Effects of Different Pre-Cooling Methods on the Shelf Life and Quality of Sweet Corn (Zea mays L.)
Abstract
:1. Introduction
2. Results and Discussion
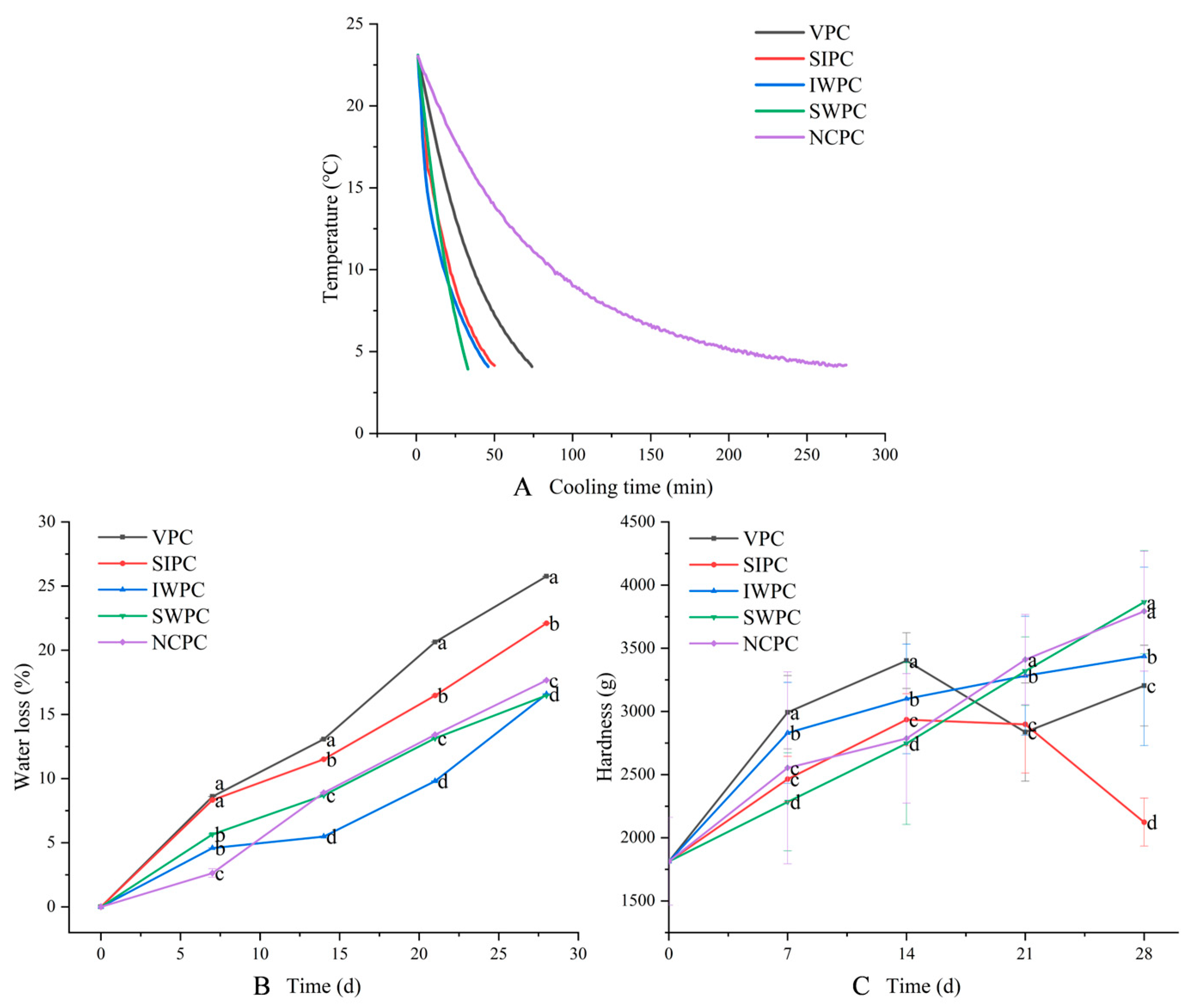
2.1. Pre-Cooling Curve
2.2. Water Loss
2.3. Hardness
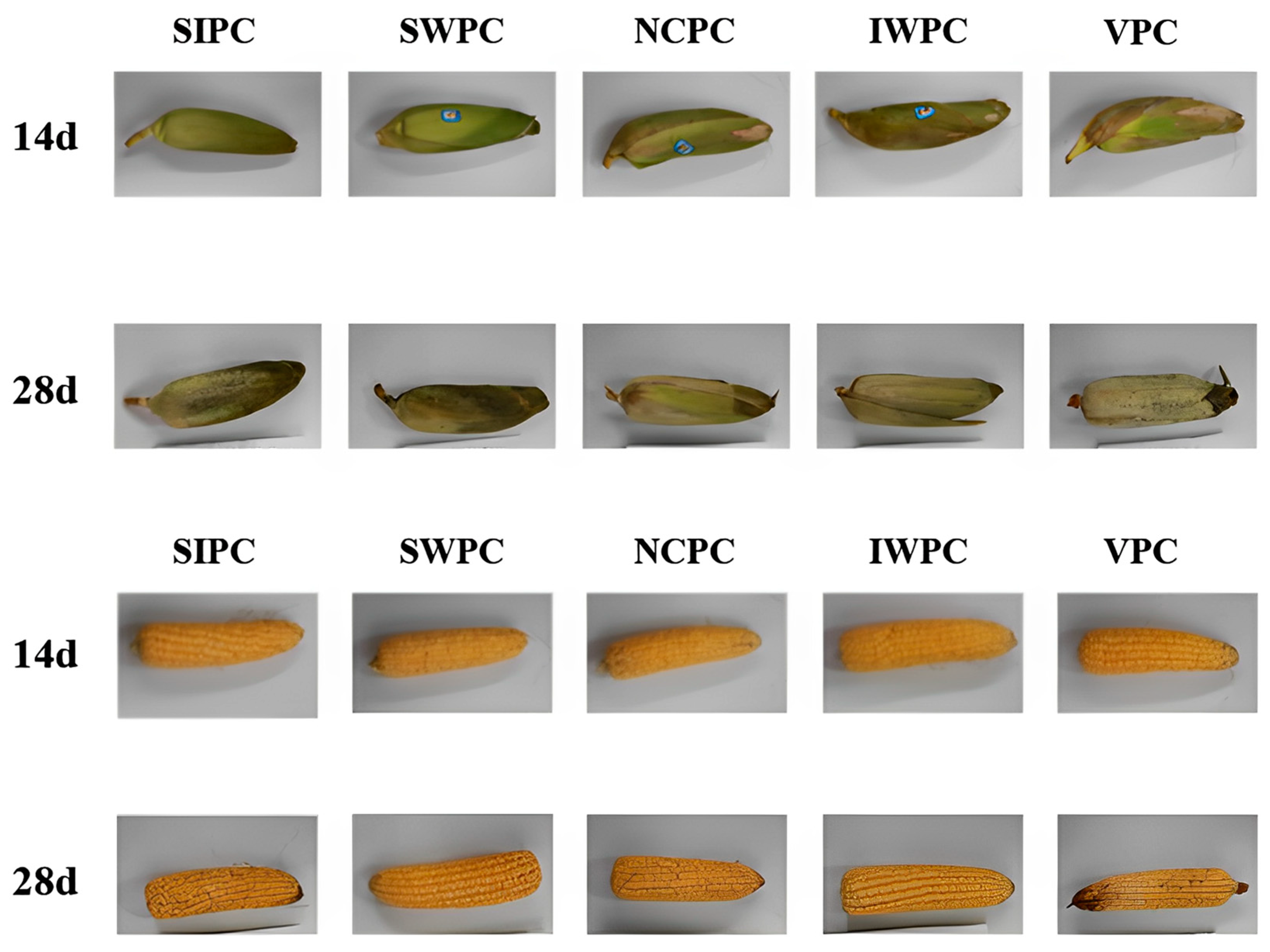
2.4. Color
2.5. Total Soluble Solids (TSS)
2.6. Soluble Sugar
2.7. Carotenoids
2.8. APX
2.9. CAT
2.10. POD
2.11. Sensory Characteristics
3. Materials and Methods
3.1. Sample Preparation
3.2. Pre-Cooling Methods
3.3. Determination of Sweet Corn Quality
3.3.1. Sensory Characteristics
3.3.2. The Weight Loss
3.3.3. Hardness
3.3.4. Color
3.3.5. Total Soluble Solids
3.3.6. Total Soluble Sugar
3.3.7. Carotenoids
3.3.8. Ascorbate-Peroxidase (APX)
3.3.9. Catalase (CAT)
3.3.10. Peroxidase (POD)
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yactayo-Chang, J.P.; Boehlein, S.; Beiriger, R.L.; Resende, M.F., Jr.; Bruton, R.G.; Alborn, H.T.; Romero, M.; Tracy, W.F.; Block, A.K. The impact of post-harvest storage on sweet corn aroma. Phytochem. Lett. 2022, 52, 33–39. [Google Scholar] [CrossRef]
- Becerra-Sanchez, F.; Taylor, G. Reducing post-harvest losses and improving quality in sweet corn (Zea mays L.): Challenges and solutions for less food waste and improved food security. Food Energy Secur. 2021, 10, e277. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, G.-B.; Fawole, O.A.; Verboven, P.; Zhang, X.-R.; Wu, D.; Opara, U.L.; Nicolai, B.; Chen, K. Postharvest precooling of fruit and vegetables: A review. Trends Food Sci. Technol. 2020, 100, 278–291. [Google Scholar] [CrossRef]
- Li, X.; Wu, W.; Li, K.; Ren, X.; Wang, Z. Experimental study on a wet precooling system for fruit and vegetables with ice slurry. Int. J. Refrig. 2022, 133, 9–18. [Google Scholar] [CrossRef]
- Wang, X.-F.; Fan, Z.-Y.; Li, B.-G.; Liu, E.-H. Variable air supply velocity of forced-air precooling of iceberg lettuces: Optimal cooling strategies. Appl. Therm. Eng. 2021, 187, 116484. [Google Scholar] [CrossRef]
- Dirapan, P.; Boonyakiat, D.; Poonlarp, P.J.H. Improving shelf life, maintaining quality, and delaying microbial growth of broccoli in supply chain using commercial vacuum cooling and package icing. Horticulturae 2021, 7, 506. [Google Scholar] [CrossRef]
- Tsang, M.; Furutani, S. A low cost hydrocooling unit for horticultural commodities. J. Hawaii Pac. Agric. 2006, 14, 1. [Google Scholar]
- Zainal, B.; Ding, P.; Ismail, I.S.; Saari, N. Physico-chemical and microstructural characteristics during postharvest storage of hydrocooled rockmelon (Cucumis melo L. reticulatus cv. Glamour). Postharvest Biol. Technol. 2019, 152, 89–99. [Google Scholar] [CrossRef]
- Liang, Y.S.; Wongmetha, O.; Wu, P.S.; Ke, L.S. Influence of hydrocooling on browning and quality of litchi cultivar Feizixiao during storage. Int. J. Refrig. 2013, 36, 1173–1179. [Google Scholar] [CrossRef]
- Álvares, V.S.; Finger, F.L.; de ASantos, R.C.; da Silva Negreiros, J.R.; Casali, V.W. Effect of pre-cooling on the postharvest of parsley leaves. J. Food Agric. Environ. 2007, 5, 31. [Google Scholar]
- Emond, J.-P.; Mercier, F.; Sadfa, S.; Bourré, M.; Gakwaya, A. Study of parameters affecting cooling rate and temperature distribution in forced-air precooling of strawberry. Trans. ASAE 1996, 39, 2185–2191. [Google Scholar] [CrossRef]
- An, R.; Luo, S.; Zhou, H.; Zhang, Y.; Zhang, L.; Hu, H.; Li, P. Effects of hydrogen-rich water combined with vacuum precooling on the senescence and antioxidant capacity of pakchoi (Brassica rapa subsp. Chinensis). Sci. Hortic. 2021, 289, 110469. [Google Scholar] [CrossRef]
- Zhang, X.; Yi, W.; Liu, G.; Kang, N.; Ma, L.; Yang, G. Colour and chlorophyll level modelling in vacuum-precooled green beans during storage. J. Food Eng. 2021, 301, 110523. [Google Scholar] [CrossRef]
- Zhu, Z.; Geng, Y.; Sun, D.-W. Effects of Pressure Reduction Modes on Vacuum Cooling Efficiency and Quality Related Attributes of Different Parts of Pakchoi (Brassica chinensis L.). Postharvest Biol. Technol. 2021, 173, 111409. [Google Scholar] [CrossRef]
- Bellas, J.; Chaer, I.; Tassou, S. Heat transfer and pressure drop of ice slurries in plate heat exchangers. Appl. Therm. Eng. 2002, 22, 721–732. [Google Scholar] [CrossRef]
- Garrido, Y.; Tudela, J.A.; Gil, M.I. Comparison of industrial precooling systems for minimally processed baby spinach. Postharvest Biol. Technol. 2015, 102, 1–8. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, X.; Geng, Y.; Sun, D.-W.; Chen, H.; Zhao, Y.; Zhou, W.; Li, X.; Pan, H. Effects of modified atmosphere vacuum cooling (MAVC) on the quality of three different leafy cabbages. LWT 2018, 94, 190–197. [Google Scholar] [CrossRef]
- Salamat, R.; Ghassemzadeh, H.R.; Ranjbar, F.; Jalali, A.; Mahajan, P.; Herppich, W.B.; Mellmann, J. The effect of additional packaging barrier, air moment and cooling rate on quality parameters of button mushroom (Agaricus bisporus). Food Packag. Shelf Life 2020, 23, 100448. [Google Scholar] [CrossRef]
- Liu, F.; Jia, B.; Li, Z.; Zhang, X. Thermodynamics analysis for forced air pre-cooling of cherry. J. Food Process Eng. 2021, 44, e13881. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, M.; Mei, J.; Xie, J. Effects of Different Postharvest Precooling Treatments on Cold-Storage Quality of Yellow Peach (Amygdalus persica). Plants 2022, 11, 2334. [Google Scholar] [CrossRef]
- Makule, E.; Dimoso, N.; Tassou, S.A. Precooling and Cold Storage Methods for Fruits and Vegetables in Sub-Saharan Africa—A Review. Horticulturae 2022, 8, 776. [Google Scholar] [CrossRef]
- Mittal, T.C.; Sharma, S.R.; Jindal, N. Effect of pre-cooling and packaging materials under ambient condition storage on postharvest quality of white button mushroom. Indian J. Sci. Res. Technol. 2014, 2, 60–72. [Google Scholar]
- Mahajan, P.V.; Oliveira, F.; Macedo, I. Effect of temperature and humidity on the transpiration rate of the whole mushrooms. J. Food Eng. 2008, 84, 281–288. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Min, W.; Zheng, M.; Li, H. Changes of moisture distribution and migration in fresh ear corn during storage. J. Integr. Agric. 2019, 18, 2644–2651. [Google Scholar] [CrossRef]
- Kozos, K.; Ochmian, I.; Chełpiński, P. The effects of rapid chilling and storage conditions on the quality of Brigitta Blue cultivar highbush blueberries (Vaccinium corymbosum L.). Folia Hortic. 2014, 26, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Fu, Y.; Yan, J.; Song, H.; Jiang, W. Forced Air Precooling Enhanced Storage Quality by Activating the Antioxidant System of Mango Fruits. J. Food Qual. 2019, 2019, 1606058. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.-W.; Zheng, L. Vacuum cooling technology for the agri-food industry: Past, present and future. J. Food Eng. 2006, 77, 203–214. [Google Scholar] [CrossRef]
- Pyatkovskyy, T.; Ranjbaran, M.; Datta, A.K.; Sastry, S.K. Factors affecting contamination and infiltration of Escherichia coli K12 into spinach leaves during vacuum cooling. J. Food Eng. 2021, 311, 110735. [Google Scholar] [CrossRef]
- Zhu, Z.; Geng, Y.; Sun, D.-W. Effects of operation processes and conditions on enhancing performances of vacuum cooling of foods: A review. Trends Food Sci. Technol. 2019, 85, 67–77. [Google Scholar] [CrossRef]
- Alique, R.; Martínez, M.A.; Alonso, J. Metabolic response to two hydrocooling temperatures in sweet cherries cv Lapins and cv Sunburst. J. Sci. Food Agric. 2006, 86, 1847–1854. [Google Scholar] [CrossRef]
- Saenz, E.; Borrás, L.; Gerde, J.A. Carotenoid profiles in maize genotypes with contrasting kernel hardness. J. Cereal Sci. 2021, 99, 103206. [Google Scholar] [CrossRef]
- Silva, G.M.C.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef]
- Johnston, J.W.; Hewett, E.W.; Hertog, M.L.A.T.M. Apple (Malus domestica) softening in the postharvest coolchain: Effects of delayed cooling and shelf-life temperatures. N. Z. J. Crop Hortic. Sci. 2005, 33, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.X.; Jin, W.W.; Chen, W.X.; Su, M.X.; Han, D.M. Physiological Response of Cold-Stored Loquat Fruit to Cool Temperature Pre-Treatments. Acta Hortic. 2007, 750, 451–456. [Google Scholar] [CrossRef]
- Calvo-Brenes, P.; O’Hare, T. Effect of freezing and cool storage on carotenoid content and quality of zeaxanthin-biofortified and standard yellow sweet-corn (Zea mays L.). J. Food Compos. Anal. 2020, 86, 103353. [Google Scholar] [CrossRef]
- He, S.Y.; Yu, Y.Q.; Zhang, G.C.; Yang, Q.R. Effects of Vacuum Pre-Cooling on Quality of Mushroom after Cooling and Storage. Adv. Mater. Res. 2013, 699, 189–193. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, J.H.; Gao, L.P.; Shi, J.Y.; Wu, B.; Yan, Z.C.; Wang, Q. Effect of Slurry Ice Precooling Treatment on Quality of Sweet Corn. J. Refrig. 2020, 41, 83–90. [Google Scholar]
- Saletnik, B.; Zaguła, G.; Saletnik, A.; Bajcar, M.; Słysz, E.; Puchalski, C. Method for prolonging the shelf life of apples after storage. Appl. Sci. 2022, 12, 3975. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Zhang, X.; Tian, J.; Yan, J.; Guo, L.; Wang, Y.; Song, L.; Yu, X. Differences in total phenolics, antioxidant activity and metabolic characteristics in peach fruits at different stages of ripening. LWT 2023, 178, 114586. [Google Scholar] [CrossRef]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.-T.; Yang, Y.-C.; Li, Y.-H.; Mau, J.-L.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.; Carvajal, F.; Jamilena, M.; Garrido, D. Putrescine treatment increases the antioxidant response and carbohydrate content in zucchini fruit stored at low temperature. Postharvest Biol. Technol. 2016, 118, 68–70. [Google Scholar] [CrossRef]
- Bello, F.A.; Henry, A.A. Storage effects and the postharvest quality of African star apple fruits (Chrysophyllum africanum) under ambient conditions. Afr. J. Food Sci. Technol. 2015, 6, 35–43. [Google Scholar]
- Marrufo-Díaz, M.d.l.L.; Vázquez-Carrillo, M.G.; Santiago-Ramos, D.; Ybarra-Moncada, M.C.; Mejía-Andrade, H. Physicochemical and sensory properties of ‘corn on the cob’ (elotes) from quality protein maize (QPM) hybrids as influenced by harvest stage and cold storage time. J. Cereal Sci. 2021, 102, 103348. [Google Scholar] [CrossRef]
- Han, Q.; Gao, H.; Chen, H.; Fang, X.; Wu, W. Precooling and ozone treatments affects postharvest quality of black mulberry (Morus nigra) fruits. Food Chem. 2017, 221, 1947–1953. [Google Scholar] [CrossRef]
- Leccese, A.; Bartolini, S.; Viti, R. Genotype, Harvest Season, and Cold Storage Influence on Fruit Quality and Antioxidant Properties of Apricot. Int. J. Food Prop. 2012, 15, 864–879. [Google Scholar] [CrossRef]
- Kachhadiya, S.; Kumar, N.; Seth, N. Process kinetics on physico-chemical and peroxidase activity for different blanching methods of sweet corn. J. Food Sci. Technol. 2018, 55, 4823–4832. [Google Scholar] [CrossRef]
- Suleiman, R.; Rosentrater, K.A.; Bern, C. Effects of deterioration parameters on storage of maize: A review. J. Nat. Sci. Res. 2013, 3, 147–165. [Google Scholar]
- Oluwalana, I.B.; Bolade, M.K.; Jolayemi, O.S.; Babarinsa, O.A.; Jeje, O.A.; Ojo, T.P. Influence of postharvest treatments on the proximate composition and sugar contents of fresh maize. J. Stored Prod. Postharvest Res. 2018, 9, 54–57. [Google Scholar]
- Ding, P.; Raja Mat, R.N. Hydro-cooling as Means to Retain Fresh Sweet Corn Ears Quality. Sains Malays. 2021, 50, 3593–3602. [Google Scholar] [CrossRef]
- Xu, J.; Tong, B.; Wang, R.; Xie, C.; Cao, M. Effect of different pre-cooling ways on freshness retaining of postharvest okra. Food Ind. Technol. 2014, 35, 312–315. [Google Scholar] [CrossRef]
- Vall-Llaura, N.; Fernández-Cancelo, P.; Nativitas-Lima, I.; Echeverria, G.; Teixidó, N.; Larrigaudière, C.; Torres, R.; Giné-Bordonaba, J. ROS-scavenging-associated transcriptional and biochemical shifts during nectarine fruit development and ripening. Plant Physiol. Biochem. 2022, 171, 38–48. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, O.F.; Ryan, L.; O’Brien, N.M. Xanthophyll carotenoids are more bioaccessible from fruits than dark green vegetables. Nutr. Res. 2007, 27, 258–264. [Google Scholar] [CrossRef]
- Walter, M.H.; Strack, D. Carotenoids and their cleavage products: Biosynthesis and functions. Nat. Prod. Rep. 2011, 28, 663–692. [Google Scholar] [CrossRef]
- Caprioli, I.; Lafuente, M.T.; Rodrigo, M.J.; Mencarelli, F. Influence of Postharvest Treatments on Quality, Carotenoids, and Abscisic Acid Content of Stored “Spring Belle” Peach (Prunus persica) Fruit. J. Agric. Food Chem. 2009, 57, 7056–7063. [Google Scholar] [CrossRef]
- Zainal, B.; Ding, P.; Ismail, I.S.; Saari, N. H NMR metabolomics profiling unveils the compositional changes of hydro-cooled rockmelon (Cucumis melo L. reticulatus cv glamour) during storage related to in vitro antioxidant activity. Sci. Hortic. 2019, 246, 618–633. [Google Scholar] [CrossRef]
- Sivapriya, M.; Leela, S. Isolation and purification of a novel antioxidant protein from the water extract of Sundakai (Solanum torvum) seeds. Food Chem. 2007, 104, 510–517. [Google Scholar] [CrossRef]
- Brehe, J.E.; Burch, H.B. Enzymatic assay for glutathione. Anal. Biochem. 1976, 74, 189–197. [Google Scholar] [CrossRef]
- Fan, J.; Ren, J.; Zhu, W.; Amombo, E.; Fu, J.; Chen, L. Antioxidant responses and gene expression in bermudagrass under cold stress. J. Am. Soc. Hortic. Sci. 2014, 139, 699–705. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, W.; Cao, J.; Zhao, Y.; Gu, Y. Effect of cold-shock treatment on chilling injury in mango (Mangifera indica L. cv.‘Wacheng’) fruit. J. Sci. Food Agric. 2006, 86, 2458–2462. [Google Scholar] [CrossRef]
- Sun, Y.; Li, W. Effects the mechanism of micro-vacuum storage on broccoli chlorophyll degradation and builds prediction model of chlorophyll content based on the color parameter changes. Sci. Hortic. 2017, 224, 206–214. [Google Scholar] [CrossRef]
- Yan, H.; Chen, H.; Zhao, J.; Yao, T.; Ding, X. Postharvest H2O2 treatment affects flavor quality, texture quality and ROS metabolism of ‘Hongshi’kiwifruit fruit kept at ambient conditions. Food Chem. 2023, 405, 134908. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Song, Y.; Li, J.; Jiang, W. Forced-air precooling treatment enhanced antioxidant capacities of apricots. J. Food Process. Preserv. 2018, 42, e13320. [Google Scholar] [CrossRef]
- Zhu, F.; Chen, J.; Xiao, X.; Zhang, M.; Yun, Z.; Zeng, Y.; Xu, J.; Cheng, Y.; Deng, X. Salicylic acid treatment reduces the rot of postharvest citrus fruit by inducing the accumulation of H2O2, primary metabolites and lipophilic polymethoxylated flavones. Food Chem. 2016, 207, 68–74. [Google Scholar] [CrossRef]
- Xu, F.; Tang, Y.; Dong, S.; Shao, X.; Wang, H.; Zheng, Y.; Yang, Z. Reducing yellowing and enhancing antioxidant capacity of broccoli in storage by sucrose treatment. Postharvest Biol. Technol. 2016, 112, 39–45. [Google Scholar] [CrossRef]
- Li, L.; He, X.; Sun, J.; Li, C.; Ling, D.; Sheng, J.; Zheng, F.; Liu, G.; Li, J.; Tang, Y.; et al. Responses of Phospholipase D and Antioxidant System to Mechanical Wounding in Postharvest Banana Fruits. J. Food Qual. 2017, 2017, 8347306. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Gu, C.; Zhu, D.; Chao, H.; Liang, Y.; Quan, S. Near-freezing temperature (NFT) storage alleviates chilling injury by enhancing antioxidant metabolism of postharvest guava (Psidium guajava L.). Sci. Hortic. 2022, 305, 111395. [Google Scholar] [CrossRef]
- Aharoni, Y.; Copel, A.; Gil, M.; Fallik, E. Polyolefin stretch films maintain the quality of sweet corn during storage and shelf-life. Postharvest Biol. Technol. 1996, 7, 171–176. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Zheng, S. Effect of Pressure Pre-Cooling, Room Pre-Cooling and Cold Storage on Quality of Sweet Corn; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2012. [Google Scholar]
- Saengrayap, R.; Tansakul, A.; Mittal, G.S. Effect of far-infrared radiation assisted microwave-vacuum drying on drying characteristics and quality of red chilli. J. Food Sci. Technol.-Mysore 2015, 52, 2610–2621. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Kachhadiya, S.; Nayi, P. Storage stability and characterization of biochemical, rehydration and colour characteristics of dehydrated sweet corn kernels. J. Stored Prod. Res. 2020, 87, 101619. [Google Scholar] [CrossRef]
- Liu, H.; Li, D.; Xu, W.; Fu, Y.; Liao, R.; Shi, J.; Chen, Y. Application of passive modified atmosphere packaging in the preservation of sweet corns at ambient temperature. LWT 2021, 136, 110295. [Google Scholar] [CrossRef]
- Deng, L.Z.; Xiong, C.H.; Pei, Y.P.; Zhu, Z.Q.; Zheng, X.; Zhang, Y.; Yang, X.H.; Liu, Z.L.; Xiao, H.W. Effects of various storage conditions on total phenolic, carotenoids, antioxidant capacity, and color of dried apricots. Food Control 2022, 136, 108846. [Google Scholar] [CrossRef]



| Color | Storage Time | Pre-Cooling Methods | ||||
|---|---|---|---|---|---|---|
| Day | VPC | SIPC | IWPC | SWPC | NCPC | |
| L* | 0 | 81.47 ± 0.03 | 81.47 ± 0.03 | 81.47 ± 0.03 | 81.47 ± 0.03 | 81.47 ± 0.03 |
| 7 | 81.44 ± 1.27 a | 81.24 ± 0.1 a | 79.33 ± 0.81 b | 82.12 ± 0.53 a | 80.87 ± 0.75 a | |
| 14 | 78.42 ± 0.07 b | 78.1 ± 1.37 b | 80.14 ± 0.1 a | 77.28 ± 1.14 b | 78.87 ± 0.61 a | |
| 21 | 77.56 ± 1 b | 75.48 ± 1.1 c | 78.86 ± 0.59 a | 76.96 ± 0.73 b | 76.12 ± 0.63 c | |
| 28 | 74.88 ± 0.8 b | 75.29 ± 0.82 b | 77.88 ± 0.49 a | 75.55 ± 1.09 b | 75.49 ± 0.23 b | |
| b* | 0 | 40.07 ± 0.03 | 40.07 ± 0.03 | 40.07 ± 0.03 | 40.07 ± 0.03 | 40.07 ± 0.03 |
| 7 | 42.38 ± 0.78 b | 39.79 ± 3.08 b | 45.68 ± 3.27 a | 31.64 ± 5.37 c | 40.27 ± 3.18 b | |
| 14 | 33.33 ± 0.23 b | 38.44 ± 2.17 a | 38.16 ± 0.59 a | 36.68 ± 0.26 a | 31.54 ± 0.99 b | |
| 21 | 40.54 ± 2.03 a | 35.85 ± 4.14 c | 38.64 ± 2.14 b | 37.99 ± 0.85 b | 36.07 ± 3.63 c | |
| 28 | 31.98 ± 1.15 c | 36.6 ± 4.8 b | 37.82 ± 2.95 a | 35.95 ± 0.27 b | 36.94 ± 6.64 b | |
| Color and Shape | Odor and Mildew |
|---|---|
| Uniform color, full granules, and smooth skin | No mildew, impurities, odor |
| Uniform color, skin micro-folds | Almost no mildew and odor, slight impurities |
| Dark color, unsaturated granules, skin folds | Mildew, impurities, odor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhou, P.; Mei, J.; Xie, J. Effects of Different Pre-Cooling Methods on the Shelf Life and Quality of Sweet Corn (Zea mays L.). Plants 2023, 12, 2370. https://doi.org/10.3390/plants12122370
Zhang C, Zhou P, Mei J, Xie J. Effects of Different Pre-Cooling Methods on the Shelf Life and Quality of Sweet Corn (Zea mays L.). Plants. 2023; 12(12):2370. https://doi.org/10.3390/plants12122370
Chicago/Turabian StyleZhang, Chi, Pengcheng Zhou, Jun Mei, and Jing Xie. 2023. "Effects of Different Pre-Cooling Methods on the Shelf Life and Quality of Sweet Corn (Zea mays L.)" Plants 12, no. 12: 2370. https://doi.org/10.3390/plants12122370
APA StyleZhang, C., Zhou, P., Mei, J., & Xie, J. (2023). Effects of Different Pre-Cooling Methods on the Shelf Life and Quality of Sweet Corn (Zea mays L.). Plants, 12(12), 2370. https://doi.org/10.3390/plants12122370








