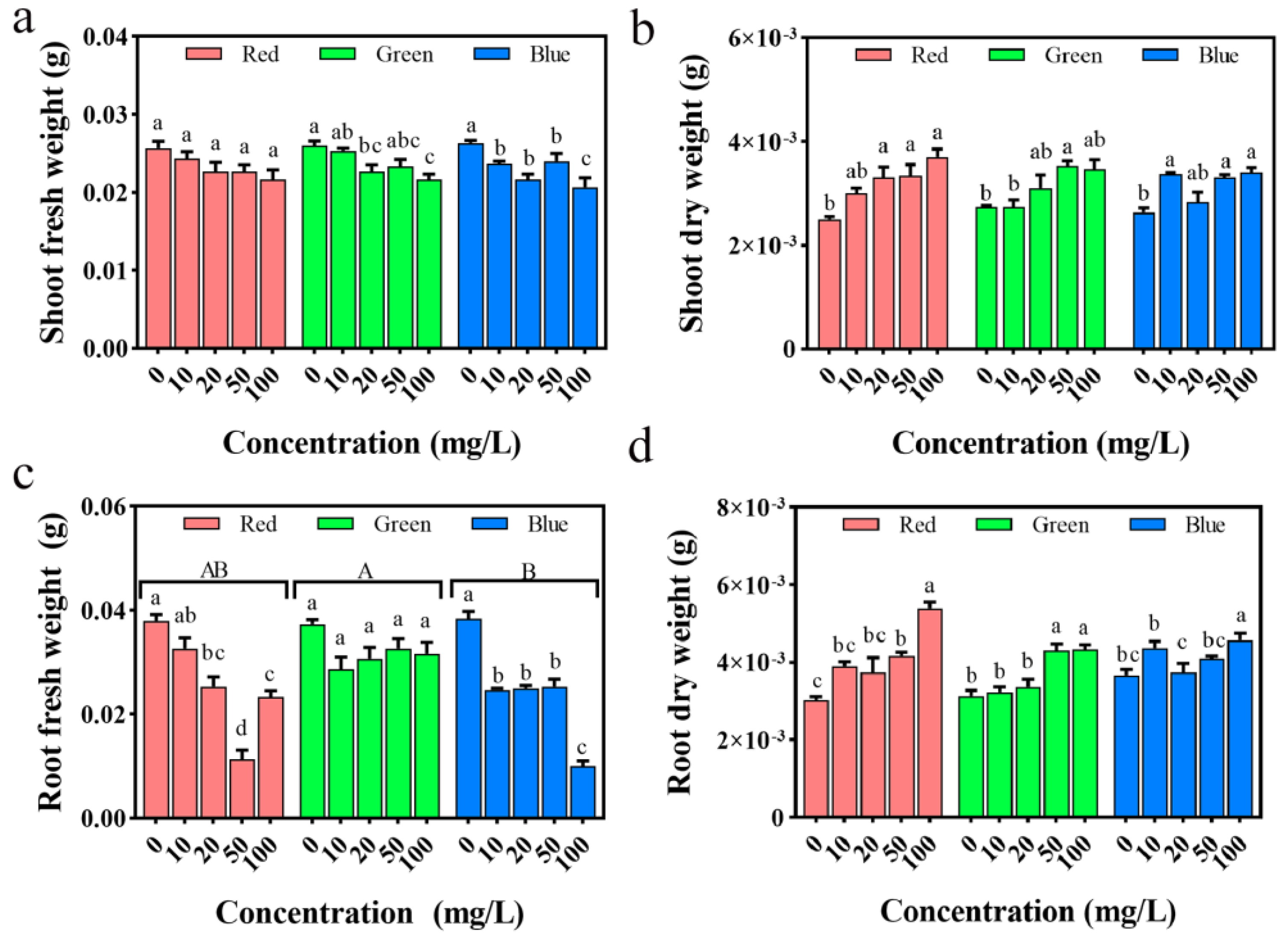
2.8. Impact on Seedling Biomass
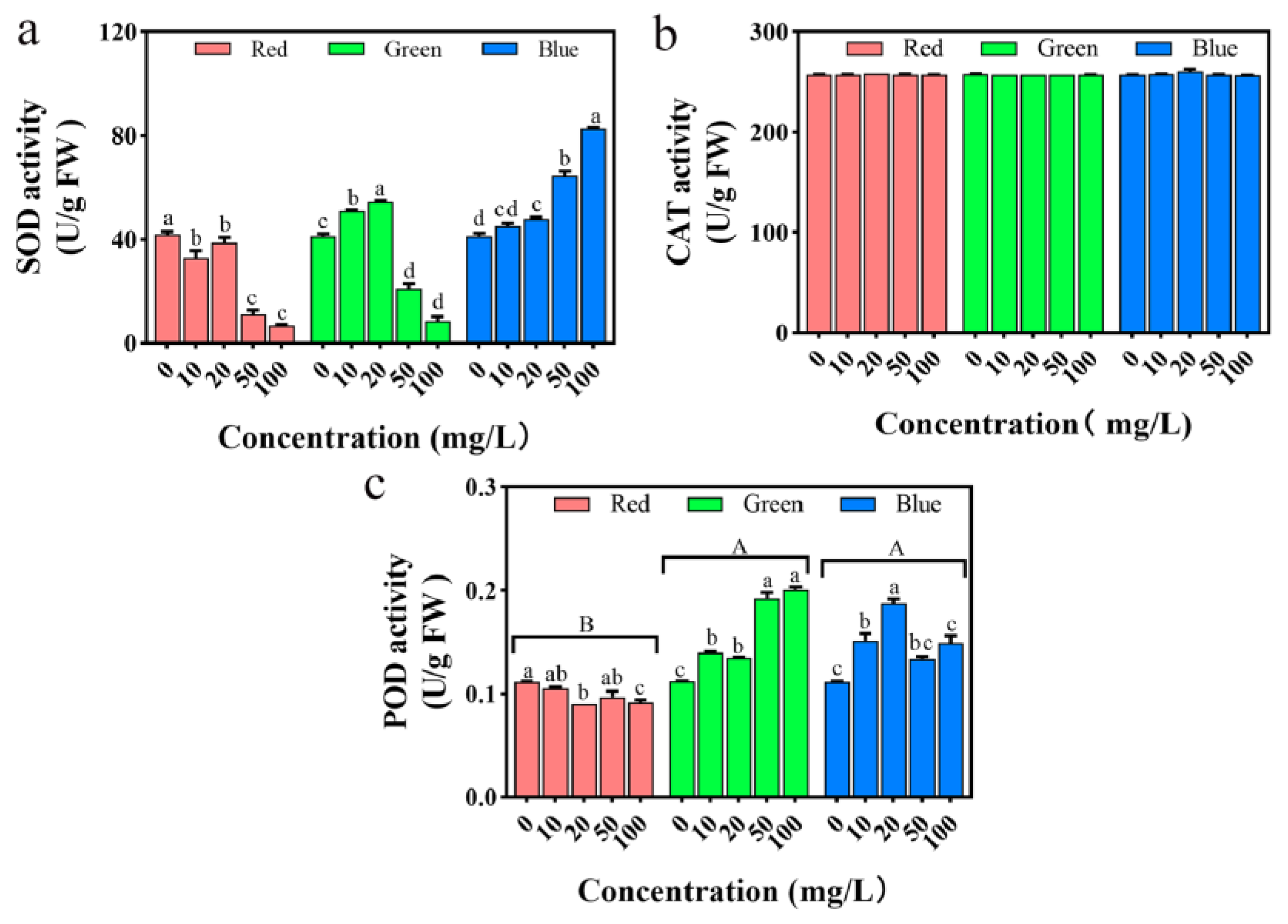
2.9. Impact on Seedling Antioxidant System
2.10. Impact of H2O2 Accumulation
2.11. Impact on Seedling Osmotic Regulatory Substances
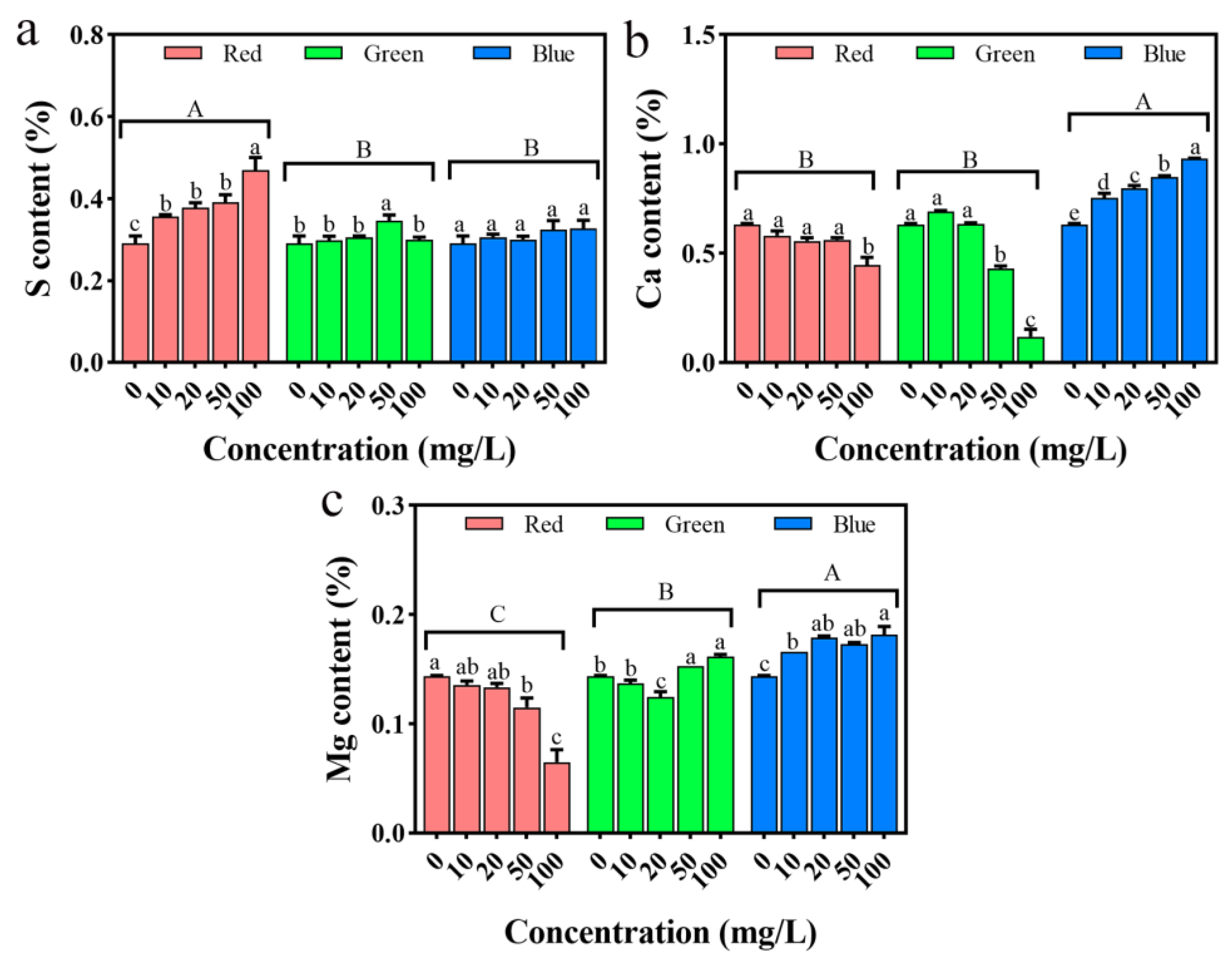
2.12. Impact on the Mineral Elements in Seedlings
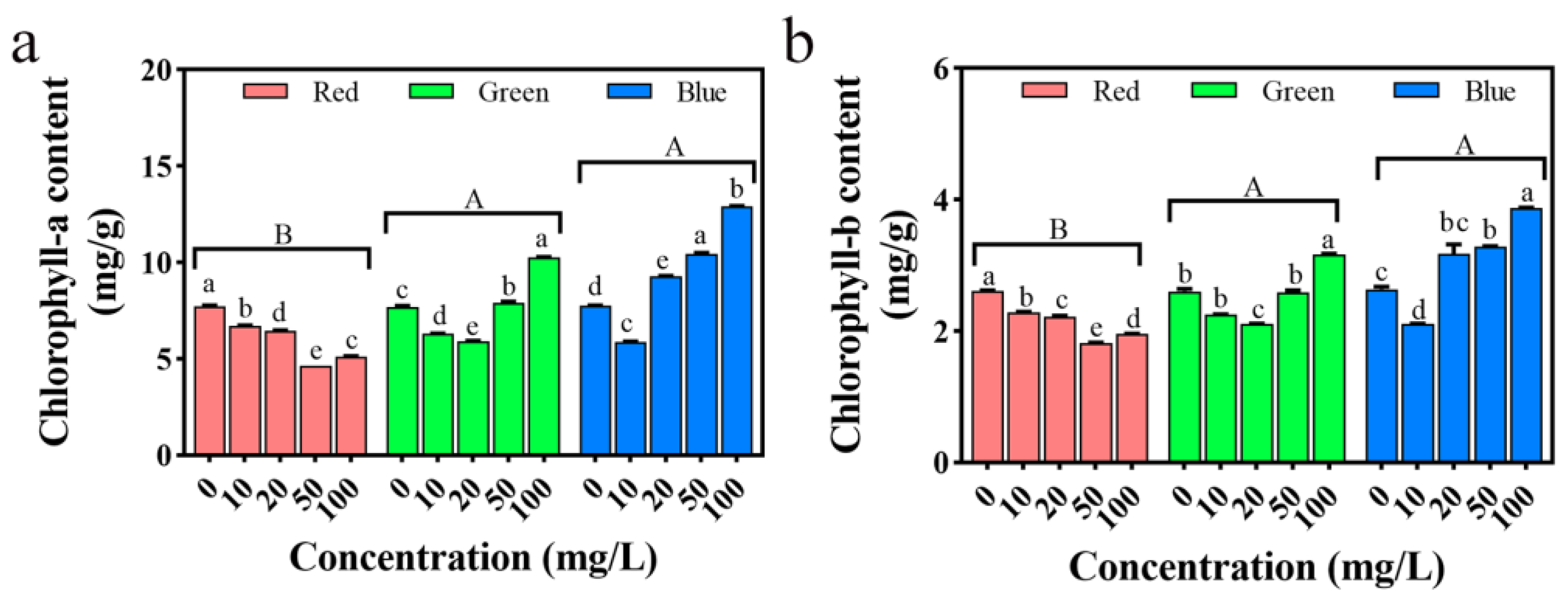
2.13. Impact on Seedling Chlorophyll
3. Discussion
4. Materials and Methods
4.1. Materials and Instruments
4.2. Experimental Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gluscevich, N.; Roy, B. Magnetic catalysis in weakly interacting hyperbolic Dirac materials. arXiv 2023, arXiv:2305.11174. [Google Scholar]
- Hiramatsu, D.; Matsumoto, T.; Berger, E.; Ransome, C.; Villar, V.A.; Gomez, S.; Cendes, Y.; De, K.; Farah, J.; Howell, D.A.; et al. Multiple Peaks and a Long Precursor in the Type IIn Supernova 2021qqp: An Energetic Explosion in a Complex Circumsteller Environment. arXiv 2023, arXiv:2305.11168. [Google Scholar]
- Zhao, D.; Lichy, D.; Perrin, P.-N.; Frahm, J.-M.; Sengupta, S. MVPSNet: Fast Generalizable Multi-view Photometric Stereo. arXiv 2023, arXiv:2305.11167. [Google Scholar] [CrossRef]
- Castaño, J.; Martínez-Fernández, S.; Franch, X.; Bogner, J. Exploring the Carbon Footprint of Hugging Face’s ML Models: A Repository Mining Study. arXiv 2023, arXiv:2305.11164. [Google Scholar] [CrossRef]
- Carosini, L.; Oddi, V.; Giorgino, F.; Hansen, L.M.; Seron, B.; Piacentini, S.; Guggemos, T.; Agresti, I.; Loredo, J.C.; Walther, P. Programmable multi-photon quantum interference in a single spatial mode. arXiv 2023, arXiv:2305.11157. [Google Scholar] [CrossRef]
- Lei, L.; Wang, Y.; Kuzmin, A.; Hua, Y.; Zhao, J.; Xu, S.; Prasad, P.N. Next generation lanthanide doped nanoscintillators and photon converters. eLight 2022, 2, 17. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, L.; Ye, R.; Zhang, J.; Zhang, X.; Xu, S. Sunlight activated ultra-stable long persistent luminescence glass ceramic for outdoor information display. J. Adv. Ceram. 2022, 11, 974–983. [Google Scholar] [CrossRef]
- Khramov, R.N.; Kreslavski, V.D.; Svidchenko, E.A.; Surin, N.M.; Kosobryukhov, A.A. Influence of photoluminophore-modified agro textile spunbond on growth and photosynthesis of cabbage and lettuce plants. Opt. Express 2019, 27, 31967–31977. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Luderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef]
- Hund-Rinke, K.; Broßell, D.; Eilebrecht, S.; Schlich, K.; Schlinkert, R.; Steska, T.; Wolf, C.; Kühnel, D. Prioritising nano- and microparticles: Identification of physicochemical properties relevant for toxicity to Raphidocelis subcapitata and Daphnia magna. Environ. Sci. Eur. 2022, 34, 116. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Q.; Yin, N.; Tu, C.; Luo, Y. Uptake and accumulation of microplastics in an edible plant. Chin. Sci. Bull. 2019, 64, 928–934. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobucar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant Vicia faba. Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Kalcikova, G.; Gotvajn, A.Z.; Kladnik, A.; Jemec, A. Impact of polyethylene microbeads on the floating freshwater plant duckweed Lemna minor. Environ. Pollut. 2017, 230, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Cardenas, A.; Scott, D.T.; Seitmaganbetova, G.; van Pelt, F.; O’Halloran, J.; Jansen, M.A.K. Polyethylene microplastics adhere to Lemna minor (L.), yet have no effects on plant growth or feeding by Gammarus duebeni (Lillj.). Sci. Total Environ. 2019, 689, 413–421. [Google Scholar] [CrossRef]
- Luo, H.W.; Xiang, Y.H.; He, D.Q.; Li, Y.; Zhao, Y.Y.; Wang, S.; Pan, X.L. Leaching behavior of fluorescent additives from microplastics and the toxicity of leachate to Chlorella vulgaris. Sci. Total Environ. 2019, 678, 1–9. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Shi, M.M.; Zhao, H.W.; Zou, J.; Yang, B.B.; Li, Y.; Wang, Z.M.; Chang, C.K.; Li, W.B. Preparation and characterization of a new long afterglow phosphor CaSnO3: Yb3+. J. Mater. Sci. Mater. Electron. 2017, 28, 10067–10072. [Google Scholar] [CrossRef]
- Sun, D.S.; Zhang, L.L.; Hao, Z.D.; Wu, H.; Wu, H.J.; Luo, Y.S.; Yang, L.; Zhang, X.; Liu, F.; Zhang, J.H. Multi-peaked broad-band red phosphor Y3Si6N11:Pr3+ for white LEDs and temperature sensing. Dalton Trans. 2020, 49, 17779–17785. [Google Scholar] [CrossRef]
- Sampedro-Guerrero, J.; Vives-Peris, V.; Gomez-Cadenas, A.; Clausell-Terol, C. Efficient strategies for controlled release of nanoencapsulated phytohormones to improve plant stress tolerance. Plant Methods 2023, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Okino, N.; Kijitori, H.; Izawa, Y.; Wada, Y.; Maki, M.; Yamamoto, T.; Yano, T. Analysis of biofilm and bacterial communities in the towel environment with daily use. Sci. Rep. 2023, 13, 7611. [Google Scholar] [CrossRef] [PubMed]
- Bastida, G.A.; Zanuttini, M.A.; Tarres, Q.; Fiol, N.; Delgado-Aguilar, M.; Galvan, M.V. Innovative system based on natural polyelectrolyte complex and cellulose micro/nanofibers to improve drainability and properties of recycled paper. Cellulose 2023, 30, 5895–5910. [Google Scholar] [CrossRef]
- Ling, X.; Osotsi, M.I.; Zhang, W.; Wu, Y.; Jin, Q.J.; Zhang, D. Bioinspired Materials: From Distinct Dimensional Architecture to Thermal Regulation Properties. J. Bionic Eng. 2023, 20, 873–899. [Google Scholar] [CrossRef]
- Cheah, Y.J.; Yunus, M.H.M.; Fauzi, M.B.; Tabata, Y.; Hiraoka, Y.; Phang, S.J.; Chia, M.R.; Buyong, M.R.; Yazid, M.D. Gelatin-chitosan-cellulose nanocrystals as an acellular scaffold for wound healing application: Fabrication, characterisation and cytocompatibility towards primary human skin cells. Cellulose 2023, 30, 5071–5092. [Google Scholar] [CrossRef]
- Singhal, R.K.; Kumar, M.; Bose, B. Eco-physiological Responses of Artificial Night Light Pollution in Plants. Russ. J. Plant Physiol. 2019, 66, 190–202. [Google Scholar] [CrossRef]
- Medvedev, A.; Proskurnikov, A.V.; Zhusubaliyev, Z.T. Design of the Impulsive Goodwin’s Oscillator: A Case Study. arXiv 2023, arXiv:2305.11136. [Google Scholar] [CrossRef]
- Lee, T.H.; Melnick, C.; Adler, R.; Lanatà, N.; Kotliar, G. Accuracy of ghost-rotationally-invariant slave-boson theory for multiorbital Hubbard models and realistic materials. arXiv 2023, arXiv:2305.11128. [Google Scholar]
- Fankhauser, F.; Tyson, J.A.; Askari, J. Satellite Optical Brightness. arXiv 2023, arXiv:2305.11123. [Google Scholar]
- Mokhalingam, A.; Gupta, S.S.; Sauer, R.A. A continuum contact model for friction between graphene sheets that accounts for surface anisotropy and curvature. arXiv 2023, arXiv:2305.11121. [Google Scholar]
- Oberdieck, G. Curve counting on the Enriques surface and the Klemm-Mariño formula. arXiv 2023, arXiv:2305.11115. [Google Scholar]
- Zhou, C.Q.; Lu, C.H.; Mai, L.; Bao, L.J.; Liu, L.Y.; Zeng, E.Y. Response of rice (Oryza sativa L.) roots to nanoplastic treatment at seedling stage. J. Hazard. Mater. 2021, 401, 123412. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.C.; Aufrecht, J.; Patino-Ramirez, F.; Cabral, H.; Arson, C.; Retterer, S.T. Modeling root system growth around obstacles. Sci. Rep. 2020, 10, 15868. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Z.; Chen, J.; Dou, R.Z.; Gao, X.; Mao, C.B.; Wang, L. Assessment of the Phytotoxicity of Metal Oxide Nanoparticles on Two Crop Plants, Maize (Zea mays L.) and Rice (Oryza sativa L.). Int. J. Environ. Res. Public Health 2015, 12, 15100–15109. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Z.; Xiao, Y.F.; Jiao, T.T.; Zhang, Y.; Chen, J.; Gao, Y. Effects of Copper Oxide Nanoparticles on the Growth of Rice (Oryza Sativa L.) Seedlings and the Relevant Physiological Responses. Int. J. Environ. Res. Public Health 2020, 17, 1260. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Aubert, T.; Burel, A.; Esnault, M.A.; Cordier, S.; Grasset, F.; Cabello-Hurtado, F. Root uptake and phytotoxicity of nanosized molybdenum octahedral clusters. J. Hazard. Mater. 2012, 219–220, 111–118. [Google Scholar] [CrossRef]
- Dong, R.Y.; Liu, R.L.; Xu, Y.M.; Liu, W.T.; Wang, L.; Liang, X.F.; Huang, Q.Q.; Sun, Y.B. Single and joint toxicity of polymethyl methacrylate microplastics and As (V) on rapeseed (Brassia campestris L.). Chemosphere 2022, 291, 133066. [Google Scholar] [CrossRef]
- Guo, M.; Zhao, F.R.; Tian, L.W.; Ni, K.J.; Lu, Y.Q.; Borah, P. Effects of polystyrene microplastics on the seed germination of herbaceous ornamental plants. Sci. Total Environ. 2022, 809, 151100. [Google Scholar] [CrossRef]
- Tripathi, S.; Kapri, S.; Datta, A.; Bhattacharyya, S. Influence of the morphology of carbon nanostructures on the stimulated growth of gram plant. RSC Adv. 2016, 6, 43864–43873. [Google Scholar] [CrossRef]
- Huang, J.K.; Huang, Z.R.; Jia, X.P.; Hu, R.F.; Xiang, C. Long-term reduction of nitrogen fertilizer use through knowledge training in rice production in China. Agric. Syst. 2015, 135, 105–111. [Google Scholar] [CrossRef]
- Holmer, M.; Pedersen, O.; Krause-Jensen, D.; Olesen, B.; Hedegård Petersen, M.; Schopmeyer, S.; Koch, M.; Lomstein, B.A.; Jensen, H.S. Sulfide intrusion in the tropical seagrasses Thalassia testudinum and Syringodium filiforme. Estuar. Coast. Shelf Sci. 2009, 85, 319–326. [Google Scholar] [CrossRef]
- Holmer, M.; Kendrick, G.A. High Sulfide Intrusion in Five Temperate Seagrasses Growing Under Contrasting Sediment Conditions. Estuaries Coasts 2012, 36, 116–126. [Google Scholar] [CrossRef]
- Lamers, L.P.; Govers, L.L.; Janssen, I.C.; Geurts, J.J.; Van der Welle, M.E.; Van Katwijk, M.M.; Van der Heide, T.; Roelofs, J.G.; Smolders, A.J. Sulfide as a soil phytotoxin-a review. Front. Plant Sci. 2013, 4, 268. [Google Scholar] [CrossRef] [PubMed]
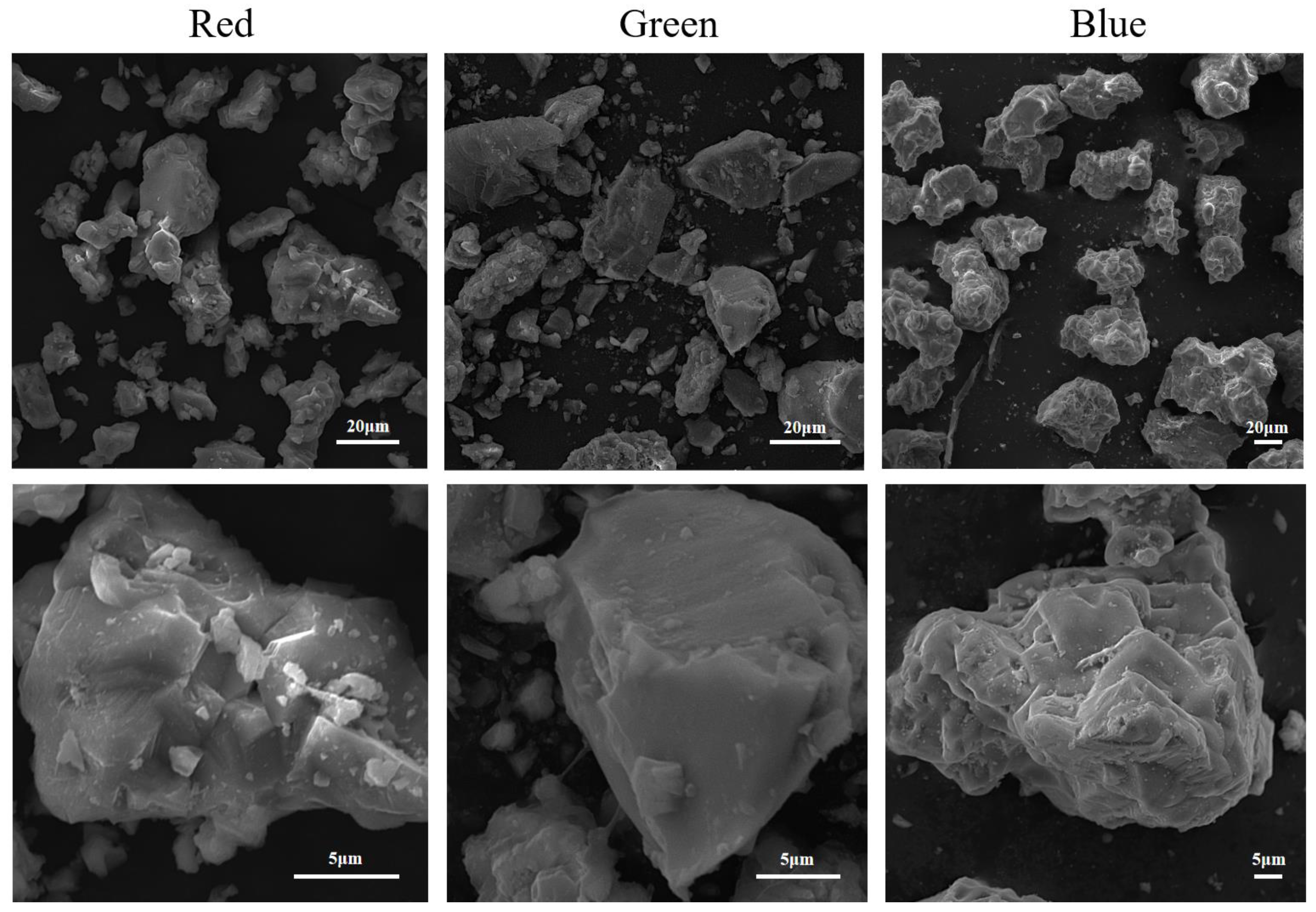


| Material | Morphology | Size | Zeta |
|---|---|---|---|
| rPLM | Irregular Block | 30 ± 5 μm | −2.96 ± 2.29 mV |
| gPLM | Angular Trihedron | 15 ± 3 μm | −13.18 ± 3.17 mV |
| bPLM | Irregular Block | 40 ± 8 μm | −10.8 ± 0.92 mVa |
| (mg/L) | rPLM | gPLM | bPLM | |||
|---|---|---|---|---|---|---|
| Germination Potential (%) | Germination Rate (%) | Germination Potential (%) | Germination Rate (%) | Germination Potential (%) | Germination Rate (%) | |
| 0 | 82.22 ± 0.08 | 93.33 ± 0.05 | 86.67 ± 0.01 | 93.33 ± 0.05 | 93.33 ± 0.05 | 91.11 ± 0.06 |
| 10 | 77.78 ± 0.03 | 95.56 ± 0.06 | 88.89 ± 0.03 | 91.11 ± 0.08 | 82.22 ± 0.03 | 93.33 ± 0.01 |
| 20 | 83.33 ± 0.39 | 95.56 ± 0.03 | 84.44 ± 0.03 | 93.33 ± 0.05 | 86.67 ± 0.05 | 93.33 ± 0.05 |
| 50 | 80.00 ± 0.05 | 93.33 | 88.89 ± 0.06 | 95.56 ± 0.06 | 82.22 ± 0.08 | 95.56 ± 0.003 |
| 100 | 90.00 ± 0.42 | 93.33 ± 0.03 | 82.22 ± 0.08 | 93.33 ± 0.05 | 91.11 ± 0.03 | 95.56 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, N.; Wei, X.; Yu, J.; Zhang, S.; Hu, D.; Li, P.; Xia, Y.; Song, K. Interference Effects of Commercial Persistent Luminescence Materials on Rice Germination and Seedling Growth. Plants 2023, 12, 2554. https://doi.org/10.3390/plants12132554
Zhu N, Wei X, Yu J, Zhang S, Hu D, Li P, Xia Y, Song K. Interference Effects of Commercial Persistent Luminescence Materials on Rice Germination and Seedling Growth. Plants. 2023; 12(13):2554. https://doi.org/10.3390/plants12132554
Chicago/Turabian StyleZhu, Nina, Xinpei Wei, Jingbo Yu, Shuo Zhang, Die Hu, Ping Li, Yunfei Xia, and Kai Song. 2023. "Interference Effects of Commercial Persistent Luminescence Materials on Rice Germination and Seedling Growth" Plants 12, no. 13: 2554. https://doi.org/10.3390/plants12132554
APA StyleZhu, N., Wei, X., Yu, J., Zhang, S., Hu, D., Li, P., Xia, Y., & Song, K. (2023). Interference Effects of Commercial Persistent Luminescence Materials on Rice Germination and Seedling Growth. Plants, 12(13), 2554. https://doi.org/10.3390/plants12132554





