Components of the Phenylpropanoid Pathway in the Implementation of the Protective Effect of Sodium Nitroprusside on Wheat under Salinity
Abstract
1. Introduction
2. Results
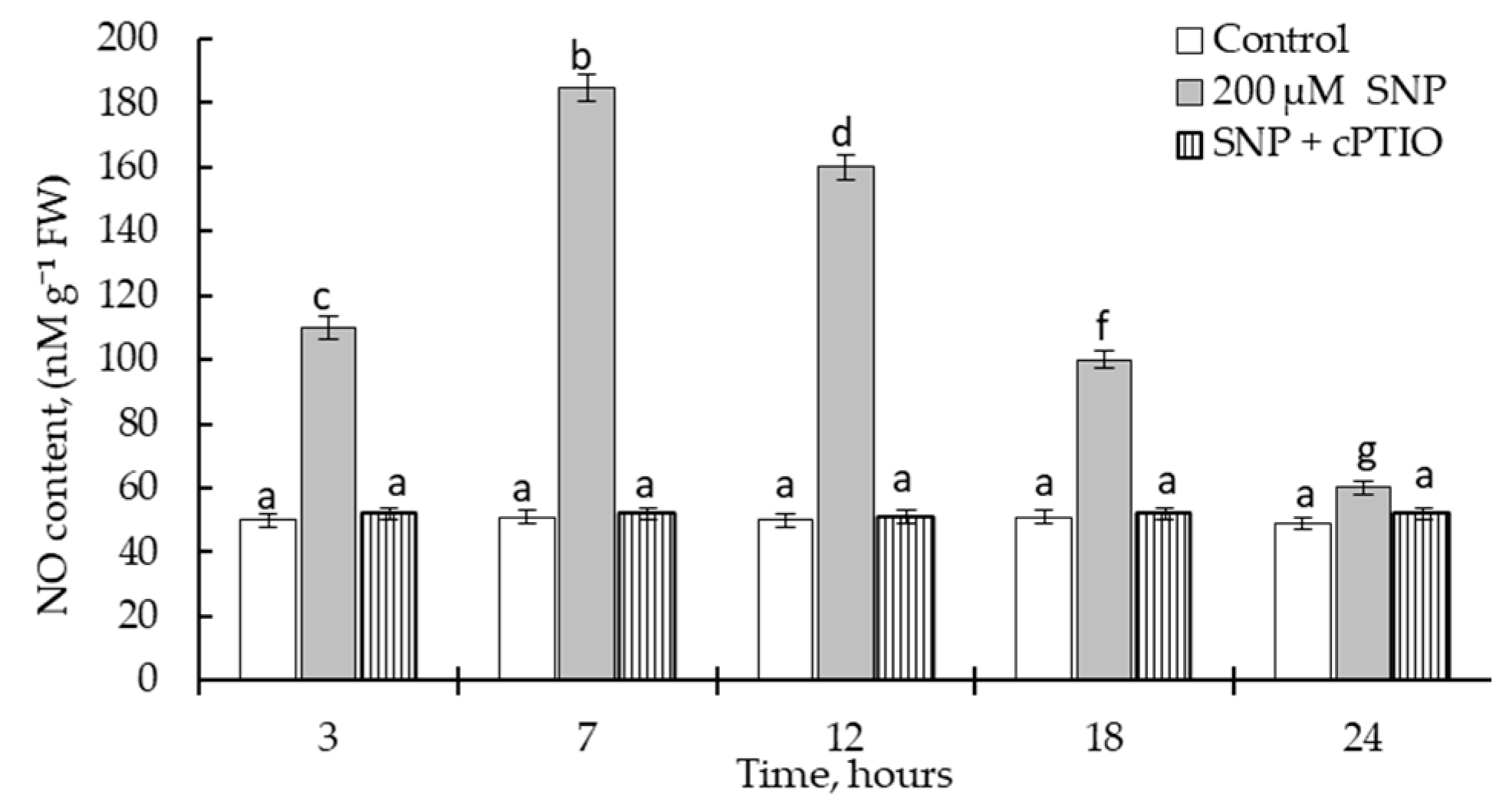
2.1. Influence of SNP and cPTIO on Growth Parameters and Endogenous NO Content in Wheat
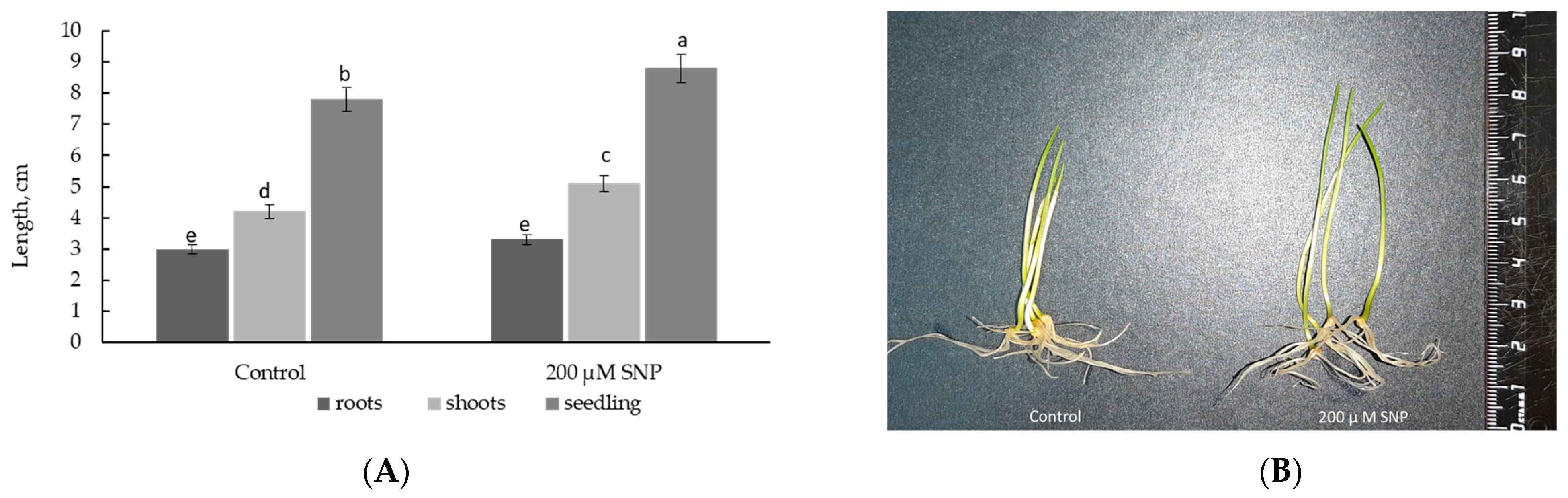
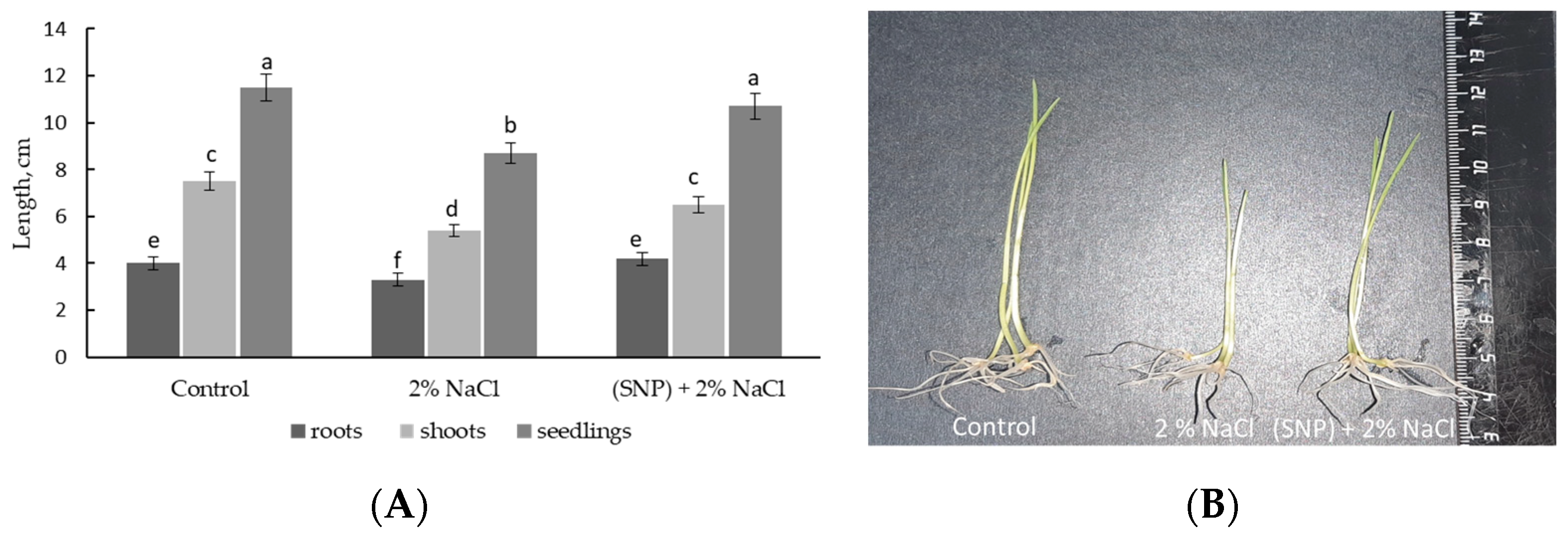
2.2. Exogenous SNP Improves Wheat Plant Growth under Normal and Salinity Conditions
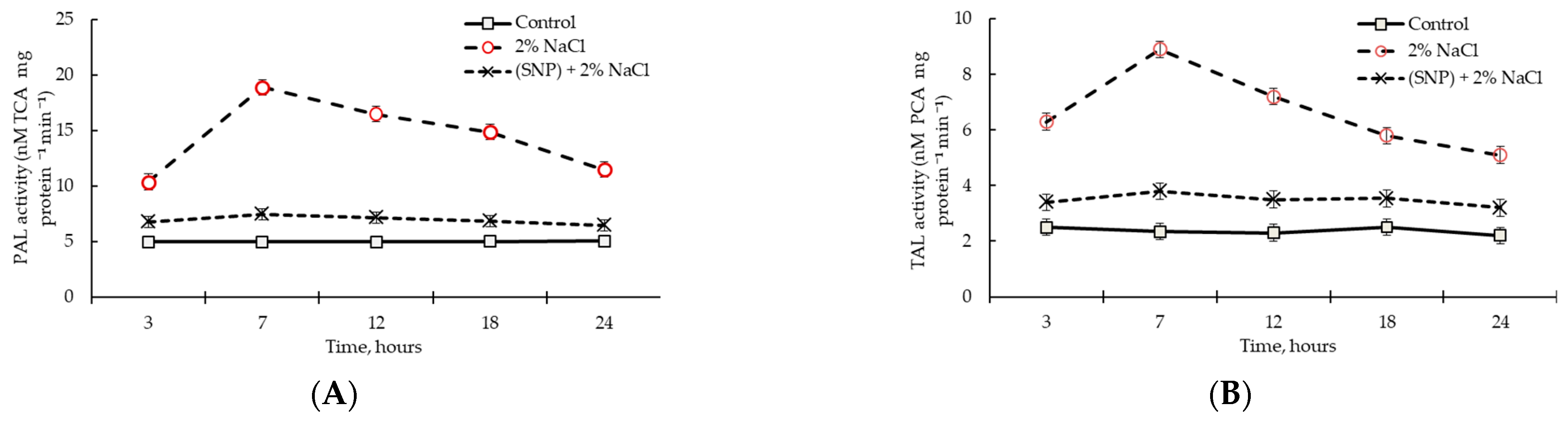
2.3. Effect of SNP Treatment on PAL and TAL Activities in Wheat Roots under Normal Conditions
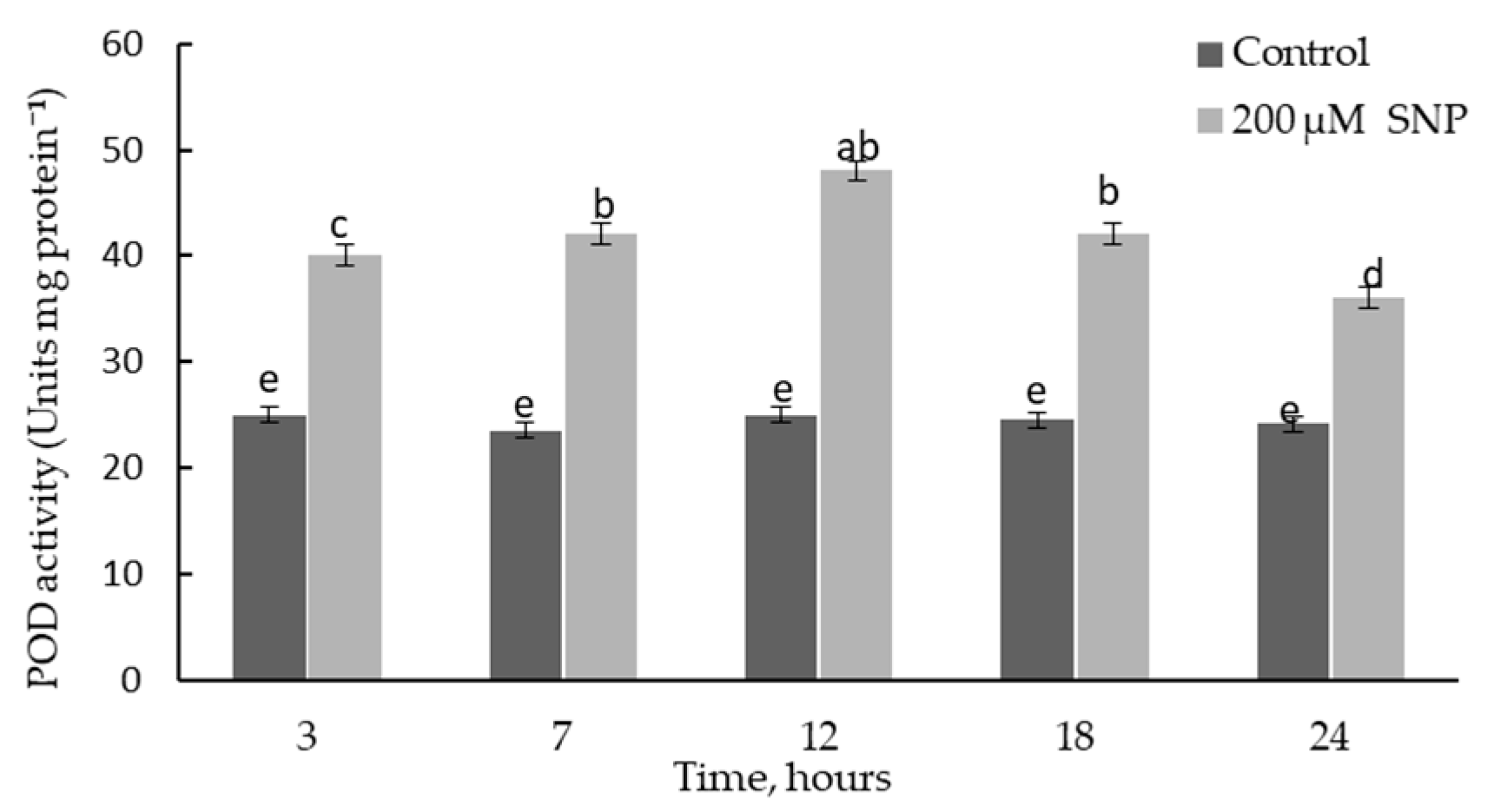
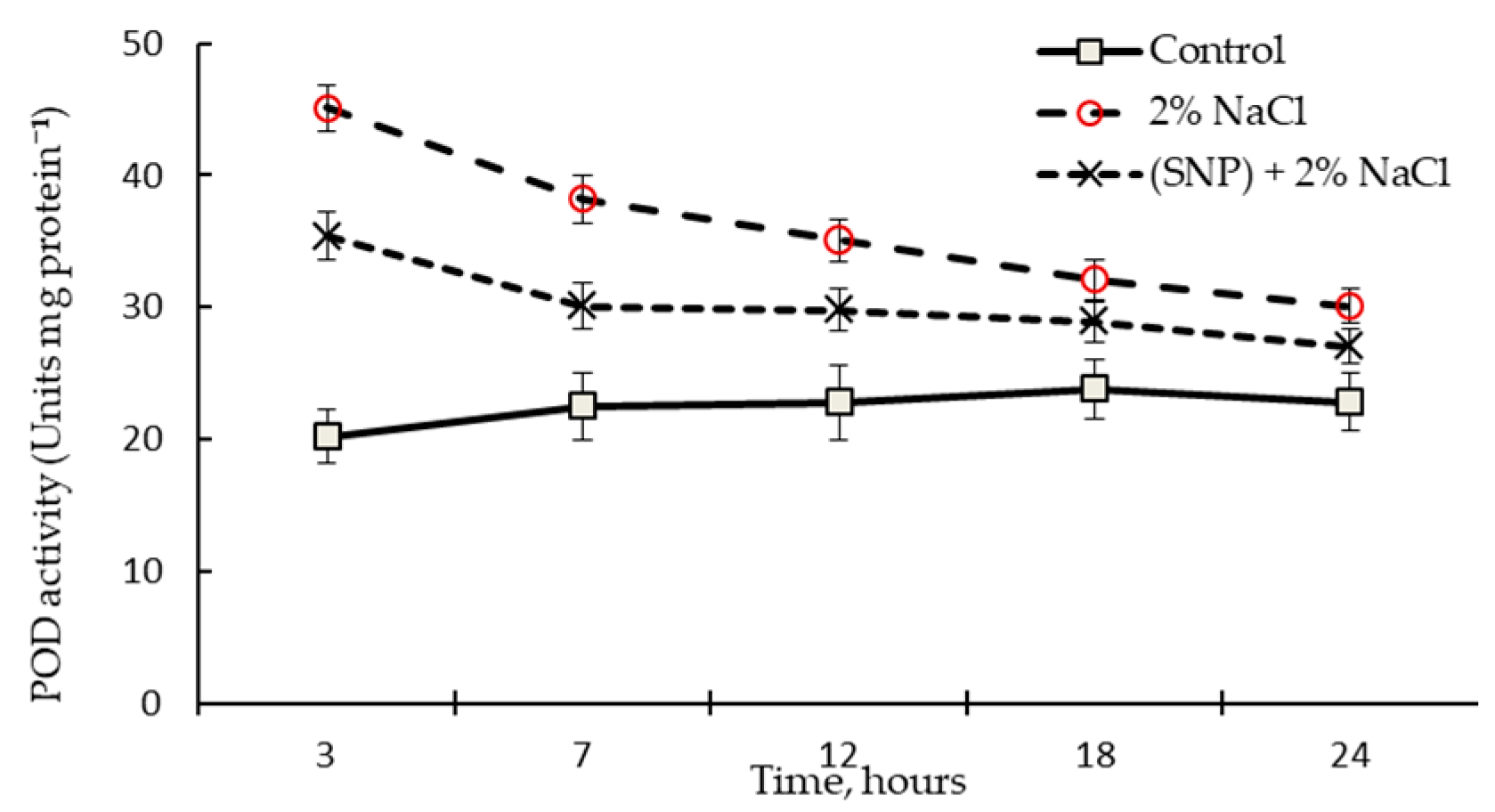
2.4. Effect of SNP on the Dynamics of POD Activity in the Roots of Wheat Seedlings and Lignin Content and Deposition in the Cell Walls of the Basal Part of the Roots
2.5. Gene Expression
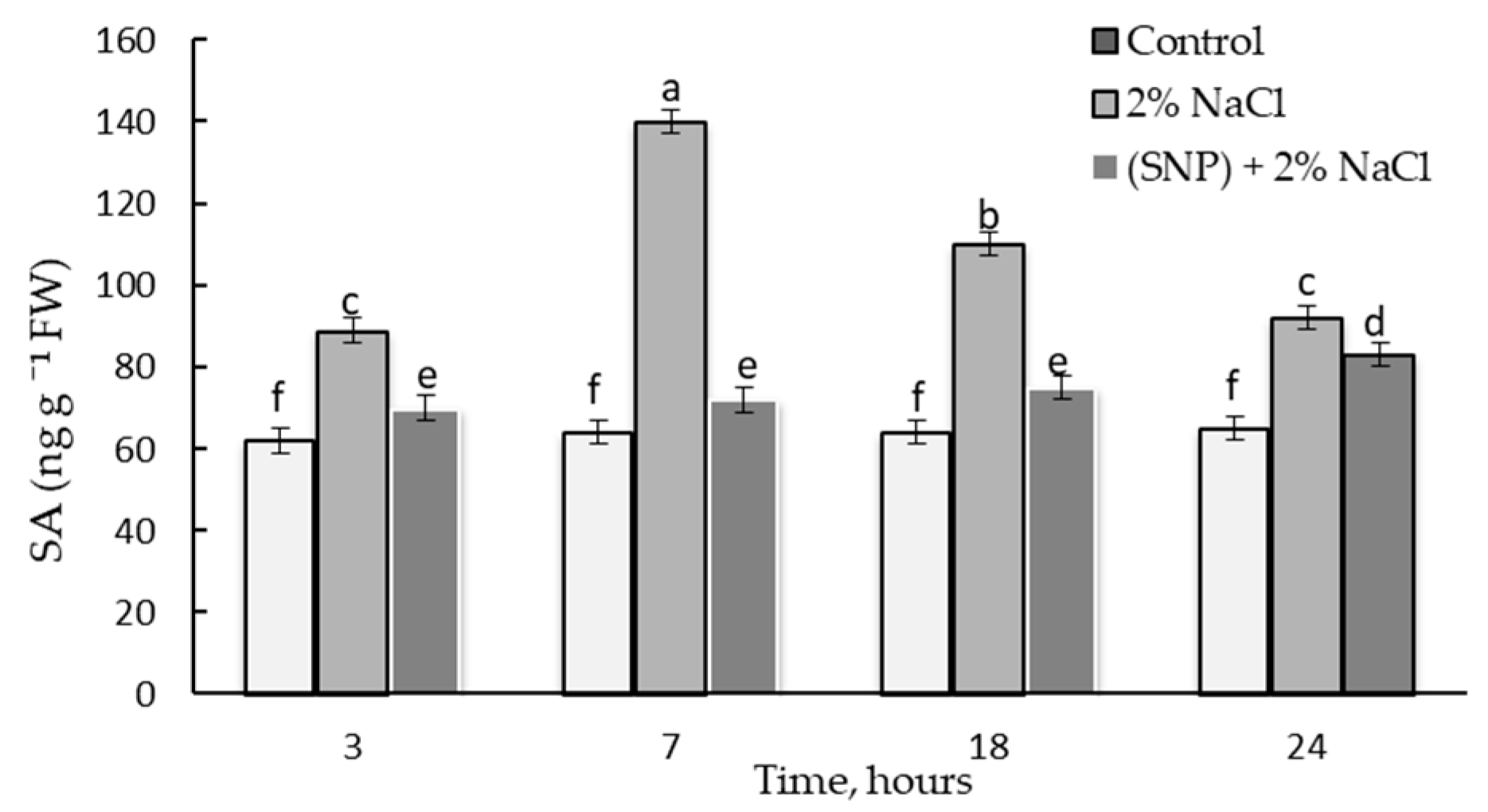
2.6. Dynamics of Endogenous SA Content in Wheat under the Action of 200 µM SNP
2.7. PAL and TAL Activities in SNP-Pretreated Wheat Roots under Salinity Conditions
2.8. Effect of 200 µM SNP on the Dynamics of POD Activity and Lignin Content and Deposition in the Cell Walls of the Basal Part of the Roots under Salinity Conditions
2.9. Effect of 200 µM SNP and Salinity on the Endogenous SA Content in Wheat Plants
3. Discussion
4. Materials and Methods
4.1. Plant Material, Seed Treatment, and Growth Conditions
4.2. Design of Experiments
4.3. Determination of Plant Growth Parameters
4.4. Assays of Enzyme Activities
4.4.1. PAL and TAL Activities
4.4.2. Peroxidase (POD) Activity
4.5. Lignin Determination
4.5.1. Quantitative Determination of Lignin
4.5.2. Lignin Deposition In-Situ
4.6. Endogenous Salicylic Acid (SA) Assay
4.7. Endogenous NO Measurement
4.8. Isolation of RNA and Performing the Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Cereal Supply and Demand Brief. 2021. Available online: http://www.fao.org/worldfoodsitua-tion/csdb/ru/ (accessed on 12 February 2023).
- Wang, D.; Gao, Y.; Sun, S.; Lu, X.; Li, Q.; Li, L.; Wang, K.; Liu, J. Effects of salt stress on the antioxidant activity and malondialdehyde, solution protein, proline, and chlorophyll contents of three malus species. Life 2022, 12, 1929. [Google Scholar] [CrossRef] [PubMed]
- Abdi, N.; Van Biljon, A.; Steyn, C.; Labuschagne, M.T. Salicylic acid improves growth and physiological attributes and salt tolerance differentially in two bread wheat cultivars. Plants 2022, 11, 1853. [Google Scholar] [CrossRef]
- Tanveer, M.; Shabala, S. Targeting Redox regulatory mechanisms for salinity stress tolerance in crops. In Salinity Responses and Tolerance in Plants; Kumar, V., Wani, S., Suprasanna, P., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, J.; Bi, C.; Li, L.; Wang, Z. Transcriptome and proteomics analysis of wheat seedling roots reveals that increasing NH4+/NO3− ratio induced root lignification and reduced nitrogen utilization. Front. Plant Sci. 2022, 12, 797260. [Google Scholar] [CrossRef]
- Zhang, C.; Meng, W.; Wang, Y.; Zhou, Y.; Wang, S.; Qi, F.; Wang, N.; Ma, J. Comparative analysis of physiological, hormonal and transcriptomic responses reveal mechanisms of saline-alkali tolerance in autotetraploid rice (Oryza sativa L.). Int. J. Mol. Sci. 2022, 23, 16146. [Google Scholar] [CrossRef] [PubMed]
- Byrt, C.S.; Munns, R.; Burton, R.A.; Gilliham, M.; Wege, S. Root cell wall solutions for crop plants in saline soils. Plant Sci. 2018, 269, 47–55. [Google Scholar] [CrossRef]
- Su, P.; Yan, J.; Li, W.; Wang, L.; Zhao, J.; Ma, X.; Li, A.; Wang, H.; Kong, L. A member of wheat class III peroxidase gene family, TaPRX-2A, enhanced the tolerance of salt stress. BMC Plant Biol. 2020, 20, 392. [Google Scholar] [CrossRef]
- Rahman, M.A.; Woo, J.H.; Lee, S.-H.; Park, H.S.; Kabir, A.H.; Raza, A.; El Sabagh, A.; Lee, K.-W. Regulation of Na+/H+ exchangers, Na+/K+ transporters, and lignin biosynthesis genes, along with lignin accumulation, sodium extrusion, and antioxidant defense, confers salt tolerance in alfalfa. Front. Plant Sci. 2022, 13, 1041764. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed Vigna angularis. Biomolecules 2020, 10, 42. [Google Scholar] [CrossRef]
- Chun, H.J.; Baek, D.; Cho, H.M.; Lee, S.H.; Jin, B.J.; Yun, D.-J.; Hong, Y.-S.; Kim, M.C. Lignin biosynthesis genes play critical roles in the adaptation of Arabidopsis plants to high-salt stress. Plant Signal. Behav. 2019, 14, 1625697. [Google Scholar] [CrossRef]
- Sehar, Z.; Masood, A.; Khan, N.A. Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ. Exp. Bot. 2019, 161, 277–289. [Google Scholar] [CrossRef]
- Al-Zahrani, H.S.; Alharby, H.F.; Hakeem, K.R.; Rehman, R.U. Exogenous application of zinc to mitigate the salt stress in Vigna radiata (L.) Wilczek—Evaluation of physiological and biochemical processes. Plants 2021, 10, 1005. [Google Scholar] [CrossRef]
- Alam, P.; Balawi, T.A.; Faizan, M. Salicylic acid’s impact on growth, photosynthesis, and antioxidant enzyme activity of Triticum aestivum when exposed to salt. Molecules 2023, 28, 100. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, J.; Tschaplinski, T.J.; Tuskan, G.A.; Chen, J.-G.; Muchero, W. Regulation of lignin biosynthesis and its role in growth-defense tradeoffs. Front. Plant Sci. 2018, 9, 1427. [Google Scholar] [CrossRef] [PubMed]
- Böhm, F.M.L.Z.; Ferrarese, M.L.L.; Zanardo, D.I.L. Nitric oxide affecting root growth, lignification and related enzymes in soybean seedlings. Acta Physiol. Plant. 2010, 32, 1039–1046. [Google Scholar] [CrossRef]
- Falahi, H.; Sharifi, M.; Chashmi, N.A.; Maivan, H.Z. Water stress alleviation by polyamines and phenolic compounds in Scrophularia striata is mediated by NO and H2O2. Plant Physiol. Biochem. 2018, 130, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Cuong, D.M.; Kwon, S.-J.; Nguyen, B.V.; Chun, S.W.; Kim, J.K.; Park, S.U. Effect of salinity stress on phenylpropanoid genes expression and related gene expression in wheat sprout. Agronomy 2020, 10, 390. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Barros, J.; Dixon, R.A. Plant phenylalanine/tyrosine ammonia-lyases. Trends Plant Sci. 2020, 1, 66–79. [Google Scholar] [CrossRef]
- Rasool, F.; Uzair, M.; Naeem, M.K.; Rehman, N.; Afroz, A.; Shah, H.; Khan, M.R. Phenylalanine ammonia-lyase (PAL) genes family in wheat (Triticum aestivum L.): Genome-wide characterization and expression profiling. Agronomy 2021, 11, 2511. [Google Scholar] [CrossRef]
- Nguyen, T.-N.; Son, S.H.; Jordan, M.C.; Levin, D.B.; Ayele, B.T. Lignin biosynthesis in wheat (Triticum aestivum L.): Its response to waterlogging and association with hormonal levels. BMC Plant Biol. 2016, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Luo, Y.; Jin, M.; Sun, S.; Wang, Z.; Li, Y. Response of lignin metabolism to light quality in wheat population. Front. Plant Sci. 2021, 12, 729647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, X.; Jia, C.; Xu, B.; Wang, J.; Liu, J.; Jin, Z. Identification and analysis of lignin biosynthesis genes related to fruit ripening and stress response in banana (Musa acuminata L. AAA group, cv. Cavendish). Front. Plant Sci. 2023, 14, 891. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Weng, J.K.; Chapple, C. Improvement of biomass through lignin modification. Plant J. 2008, 54, 569–581. [Google Scholar] [CrossRef]
- Cuong, D.M.; Ha, T.W.; Park, C.H.; Kim, N.S.; Yeo, H.J.; Chun, S.W.; Kim, C.; Park, S.U. Effects of LED lights on expression of genes involved in phenylpropanoid biosynthesis and accumulation of phenylpropanoids in wheat sprout. Agronomy 2019, 9, 307. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Blaschek, L.; Pesquet, E. Phenoloxidases in plants—How structural diversity enables functional specificity. Front. Plant Sci. 2021, 12, 754601. [Google Scholar] [CrossRef]
- Nadarajah, K.; Abdul Hamid, N.W.; Abdul Rahman, N.S.N. SA-mediated regulation and control of abiotic stress tolerance in rice. Int. J. Mol. Sci. 2021, 22, 5591. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, G.; Liu, C.; Li, H.; Chen, X.; Fu, Q.; Lin, Y.; Guo, B. Salicylic acid, a multifaceted hormone, combats abiotic stresses in plants. Life 2022, 12, 886. [Google Scholar] [CrossRef]
- Shakirova, F.M. Role of hormonal system in the manifestation of growth promoting and antistress action of salicylic acid. In Salicylic Acid: A Plant Hormone; Hayat, S., Ahmad, A., Eds.; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Khan, M.I.R.; Poor, P.; Janda, T. Salicylic acid: A versatile signaling molecule in plants. J. Plant Growth Regul. 2022, 41, 1887–1890. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, F.; Mir, I.R.; Sehar, Z.; Fatma, M.; Gautam, H.; Khan, S.; Anjum, N.A.; Masood, A.; Sofo, A.; Khan, N.A. Nitric oxide and salicylic acid regulate glutathione and ethylene production to enhance heat stress acclimation in wheat involving sulfur assimilation. Plants 2022, 11, 3131. [Google Scholar] [CrossRef]
- Ren, R.; Wei, Y.; Ahmad, S.; Jin, J.; Gao, J.; Lu, C.; Zhu, G.; Yang, F. Identification and characterization of NPR1 and PR1 homologs in Cymbidium orchids in response to multiple hormones, salinity and viral stresses. Int. J. Mol. Sci. 2020, 21, 1977. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, M.; Zribi, I.; Haddaji, N.; Besbes, M.; Bouali, N.; Brini, F. The wheat pathogenesis related protein (TdPR1.2) ensures contrasting behaviors to E. coli transformant cells under stress conditions. Adv. Microbiol. 2021, 11, 453–468. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Tang, B.; Zheng, L.; Chen, H.; Cui, X.; Ge, F.; Liu, D. A pathogenesis-related protein-like gene is involved in the Panax notoginseng defense response to the root rot pathogen. Front. Plant Sci. 2021, 11, 610176. [Google Scholar] [CrossRef]
- Valderrama, R.; Mounira, C.; Juan Carlos, B.-M.; Petrivalský, M.; Barroso, J.B. Editorial: Nitric oxide in plants. Front. Plant Sci. 2021, 12, 705157. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhang, Z.; Zhang, P.; Zhu, X.; Jing, Y.; Wei, J.; Wu, B. Nitric oxide enhances resistance against black spot disease in muskmelon and the possible mechanisms involved. Sci. Hortic. 2019, 256, 108650. [Google Scholar] [CrossRef]
- Floryszak-Wieczorek, J.; Milczarek, G.; Arasimowicz, M.; Ciszewski, A. Do nitric oxide donors mimic endogenous NO-related response in plants? Planta 2006, 224, 1363–1372. [Google Scholar] [CrossRef]
- Jahan, B.; Rasheed, F.; Sehar, Z.; Fatma, M.; Iqbal, N.; Masood, A.; Anjum, N.A.; Khan, N.A. Coordinated role of nitric oxide, ethylene, nitrogen, and sulfur in plant salt stress tolerance. Stresses 2021, 1, 181–199. [Google Scholar] [CrossRef]
- Maslennikova, D.R.; Allagulova, C.h.R.; Fedorova, K.A.; Plotnikov, A.A.; Avalbaev, A.M.; Shakirova, F.M. Cytokinins contribute to realization of nitric oxide growth-stimulating and protective effects on wheat plants. Russ. J. Plant Physiol. 2017, 64, 665–671. [Google Scholar] [CrossRef]
- Maslennikova, D.R.; Lastochkina, O.V.; Shakirova, F.M. Exogenous sodium nitroprusside improves salt stress tolerance of wheat (Triticum aestivum L.) via regulating the components of ascorbate-glutathione cycle, chlorophyll content and stabilization of cell membranes state. Russ. J. Plant Physiol. 2022, 69, 130. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Nitric oxide induced modulations in adventitious root growth, lignin content and lignin synthesizing enzymes in the hypocotyls of Vigna radiata. Plant Physiol. Biochem. 2019, 141, 225–230. [Google Scholar] [CrossRef]
- Hao, G.; Du, X.; Zhao, F.; Shi, R.; Wang, J. Role of nitric oxide in UV-B-induced activation of PAL and stimulation of flavonoid biosynthesis in Ginkgo biloba callus. Plant Cell Tiss. Organ Cult. 2009, 97, 175–185. [Google Scholar] [CrossRef]
- Klessig, D.F.; Durner, J.; Noad, R.; Navarre, D.A.; Wendehenne, D.; Kumar, D.; Zhou, J.M.; Shah, J.; Zhang, S.; Kachroo, P. Nitric oxide and salicylic acid signaling in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 8849–8855. [Google Scholar] [CrossRef] [PubMed]
- Rezayian, M.; Ebrahimzadeh, H.; Niknam, V. Nitric oxide stimulates antioxidant system and osmotic adjustment in soybean under drought stress. J. Soil Sci. Plant Nutr. 2020, 20, 1122–1132. [Google Scholar] [CrossRef]
- Keisham, M.; Jain, P.; Singh, N.; von Toerne, C.; Bhatla, S.C.; Lindermayr, C. Deciphering the nitric oxide, cyanide and iron-mediated actions of sodium nitroprusside in cotyledons of salt stressed sunflower seedlings. Nitric Oxide 2019, 88, 10–26. [Google Scholar] [CrossRef]
- Planchet, E.; Kaiser, W.M. Nitric oxide production in plants: Facts and fictions. Plant Signal. Behav. 2006, 1, 46–51. [Google Scholar] [CrossRef]
- Soares, C.; Rodrigues, F.; Sousa, B.; Pinto, E.; Ferreira, I.M.P.L.V.O.; Pereira, R.; Fidalgo, F. Foliar application of sodium nitroprusside boosts Solanum lycopersicum L. tolerance to glyphosate by preventing redox disorders and stimulating herbicide detoxification pathways. Plants 2021, 10, 1862. [Google Scholar] [CrossRef]
- Feduraev, P.; Riabova, A.; Skrypnik, L.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Assessment of the role of PAL in lignin accumulation in wheat (Triticum aestivum L.) at the early stage of ontogenesis. Int. J. Mol. Sci. 2021, 22, 9848. [Google Scholar] [CrossRef]
- Tugbaeva, A.; Ermoshin, A.; Plotnikov, D.; Wuriyanghan, H.; Kiseleva, I. Role of Class III Peroxidases in Stem Lignification of Zinnia elegans Jacq. Biol. Life Sci. Forum 2021, 4, 22. [Google Scholar] [CrossRef]
- Bhai, R.S.; Alex, R.; Vadivukkarasi, P.; Shivakumar, M.S. Peroxidase activity as a marker to evaluate resistance in Black pepper against Phytophthora infection. Indian Phytopathol. 2021, 74, 1099–1104. [Google Scholar] [CrossRef]
- Wu, L.; Jiang, Q.; Zhang, Y.; Du, M.; Ma, L.; Ma, Y. Peroxidase activity in tomato leaf cells under salt stress based on micro-hyperspectral imaging technique. Horticulturae 2022, 8, 813. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Singh, S. Salicylic acid elicitation improves antioxidant activity of spinach leaves by increasing phenolic content and enzyme levels. Food Chem. Adv. 2023, 2, 100156. [Google Scholar] [CrossRef]
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic acid in root growth and development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef] [PubMed]
- Mokronosova, A.T. Small Workshop on Plant Physiology; Moscow State University: Moscow, Russia, 1994; 184p. [Google Scholar]
- Jiang, H.; Wang, B.; Ma, L.; Zheng, X.; Gong, D.; Xue, H.; Bi, Y.; Wang, Y.; Zhang, Z.; Prusky, D. Benzo-(1,2,3)-thiadiazole-7-carbothioic acid s-methyl ester (BTH) promotes tuber wound healing of potato by elevation of phenylpropanoid metabolism. Postharvest Biol. Technol. 2019, 153, 125–132. [Google Scholar] [CrossRef]
- Rösler, J.; Krekel, F.; Amrhein, N.; Schmid, J. Maize phenylalanine ammonia-lyase has tyrosine ammonia-lyase activity. Plant Physiol. 1997, 113, 175–179. [Google Scholar] [CrossRef]
- Pütter, J. Peroxidases. In Methods of Enzymatic Analysis; Academic Press: San Diego, CA, USA, 1974; pp. 685–690. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sancho, M.A.; de Forchetti, S.M.; Pliego, F.; Valpuesta, V.; Quesada, M.A. Peroxidase activity and isoenzymes in the culture medium of NaCl adapted tomato suspension cells. Plant Cell Tissue Organ Cult. 1996, 44, 161–167. [Google Scholar] [CrossRef]
- Tao, S.; Khanizadeh, S.; Zhang, H.; Zhang, S. Anatomy, ultrastructure and lignin distribution of stone cells in two Pyrus species. Plant Sci. 2009, 176, 413–419. [Google Scholar] [CrossRef]
- Temerdashev, Z.; Frolova, N.A.; Kolychev, I.A. Determination of phenolic compounds in medicinal herbs by reversed-phase HPLC. J. Anal. Chem. 2011, 66, 407–414. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Wany, A.; Pandey, S.; Bulle, M.; Kumari, A.; Kishorekumar, R.; Igamberdiev, A.U.; Mur, L.A.J.; Gupta, K.J. Current approaches to measure nitric oxide in plants. J. Exp. Bot. 2019, 70, 4333–4343. [Google Scholar] [CrossRef]
- Rumyantsev, S.D.; Alekseev, V.Y.; Sorokan, A.V.; Burkhanova, G.F.; Cherepanova, E.A.; Garafutdinov, R.R.; Maksimov, I.V.; Veselova, S.V. Additive effect of the composition of endophytic bacteria Bacillus subtilis on systemic resistance of wheat against greenbug aphid Schizaphis graminum due to lipopeptides. Life 2023, 13, 214. [Google Scholar] [CrossRef]




| Visual Assessment of Lignin Deposition in Roots | Lignin Content (ΔA 540 nm/g FW) |
|---|---|
 Control | 0 |
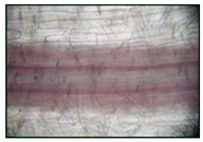 200 µM SNP | 1.35 ± 0.062 |
| Visual Assessment of Lignin Deposition in Roots | Lignin Content in Roots (Δ A 540 nm/g FW) |
|---|---|
 Control | 0 |
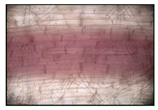 2% NaCl | 1.92 ± 0.076 b |
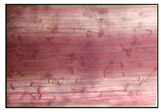 (SNP) + 2% NaCl | 2.89 ± 0.115 a |
| Gene’s Product | Genes | GenBank Accession Number | Sequence (5′-3′) | |
|---|---|---|---|---|
| Forward Primers | Reverse Primer | |||
| RNase L inhibitor | TaRLI | AY059462 | ttgagcaactcatggaccag | gctttccaaggcacaaacat |
| Phenylalanine ammonia-lyase | TaPAL | X99725 | ggcgtcaaaacatggcgtc | agtccgagaagtccgaga |
| PR1 | Ta PR1 | AF384143 | ataacctcggcgtcttcatc | gcttattacggcattcctttt |
| Peroxidase, PR9 | TaPR9 | TC 151917 | tcgacaagcagtactaccacaa | ccgaagtccgagaagaactg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslennikova, D.; Ivanov, S.; Petrova, S.; Burkhanova, G.; Maksimov, I.; Lastochkina, O. Components of the Phenylpropanoid Pathway in the Implementation of the Protective Effect of Sodium Nitroprusside on Wheat under Salinity. Plants 2023, 12, 2123. https://doi.org/10.3390/plants12112123
Maslennikova D, Ivanov S, Petrova S, Burkhanova G, Maksimov I, Lastochkina O. Components of the Phenylpropanoid Pathway in the Implementation of the Protective Effect of Sodium Nitroprusside on Wheat under Salinity. Plants. 2023; 12(11):2123. https://doi.org/10.3390/plants12112123
Chicago/Turabian StyleMaslennikova, Dilara, Sergey Ivanov, Svetlana Petrova, Guzel Burkhanova, Igor Maksimov, and Oksana Lastochkina. 2023. "Components of the Phenylpropanoid Pathway in the Implementation of the Protective Effect of Sodium Nitroprusside on Wheat under Salinity" Plants 12, no. 11: 2123. https://doi.org/10.3390/plants12112123
APA StyleMaslennikova, D., Ivanov, S., Petrova, S., Burkhanova, G., Maksimov, I., & Lastochkina, O. (2023). Components of the Phenylpropanoid Pathway in the Implementation of the Protective Effect of Sodium Nitroprusside on Wheat under Salinity. Plants, 12(11), 2123. https://doi.org/10.3390/plants12112123









