Ecological Stoichiometry and Stock Distribution of C, N, and P in Three Forest Types in a Karst Region of China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Sampling Collection
2.3. Chemical Analysis
2.4. Data Analysis
3. Results
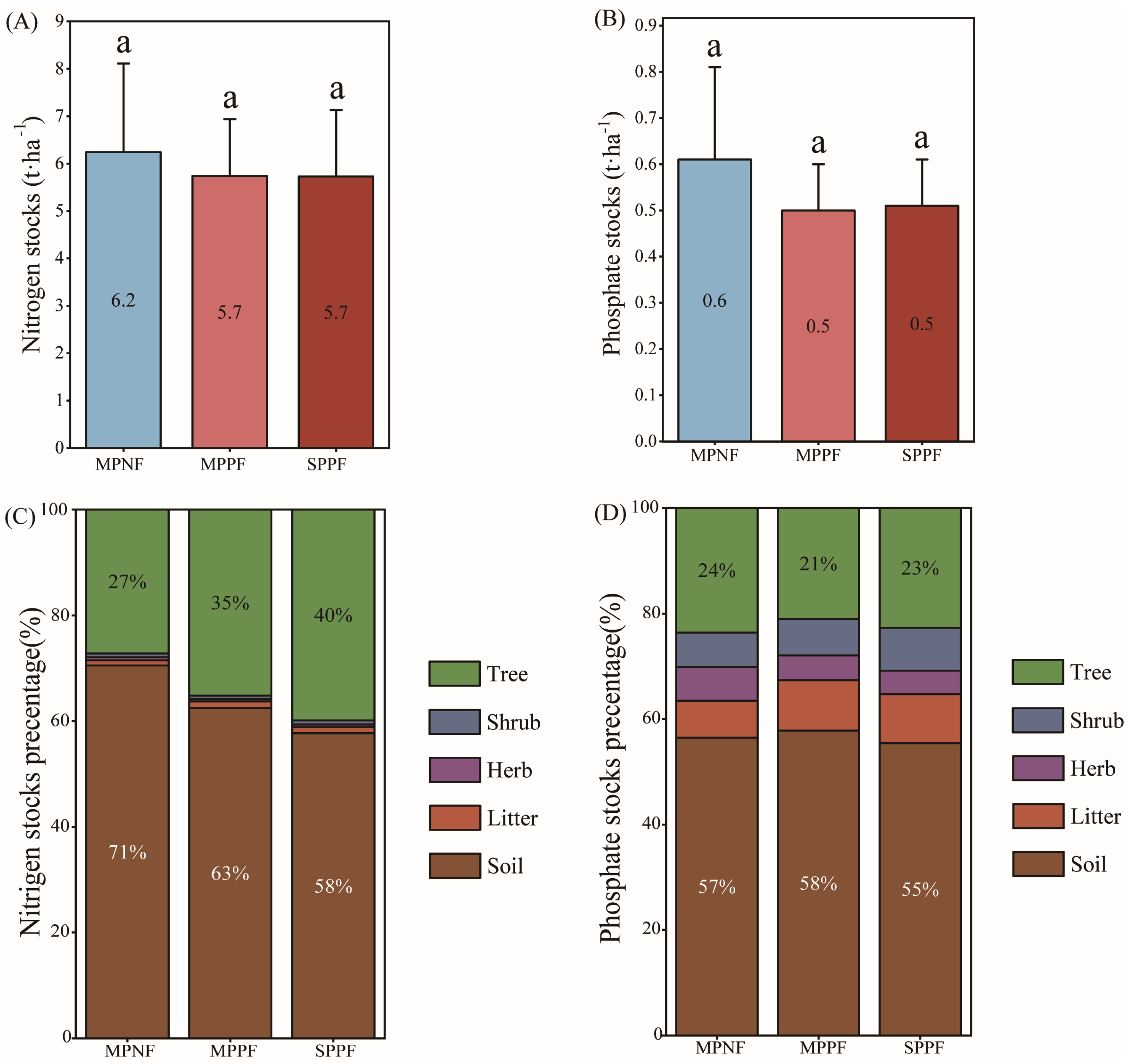
3.1. The Concentration, Distribution, and Stocks of N and P
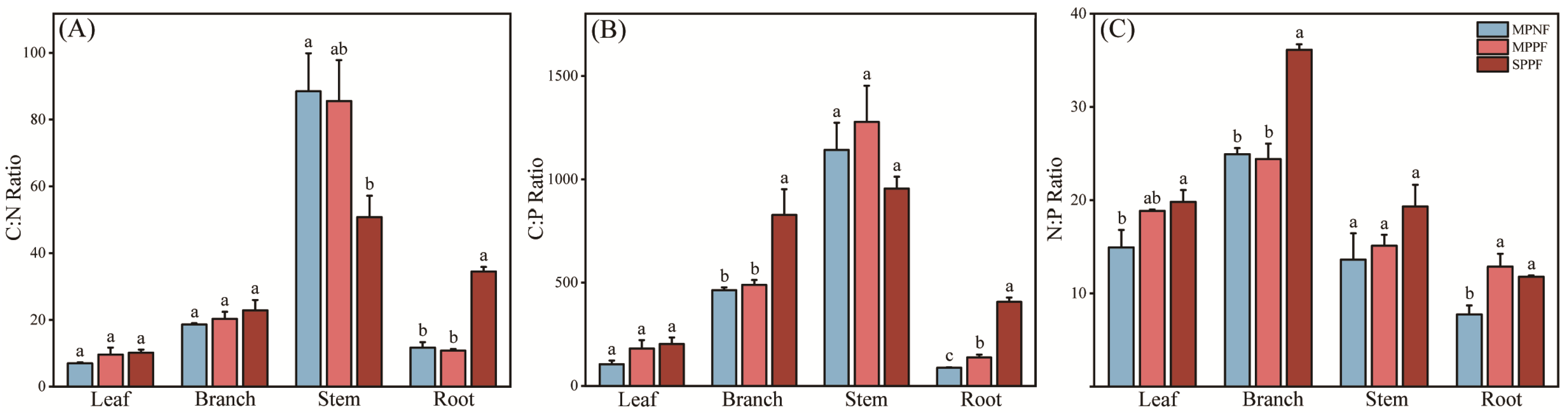
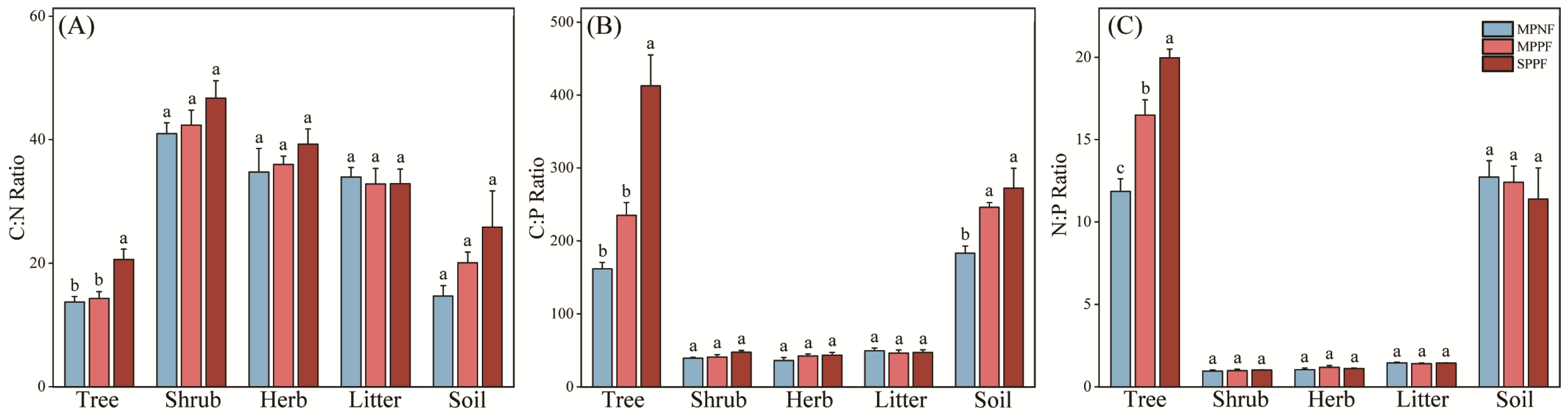
3.2. The Stoichiometric Characteristics of C, N, and P
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; p. 464. [Google Scholar]
- McGroddy, M.; Daufresne, T.; Hedin, L.O. Scaling of c:n:p stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, T.; Peñuelas, J.; Sardans, J.; Tan, W.; Wei, X.; Cui, Y.; Cui, Q.; Wu, C.; Liu, L.; et al. Crop residue return sustains global soil ecological stoichiometry balance. Glob. Chang. Biol. 2023, 29, 2203–2226. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.; Chen, X.; Liu, S.; Lu, X.; Chen, H.Y.H.; Ruan, H. Phosphorus additions imbalance terrestrial ecosystem C:N:P stoichiometry. Glob. Chang. Biol. 2022, 28, 7353–7365. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.; Fagan, W.; Kerkhoff, A.; Swenson, N.; Enquist, B. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, N.; Liu, C.; Yang, H.; Li, M.; Yu, C.; Wilcox, K.; Yu, Q.; He, N. C:N:P stoichiometry in China’s forests: From organs to ecosystems. Funct. Ecol. 2018, 32, 50–60. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, J.; Chen, B.; Hu, Y. Nutrient resorption efficiency of three tree species in Beijing plain afforestation and its C:N:P stoichiometry. Ann. For. Res. 2020, 63, 91–102. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Xu, M.; Deng, J.; Han, X.; Yang, G.; Fen, Y.; Ren, G. Response of forest growth to C:N:P stoichiometry in plants and soils during Robinia seudoacacia afforestation on the Loess Plateau, China. Geoderma 2019, 337, 280–289. [Google Scholar] [CrossRef]
- Sistla, S.A.; Schimel, J.P. Stoichiometric flexibility as a regulator of carbon and nutrient cycling in terrestrial ecosystems under change. New Phytol. 2012, 196, 68–78. [Google Scholar] [CrossRef]
- Karhu, K.; Alaei, S.; Li, J.; Merilä, P.; Ostonen, I.; Bengtson, P. Microbial carbon use efficiency and priming of soil organic matter mineralization by glucose additions in boreal forest soils with different C:N ratios. Soil Biol. Biochem. 2022, 167, 108615. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; Van derPutten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Hu, Q.; Sheng, M.; Bai, Y.; Jie, Y.; Xiao, H. Response of C, N, and P stoichiometry characteristics of Broussonetia papyrifera to altitude gradients and soil nutrients in the karst rocky ecosystem, SW China. Plant Soil 2022, 475, 123–136. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, G.; Zhang, D.; Liu, S.; Zhu, G.; Yan, J. N and P stoichiometry of plant and soil in lower subtropical forest successional series in southern China. Chin. J. Plant Ecol. 2010, 34, 64–71. [Google Scholar]
- Zeng, Q.; Li, X.; Dong, Y.; An, S.; Darboux, F. Soil and plant components ecological stoichiometry in four steppe communities in the Loess Plateau of China. Catena 2016, 147, 481–488. [Google Scholar] [CrossRef]
- Tian, D.; Reich, P.B.; Chen, H.; Xiang, Y.; Luo, Y.; Shen, Y.; Meng, C.; Han, W.; Niu, S. Global changes alter plant multi-element stoichiometric coupling. New Phytol. 2018, 221, 807–817. [Google Scholar] [CrossRef]
- Xu, E.; Zhang, H. Human–desertification coupling relationship in a karst region of China. Land Degrad. Dev. 2021, 32, 4988–5003. [Google Scholar] [CrossRef]
- Wang, S.-J.; Liu, Q.-M.; Zhang, D.-F. Karst rocky desertification in southwestern China: Geomorphology, landuse, impact and rehabilitation. Land Degrad. Dev. 2004, 15, 115–121. [Google Scholar] [CrossRef]
- Pu, J.; Zhao, X.; Huang, P.; Gu, Z.; Shi, X.; Chen, Y.; Shi, X.; Tao, J.; Xu, Y.; Xiang, A. Ecological risk changes and their relationship with exposed surface fraction in the karst region of southern China from 1990 to 2020. J. Environ. Manag. 2022, 323, 116206. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.M.; Wang, K.L.; Zhang, B.; Jiao, Q.J.; Liu, B.; Zhang, M.Y. Remote sensing of fractional cover of vegetation and exposed bedrock for karst rocky desertification assessment. Procedia Environ. Sci. 2012, 13, 847–853. [Google Scholar] [CrossRef]
- Li, D.; Wen, L.; Jiang, S.; Song, T.; Wang, K. Responses of soil nutrients and microbial communities to three restoration strategies in a karst area, southwest China. J. Environ. Manag. 2018, 207, 456–464. [Google Scholar] [CrossRef]
- Wang, M.; Chen, H.; Zhang, W.; Wang, K. Soil nutrients and stoichiometric ratios as affected by land use and lithology at county scale in a karst area, southwest China. Sci. Total Environ. 2018, 619–620, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wang, W.; Peng, Y.; Chen, X. Evaluation of Biomass and Carbon Stocks in Three Pine Forest Types in Karst Area of Southwestern China. J. Sustain. For. 2020, 41, 18–32. [Google Scholar] [CrossRef]
- Nelson, D.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods of soil analysis Part 2. Chem. Microbiol. Prop. 1983, 9, 539–579. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis. Part 3—Chemical Methods; Soil Science Society of America Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Parkinson, J.A.; Allen, S.E. A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun. Soil Sci. Plant Anal. 1975, 6, 1–11. [Google Scholar] [CrossRef]
- He, J.S.; Wang, J.; Dan, F.B.; Flynn, D.F.B.; Wang, X.; Ma, W.; Fang, J. Leaf nitrogen:phosphorus stoichiometry across Chinese grassland biomes. Oecologia 2007, 155, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wu, J.; Liu, W.; Yuan, Y.; Hu, L.; Cai, Q. Linkages of plant and soil C:N:P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Ågren, G.I. Stoichiometry and Nutrition of Plant Growth in Natural Communities. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 153–170. [Google Scholar] [CrossRef]
- Hedin, L.O. Global organization of terrestrial plant–nutrient interactions. Proc. Natl. Acad. Sci. USA 2004, 101, 10849–10850. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, J.; Zhao, X.; Chao, Z.; Wang, Y.; Zhang, X.; Wang, D. The linkages of plant, litter and soil C:N:P stoichiometry and nutrient stock in different secondary mixed forest types in the Qinling Mountains, China. PeerJ 2020, 8, e9274. [Google Scholar] [CrossRef]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- Niklas, K.J.; Cobb, E.D. N, P, and C stoichiometry of Eranthis hyemalis (Ranunculaceae) and the allometry of plant growth. Am. J. Bot. 2005, 92, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Yu, G.R. Ecological stoichiometry characteristics of ecosystem carbon, nitrogen and phosphorus elements. Acta Ecol. Sinica 2008, 28, 3937–3947. [Google Scholar]
- Gren, G.I. The C:N:P stoichiometry of autotrophs–theory and observations. Ecol. Lett. 2004, 7, 185–191. [Google Scholar]
- Knecht, M.F.; Gǒransson, A. Terrestrial plants require nutrients in similar proportions. Tree Physiol. 2004, 24, 447–460. [Google Scholar] [CrossRef]
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.; Li, D.; Chen, H.; Wang, K. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Bui, E.N.; Henderson, B.L. C:N:P stoichiometry in Australian soils with respect to vegetation and environmental factors. Plant Soil 2013, 373, 553–568. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiong, G.; Li, J.; Lu, Z.; Li, Y.; Xu, W.; Wang, Y.; Zhao, C.; Tang, Z.; Xie, Z. Nitrogen and phosphorus concentrations and allocation strategies among shrub organs: The effects of plant growth forms and nitrogen-fixation types. Plant Soil 2018, 427, 305–319. [Google Scholar] [CrossRef]
| Forest Types | Age (Year) | Tree Density (Tree/ha) | DBH (cm) | Tree Height (m) | Shrubs | Herbs |
|---|---|---|---|---|---|---|
| MPNF | 18 | 2242 (125.83) | 13.0 (1.09) | 12.2 (0.63) | 1,2,3,4,5 | 9,10,11,12 |
| MPPF | 19 | 1217 (625.17) | 17.5 (0.35) | 15.6 (0.59) | 1,2,3,4,6 | 9,10,11,12 |
| SPPF | 20 | 950 (163.94) | 21.0 (1.13) | 14.8 (0.51) | 2,4,5,7,8 | 9,10,11 |
| Element | Forest Types | Overstory | Stem | Root | Understory | Litter | Soil | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | Branch | Shrub | Herb | 0–15 cm | 15–30 cm | 30–45 cm | 45–60 cm | |||||
| N | MPNF | 60.79a (1.8) | 22.98a (0.2) | 5.21a (0.6) | 42.03a (0.6) | 8.80a (0.5) | 9.84a (0.4) | 10.70a (0.2) | 0.50a (0.1) | 0.42a (0.1) | 0.33a (0.1) | 0.29a (0.1) |
| MPPF | 67.34a (0.5) | 23.59a (0.6) | 5.12a (0.1) | 5.36b (1.9) | 9.39a (0.8) | 9.28a (1.0) | 11.23a (0.2) | 0.44a (0.1) | 0.32a (0.1) | 0.32a (0.1) | 0.35a (0.1) | |
| SPPF | 51.13b (0.5) | 22.34a (0.1) | 10.39b (0.1) | 12.54c (0.1) | 8.19a (0.1) | 8.69a (0.4) | 12.01a (0.5) | 0.56a (0.2) | 0.42a (0.1) | 0.42a (0.1) | A/N | |
| P | MPNF | 4.22a (0.6) | 0.92a (0.0) | 0.42a (0.1) | 5.60a (0.7) | 9.17a (0.2) | 9.51a (0.7) | 7.39a (0.4) | 0.03a (0.0) | 0.04a (0.0) | 0.03a (0.0) | 0.03a (0.0) |
| MPPF | 3.57a (0.1) | 0.97a (0.1) | 0.34a (0.0) | 4.23b (0.4) | 9.65a (0.3) | 7.80b (0.3) | 8.00a (0.2) | 0.03a (0.0) | 0.03a (0.0) | 0.03a (0.0) | 0.03a (0.0) | |
| SPPF | 2.60b (0.1) | 0.62b (0.0) | 0.55a (0.1) | 1.06b (0.0) | 8.06b (0.1) | 7.83b (0.3) | 8.35a (0.4) | 0.04a (0.0) | 0.04a (0.0) | 0.04a (0.0) | A/N | |
| Forest Types | Leaf | Branch | Stem | Root | Tree | Shrub | Herb | Litter | Soil |
|---|---|---|---|---|---|---|---|---|---|
| MPNF | 0.43b (0.01) | 0.42b (0.01) | 0.35b (0.05) | 0.50a (0.10) | 1.70a (0.50) | 0.04a (0.00) | 0.04a (0.00) | 0.06a (0.00) | 4.40a (0.98) |
| MPPF | 0.68a (0.01) | 0.85a (0.03) | 0.41b (0.07) | 0.07a (0.05) | 2.02a (1.13) | 0.03a (0.00) | 0.03a (0.00) | 0.07a (0.00) | 3.59a (0.75) |
| SPPF | 0.46b (0.01) | 0.33b (0.01) | 1.31a (0.21) | 0.19a (0.10) | 2.28a (1.03) | 0.04a (0.00) | 0.03a (0.00) | 0.07a (0.00) | 3.31a (0.87) |
| Forest Types | Leaf | Branch | Stem | Root | Tree | Shrub | Herb | Litter | Soil |
|---|---|---|---|---|---|---|---|---|---|
| MPNF | 0.03a (0.00) | 0.02a (0.00) | 0.03a (0.00) | 0.07a (0.00) | 0.14a (0.01) | 0.04a (0.00) | 0.04a (0.00) | 0.04a (0.00) | 0.35a (0.09) |
| MPPF | 0.04a (0.00) | 0.04a (0.00) | 0.03a (0.00) | 0.01a (0.00) | 0.11a (0.01) | 0.03a (0.00) | 0.02a (0.00) | 0.05a (0.00) | 0.29a (0.08) |
| SPPF | 0.02a (0.00) | 0.01a (0.00) | 0.07a (0.00) | 0.02a (0.00) | 0.12a (0.01) | 0.04a (0.00) | 0.02a (0.00) | 0.05a (0.00) | 0.28a (0.08) |
| Forest Type | Leaf | Litter | Soil |
|---|---|---|---|
| MPNF | 100:14:1 | 49:2:1 | 255:16:1 |
| MPPF | 181:19:1 | 46:2:1 | 310:14:1 |
| SPPF | 200:20:1 | 47:2:1 | 317:13:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Peng, Y.; Chen, Y.; Lei, S.; Wang, X.; Farooq, T.H.; Liang, X.; Zhang, C.; Yan, W.; Chen, X. Ecological Stoichiometry and Stock Distribution of C, N, and P in Three Forest Types in a Karst Region of China. Plants 2023, 12, 2503. https://doi.org/10.3390/plants12132503
Wang W, Peng Y, Chen Y, Lei S, Wang X, Farooq TH, Liang X, Zhang C, Yan W, Chen X. Ecological Stoichiometry and Stock Distribution of C, N, and P in Three Forest Types in a Karst Region of China. Plants. 2023; 12(13):2503. https://doi.org/10.3390/plants12132503
Chicago/Turabian StyleWang, Wancai, Yuanying Peng, Yazhen Chen, Shilong Lei, Xiaojun Wang, Taimoor Hassan Farooq, Xiaocui Liang, Chao Zhang, Wende Yan, and Xiaoyong Chen. 2023. "Ecological Stoichiometry and Stock Distribution of C, N, and P in Three Forest Types in a Karst Region of China" Plants 12, no. 13: 2503. https://doi.org/10.3390/plants12132503
APA StyleWang, W., Peng, Y., Chen, Y., Lei, S., Wang, X., Farooq, T. H., Liang, X., Zhang, C., Yan, W., & Chen, X. (2023). Ecological Stoichiometry and Stock Distribution of C, N, and P in Three Forest Types in a Karst Region of China. Plants, 12(13), 2503. https://doi.org/10.3390/plants12132503








