Optimization of Heterotrophic Culture Conditions for the Algae Graesiella emersonii WBG-1 to Produce Proteins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Carbon Source and Initial Concentration on Growth and Protein Yield of G. emersonii WBG-1
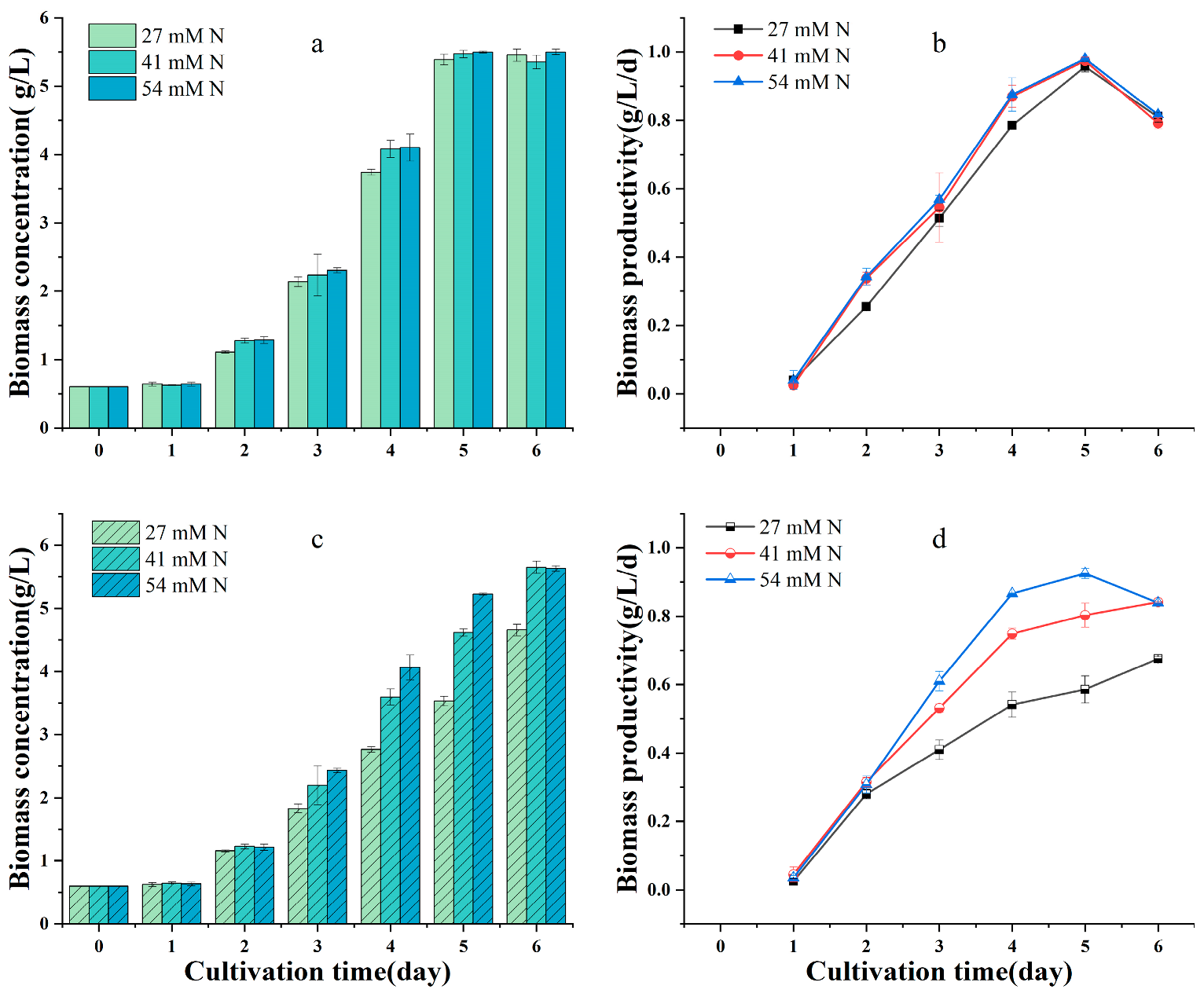
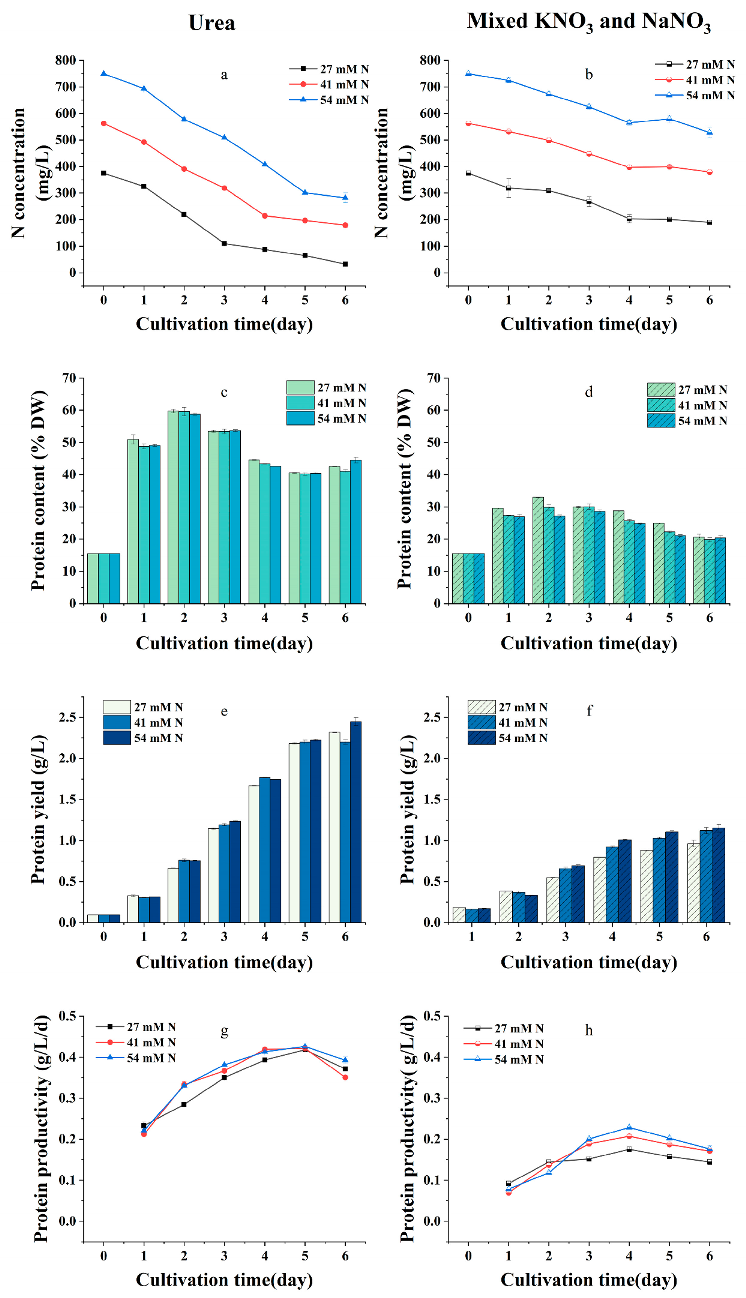
2.2. Effects of Nitrogen Sources and Initial Nitrogen Concentration on the Growth, Protein Content, and Yield of G. emersonii WBG-1
2.3. Effect of Temperature on Heterotrophic Growth, Protein Content, and Yield of G. emersonii WBG-1
3. Materials and Methods
3.1. Microalgal Strain
3.2. Algae Seed Culture Conditions
3.3. Optimization of the Carbon Source
3.4. Optimization of Nitrogen Source
3.5. Optimization of Fermentation Temperature
3.6. Analytical Procedures
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, Y.L.; Chen, T.P.; Chen, S.H.Y.; Liu, B.; Sun, P.P.; Sun, H.; Chen, F. The potentials and challenges of using microalgae as an ingredient to produce meat analogues. Trends Food Sci. Technol. 2021, 112, 188–200. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madeira, M.S.; Cardoso, C.; Lopes, P.A.; Coelho, D.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Microalgae as feed ingredients for livestock production and meat quality: A review. Livest. Sci. 2017, 205, 111–121. [Google Scholar] [CrossRef]
- Matsuda, F.; Hayashi, M.; Kondo, A. Comparative profiling analysis of central metabolites in Euglena gracilis under various cultivation conditions. Biosci. Biotechnol. Biochem. 2011, 75, 2253–2256. [Google Scholar] [CrossRef] [Green Version]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Abu-Ghosh, S.; Dubinsky, Z.; Verdelho, V.; Iluz, D. Unconventional high-value products from microalgae: A review. Bioresour. Technol. 2021, 329, 124895. [Google Scholar] [CrossRef]
- Nitsos, C.; Filali, R.; Taidi, B.; Lemaire, J. Current and novel approaches to downstream processing of microalgae: A review. Biotechnol. Adv. 2020, 45, 107650. [Google Scholar] [CrossRef]
- Avinash, A.; Sasikumar, P.; Pugazhendhi, A. Analysis of the limiting factors for large scale microalgal cultivation: A promising future for renewable and sustainable biofuel industry. Renew. Sustain. Energy Rev. 2020, 134, 110250. [Google Scholar] [CrossRef]
- Liang, M.H.; Wang, L.; Wang, Q.; Zhu, J.; Jiang, J.G. High-value bioproducts from microalgae: Strategies and progress. Crit. Rev. Food Sci. Nutr. 2019, 59, 2423–2441. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.C.; Sun, Z.; Zhong, Y.J.; Jiang, Y.; Chen, F. Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: Assessment of algal oils for biodiesel production. Bioresour. Technol. 2011, 102, 106–110. [Google Scholar] [CrossRef]
- Morales-Sánchez, D.; Martinez-Rodriguez, O.A.; Martinez, A. Heterotrophic cultivation of microalgae: Production of metabolites of commercial interest. J. Chem. Technol. Biotechnol. 2017, 92, 925–936. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Chojnacka, K.; Noworyta, A. Evaluation of Spirulina sp. growth in photoautotrophic, heterotrophic and mixotrophic cultures. Enzym. Microb. Technol. 2004, 34, 461–465. [Google Scholar] [CrossRef]
- Li, X.F.; Xu, H.; Wu, Q.Y. Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol. Bioeng. 2007, 98, 764–771. [Google Scholar] [CrossRef]
- Bouyam, S.; Choorit, W.; Sirisansaneeyakul, S.; Chisti, Y. Heterotrophic production of Chlorella sp. TISTR 8990-biomass growth and composition under various production conditions. Biotechnol. Prog. 2017, 33, 1589–1600. [Google Scholar] [CrossRef]
- Vyas, S.; Patel, A.; Nabil Risse, E.; Krikigianni, E.; Rova, U.; Christakopoulos, P.; Matsakas, L. Biosynthesis of microalgal lipids, proteins, lutein, and carbohydrates using fish farming wastewater and forest biomass under photoautotrophic and heterotrophic cultivation. Bioresour. Technol. 2022, 359, 127494. [Google Scholar] [CrossRef]
- Klamczynska, B.; Mooney, W.D. Heterotrophic Microalgae A Scalable and Sustainable Protein Source. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Laurie, S., Eds.; Academic Press: South San Francisco, CA, USA, 2016; pp. 327–339. ISBN 978-0-12-802778-3. [Google Scholar]
- Ogbonna, J.C.; Masui, H.; Tanaka, H. Sequential heterotrophic/autotrophic cultivation—An efficient method of producing Chlorella biomass for health food and animal feed. J. Appl. Phycol. 1997, 9, 359–366. [Google Scholar] [CrossRef]
- Mahboob, S.; Rauf, A.; Ashraf, M.; Sultana, T.; Sultana, S.; Jabeen, F.; Rajoka, M.I.; Al-Balawi, H.F.A.; Al-Ghanim, K.A. High-density growth and crude protein productivity of a thermotolerant Chlorella vulgaris: Production kinetics and thermodynamics. Aquac. Int. 2011, 20, 455–466. [Google Scholar] [CrossRef]
- Fan, J.H.; Huang, J.K.; Li, Y.G.; Han, F.F.; Wang, J.; Li, X.W.; Wang, W.L.; Li, S.L. Sequential heterotrophy-dilution-photoinduction cultivation for efficient microalgal biomass and lipid production. Bioresour. Technol. 2012, 112, 206–211. [Google Scholar] [CrossRef]
- Xie, T.H.; Xia, Y.; Zeng, Y.; Li, X.R.; Zhang, Y.K. Nitrate concentration-shift cultivation to enhance protein content of heterotrophic microalga Chlorella vulgaris: Over-compensation strategy. Bioresour. Technol. 2017, 233, 247–255. [Google Scholar] [CrossRef]
- Barka, A.; Blecker, C. Microalgae as a potential source of single-cell proteins. A review. Biotechnol. Agron. Soc. Environ. 2016, 20, 427–436. [Google Scholar] [CrossRef]
- Santhana Kumar, V.; Das Sarkar, S.; Das, B.K.; Sarkar, D.J.; Gogoi, P.; Maurye, P.; Mitra, T.; Talukder, A.K.; Ganguly, S.; Nag, S.K.; et al. Sustainable biodiesel production from microalgae Graesiella emersonii through valorization of garden wastes-based vermicompost. Sci. Total Environ. 2022, 807, 150995. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.S.; Cho, K.; An, S.M.; Kim, E.S.; Ki, H.; Lee, C.H.; Choi, G.; Hong, J.W. Taxonomic and Biochemical Characterization of Microalga Graesiella emersonii GEGS21 for Its Potential to Become Feedstock for Biofuels and Bioproducts. Energies 2022, 15, 8725. [Google Scholar] [CrossRef]
- Do, J.M.; Yeo, H.T.; Suh, H.S.; Yoon, H.S. Effect of salt stress on the biomass productivity and potential bioenergy feedstock of Graesiella emersonii KNUA204 isolated from Ulleungdo Island, South Korea. Front. Energy Res. 2023, 11, 1056835. [Google Scholar] [CrossRef]
- Wen, X.B.; Du, K.; Wang, Z.J.; Peng, X.A.; Luo, L.M.; Tao, H.P.; Xu, Y.; Zhang, D.; Geng, Y.H.; Li, Y.G. Effective cultivation of microalgae for biofuel production: A pilot-scale evaluation of a novel oleaginous microalga Graesiella sp. WBG-1. Biotechnol. Biofuels 2016, 9, 123. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.B.; Tao, H.P.; Peng, X.A.; Wang, Z.J.; Ding, Y.; Xu, Y.; Liang, L.; Du, K.; Zhang, A.Q.; Liu, C.X.; et al. Sequential phototrophic-mixotrophic cultivation of oleaginous microalga Graesiella sp. WBG-1 in a 1000 m2 open raceway pond. Biotechnol. Biofuels 2019, 12, 27. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Science: London, UK, 2004; pp. 97–115. ISBN 978-0-47-099528-0. [Google Scholar]
- Heredia-Arroyo, T.; Wei, W.; Hu, B. Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Appl. Biochem. Biotechnol. 2010, 162, 1978–1995. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, W.K.; Lee, B.; Seon, G.; Suh, W.I.; Moon, M.; Chang, Y.K. Optimization of heterotrophic cultivation of Chlorella sp. HS2 using screening, statistical assessment, and validation. Sci. Rep. 2019, 9, 19383. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.K.; Wang, X.; Tao, H.H.; Sun, X.S.; Tian, Y.T. Heterotrophic culture of Chlorella pyrenoidosa using sucrose as the sole carbon source by co-culture with immobilized yeast. Bioresour. Technol. 2018, 249, 425–430. [Google Scholar] [CrossRef]
- Patel, A.; Matsakas, L.; Rova, U.; Christakopoulos, P. Heterotrophic cultivation of Auxenochlorella protothecoides using forest biomass as a feedstock for sustainable biodiesel production. Biotechnol. Biofuels 2018, 11, 169. [Google Scholar] [CrossRef]
- Tan, X.B.; Zhao, X.C.; Yang, L.B.; Liao, J.Y.; Zhou, Y.Y. Enhanced biomass and lipid production for cultivating Chlorella pyrenoidosa in anaerobically digested starch wastewater using various carbon sources and up-scaling culture outdoors. Biochem. Eng. J. 2018, 135, 105–114. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Tian, X.W.; Liu, T.T.; Wang, Z.J.; Guan, W.Y.; Guo, M.J.; Chu, J.; Zhuang, Y.P. A two-stage fed-batch heterotrophic culture of Chlorella protothecoides that combined nitrogen depletion with hyperosmotic stress strategy enhanced lipid yield and productivity. Process Biochem. 2017, 60, 74–83. [Google Scholar] [CrossRef]
- Wen, Z.Y.; Chen, F. Heterotrophic production of eicosapentaenoic acid by the diatom Nitzschia laevis: Effects of silicate and glucose. J. Ind. Microbiol. Biotechnol. 2000, 25, 218–224. [Google Scholar] [CrossRef]
- Xiao, X.H.; Zhou, Y.C.; Liang, Z.B.; Lin, R.Z.; Zheng, M.M.; Chen, B.L.; He, Y.J. A novel two-stage heterotrophic cultivation for starch-to-protein switch to efficiently enhance protein content of Chlorella sp. MBFJNU-17. Bioresour. Technol. 2022, 344, 126187. [Google Scholar] [CrossRef]
- Xu, Q.; Hou, G.L.; Chen, J.P.; Wang, H.X.; Yuan, L.; Han, D.X.; Hu, Q.; Jin, H. Heterotrophically ultrahigh-cell-density cultivation of a high protein-yielding unicellular alga Chlorella with a novel nitrogen-supply strategy. Front. Bioeng. Biotechnol. 2021, 9, 774854. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, H.; Zhou, Z.W.; Li, K.P.; Hou, G.L.; Xu, Q.; Chuai, W.H.; Zhang, C.W.; Han, D.X.; Hu, Q. Ultrahigh-cell-density heterotrophic cultivation of the unicellular green microalga Scenedesmus acuminatus and application of the cells to photoautotrophic culture enhance biomass and lipid production. Biotechnol. Bioeng. 2020, 117, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.C.; Chang, C.H.; Chen, C.Y.; Chang, J.S.; Ng, I.S. Towards protein production and application by using Chlorella species as circular economy. Bioresour. Technol. 2019, 289, 121625. [Google Scholar] [CrossRef]
- Endo, H.; Nakajima, K.; Chino, R.; Shirota, M. Growth characteristics and cellular components of Chlorella regularis, heterotrophic fast growing strain. Agric. Biol. Chem. 2014, 38, 9–18. [Google Scholar] [CrossRef]
- Norihiko, H.; Ogbonna, J.C.; Hasegawa, Y.; Taroda, H.; Tanaka, H. Production of astaxanthin by Haematococcus pluvialis in a sequential heterotrophic-photoautotrophic culture. J. Appl. Phycol. 2001, 13, 395–402. [Google Scholar] [CrossRef]
- Schuler, L.; Greque de Morais, E.; Trovao, M.; Machado, A.; Carvalho, B.; Carneiro, M.; Maia, I.; Soares, M.; Duarte, P.; Barros, A.; et al. Isolation and characterization of novel Chlorella vulgaris mutants with low chlorophyll and improved protein contents for food applications. Front. Bioeng. Biotechnol. 2020, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Doucha, J.; Lívanský, K. Production of high-density Chlorella culture grown in fermenters. J. Appl. Phycol. 2011, 24, 35–43. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, L.; Chen, Y.; Zhu, M.M.; Xu, Q.; Wu, M.C.; Han, D.X.; Hu, Q. Trophic transition enhanced biomass and lipid production of the unicellular green alga Scenedesmus acuminatus. Front. Bioeng. Biotechnol. 2021, 9, 638–726. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.W.; Chinnasamy, S.; Bhatnagar, A.; Das, K.C. Effect of biochemical stimulants on biomass productivity and metabolite content of the microalga, Chlorella sorokiniana. Appl. Biochem. Biotechnol. 2010, 162, 2400–2414. [Google Scholar] [CrossRef]
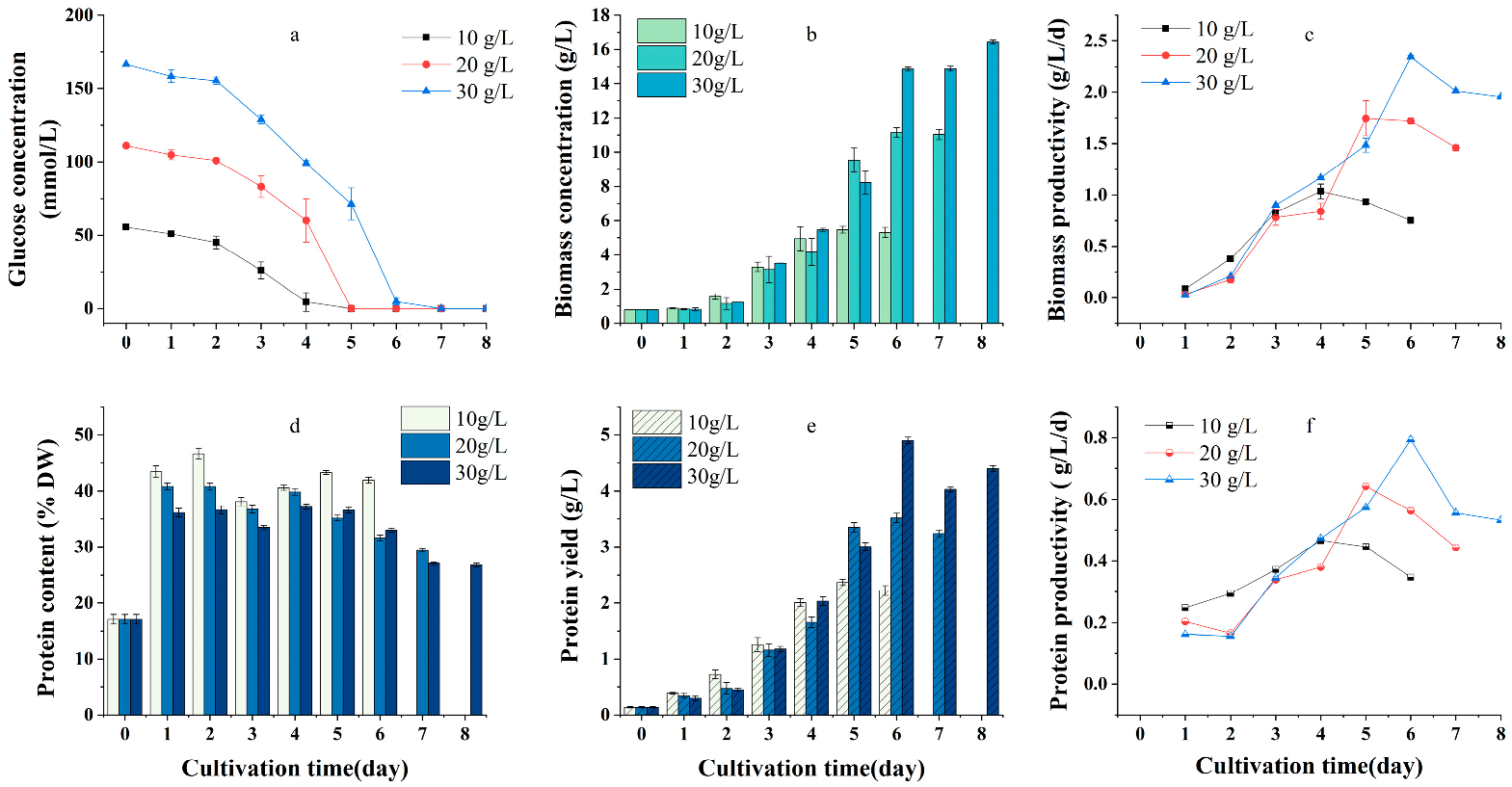

| Carbon Source | Initial Concentration (g·L−1) | Biomass (g·L−1) | Protein Content (%, DW) | Protein Yield (g·L−1) |
|---|---|---|---|---|
| Glucose | 30 | 15.36 ± 0.23 | 34.19 ± 0.53 | 5.25 ± 0.12 |
| Sodium acetate | 30 | 4.45 ± 0.15 | 38.89 ± 0.42 | 1.73 ± 0.06 |
| Sucrose | 30 | — | — | — |
| Species | Cultivation Mode | Protein Content (% DW) | Reference |
|---|---|---|---|
| Graesiella emersonii WBG-1 | heterotrophic | 66.1 | This study |
| Chlorella sp. CMBB276 | heterotrophic | 37.3 | [38] |
| Chlorella vulgaris | heterotrophic | 48.7 | [43] |
| Chlorella sorokiniana | heterotrophic | 16.3 | [16] |
| Chlorella vulgaris | heterotrophic | 36.5 | [44] |
| Chlorella vulgaris | two-stage heterotrophic | 44.3 | [21] |
| Chlorella vulgaris | two-stage heterotrophic | 59.8 | [37] |
| Chlorella | sequential heterotrophic/autotrophic | 60.1 | [18] |
| Chlorella vulgaris | Heterotrophy–dilution–photoinduction | 50.9 | [20] |
| Chlorella vulgaris | mixotrophic | 58.4 | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Wang, Z.; Ding, Y.; Yu, Y.; Wang, Y.; Geng, Y.; Li, Y.; Wen, X. Optimization of Heterotrophic Culture Conditions for the Algae Graesiella emersonii WBG-1 to Produce Proteins. Plants 2023, 12, 2255. https://doi.org/10.3390/plants12122255
Wang K, Wang Z, Ding Y, Yu Y, Wang Y, Geng Y, Li Y, Wen X. Optimization of Heterotrophic Culture Conditions for the Algae Graesiella emersonii WBG-1 to Produce Proteins. Plants. 2023; 12(12):2255. https://doi.org/10.3390/plants12122255
Chicago/Turabian StyleWang, Kaixuan, Zhongjie Wang, Yi Ding, Youzhi Yu, Yali Wang, Yahong Geng, Yeguang Li, and Xiaobin Wen. 2023. "Optimization of Heterotrophic Culture Conditions for the Algae Graesiella emersonii WBG-1 to Produce Proteins" Plants 12, no. 12: 2255. https://doi.org/10.3390/plants12122255
APA StyleWang, K., Wang, Z., Ding, Y., Yu, Y., Wang, Y., Geng, Y., Li, Y., & Wen, X. (2023). Optimization of Heterotrophic Culture Conditions for the Algae Graesiella emersonii WBG-1 to Produce Proteins. Plants, 12(12), 2255. https://doi.org/10.3390/plants12122255






