Metal-Resistant PGPR Strain Azospirillum brasilense EMCC1454 Enhances Growth and Chromium Stress Tolerance of Chickpea (Cicer arietinum L.) by Modulating Redox Potential, Osmolytes, Antioxidants, and Stress-Related Gene Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Evaluating its Cr Stress Tolerance
2.2. Examination of Plant Growth-Promoting Characteristics of A. brasilense EMCC1454
2.2.1. Nitrogen Fixation Ability
2.2.2. Siderophore Formation
2.2.3. Phosphate Solubilization Potential
2.2.4. Hydrolytic Enzyme Activities
2.3. Assessing the Capability of A. brasilense EMCC1454 to Secrete Acid Compounds
2.4. Assessing Cr Levels on Stress Tolerance Characteristics of A. brasilense EMCC1454
2.4.1. Survivability of A. brasilense EMCC1454 under Cr Stress Conditions
2.4.2. Trehalose Content of A. brasilense EMCC1454 under Cr Stress Conditions
2.4.3. ACC (1-Aminocyclopropane-1-Carboxylic Acid) Deaminase Activity of A. brasilense EMCC1454 under Cr Stress Conditions
2.4.4. Indole Acetic Acid (IAA) Production by A. brasilense EMCC1454 under Cr Stress Conditions
2.4.5. Exopolysaccharide (EPS) Production by A. brasilense EMCC1454 under Cr Stress Conditions
2.4.6. Antioxidant Enzyme Activities of A. brasilense EMCC1454 under Cr Stress Conditions
2.4.7. Antioxidant Capacity of A. brasilense EMCC1454 under Cr Stress Conditions
2.5. Pot Experiment and Chickpea Growth
2.6. Determination of Plant Morphological Characteristics
2.7. Estimation of Mineral Content in Plant Leaves
2.8. Determination of Leaf Photosynthetic Pigment Levels
2.9. Estimation of Content of Leaf Soluble Sugar, Soluble Proteins, Proline, and Glycine Betaine
2.10. Determination of Leaf Gas-Exchange Properties and Relative Water Content
2.11. Determination of Concentration of Hydrogen Peroxide and Lipid Peroxidation in Plant Leaves
2.12. Determination of the Contents of Total Phenol and Flavonoids in Plant Leaves
2.13. Estimation of Leaf Enzymatic and Non-Enzymatic Antioxidants in Plant Leaves
2.14. Transcriptional Analysis of Stress-Related Genes in Plant Leaves
2.15. Data Statistical Analysis
3. Results and Discussion
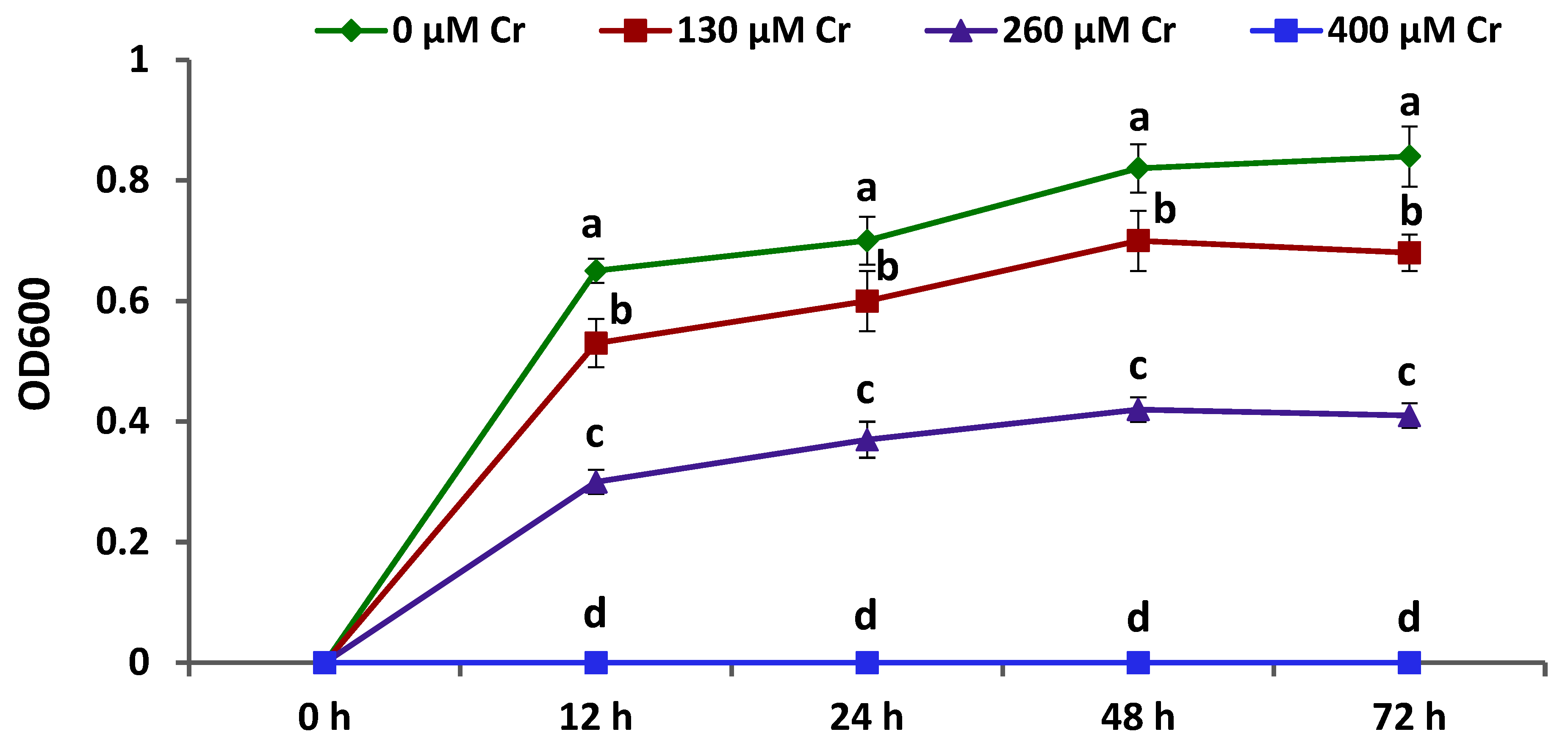
3.1. Growth and Chromium Tolerance of A. brasilense EMCC1454
3.2. Plant Growth-Promoting Characteristics of A. brasilense EMCC1454
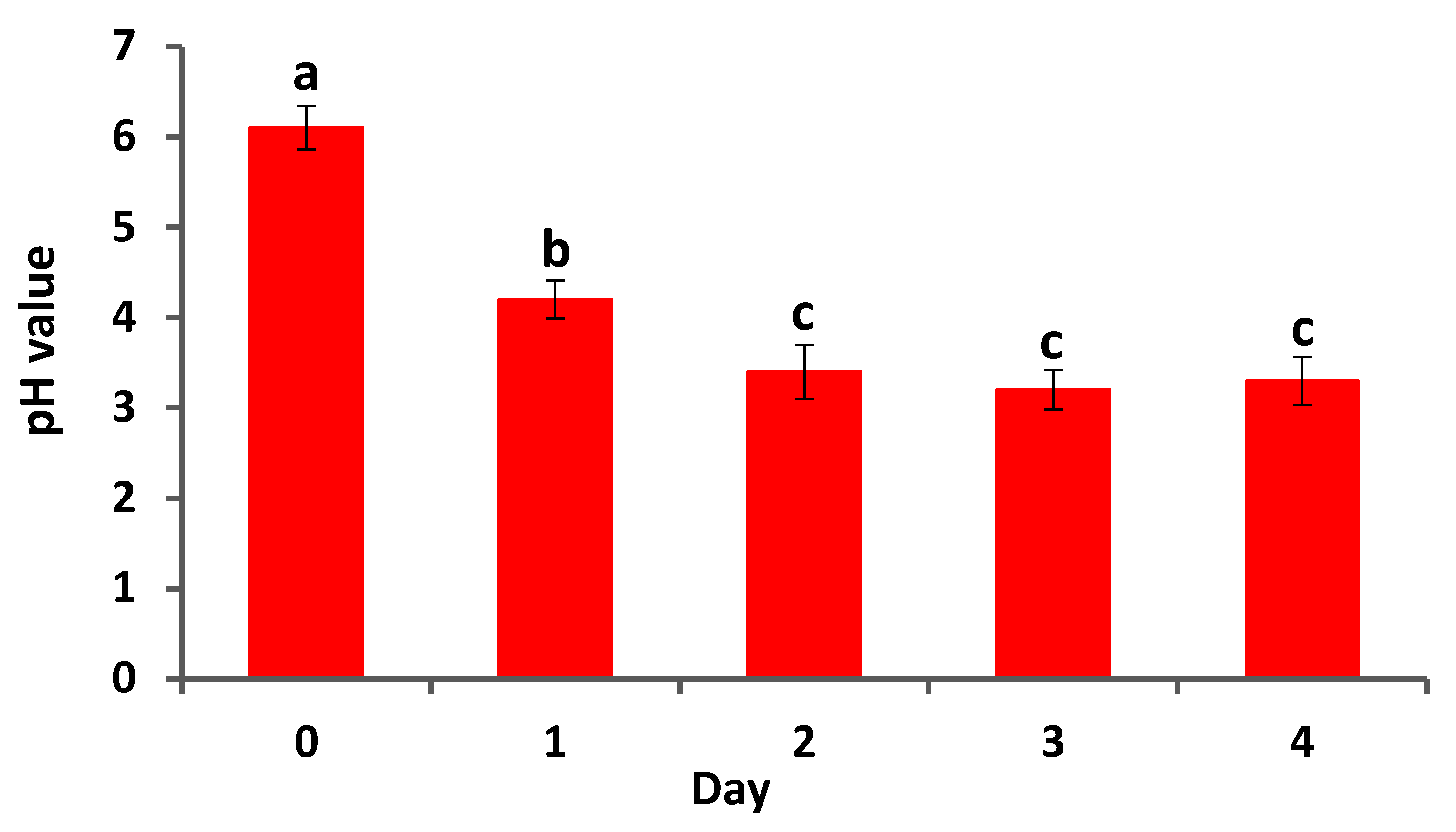
3.3. Ability of A. brasilense EMCC1454 to Generate Acid Compounds
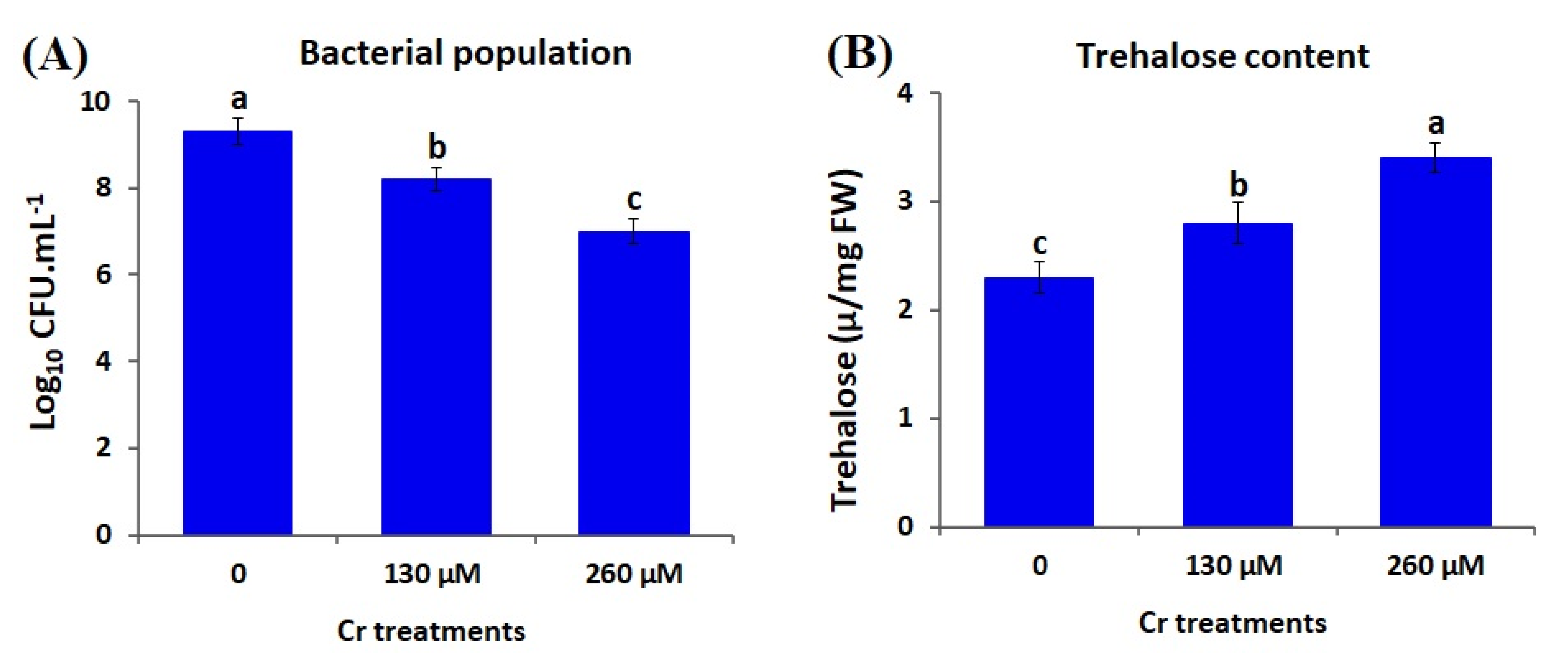
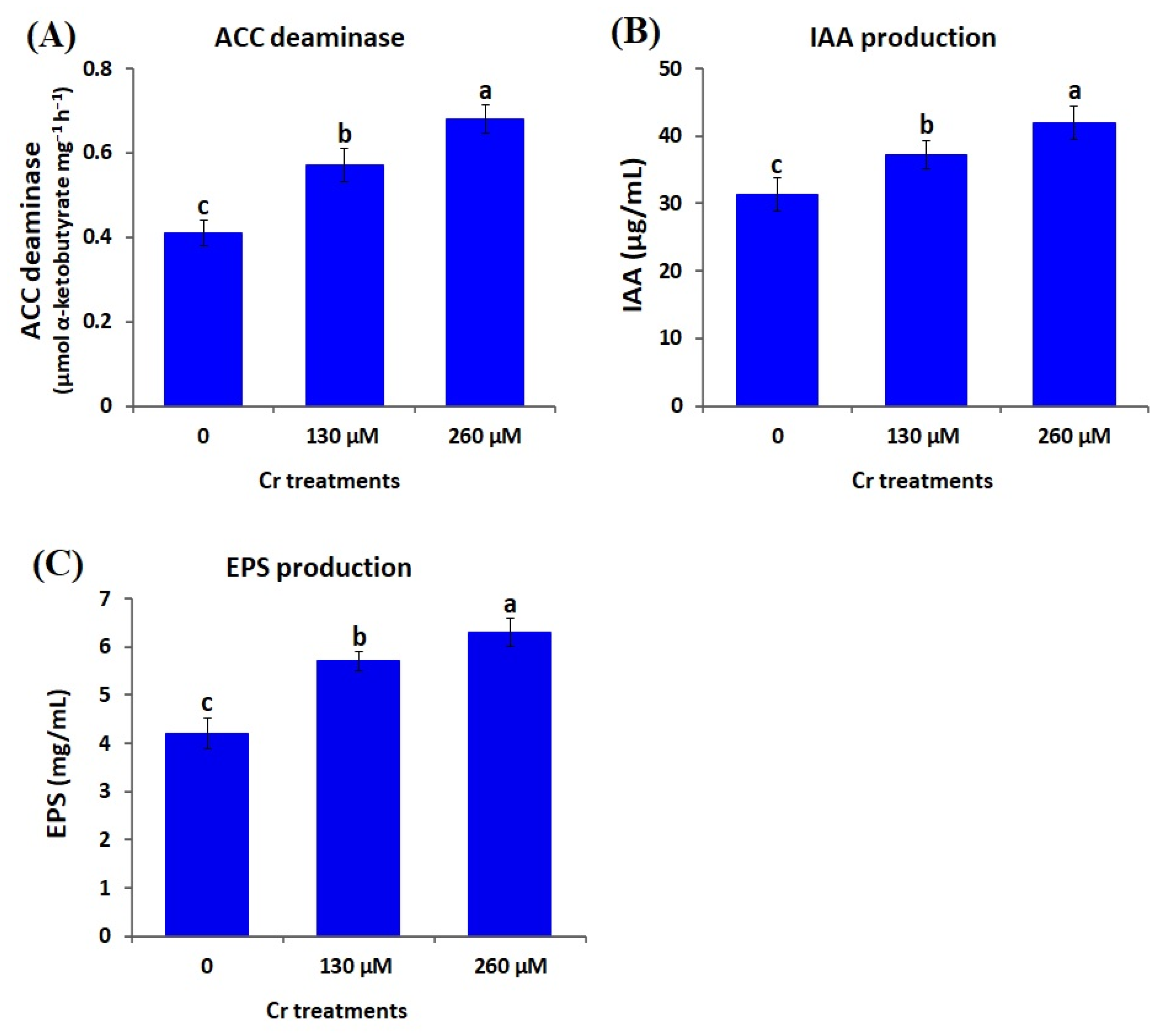
3.4. Impacts of Chromium Stress on PGP Traits of A. brasilense EMCC1454
3.5. A. brasilense EMCC1454 Enhances Chickpea Growth and Regulates its Minerals Absorption under Chromium Stress
3.6. A. Brasilense EMCC1454 Improves Photosynthetic Pigment Biosynthesis, Relative Water Content, and Gas-Exchange Traits of Chickpea under Chromium Stress
3.7. A. Brasilense EMCC1454 Increases the Levels of Osmolytes of Chickpea under Chromium Stress
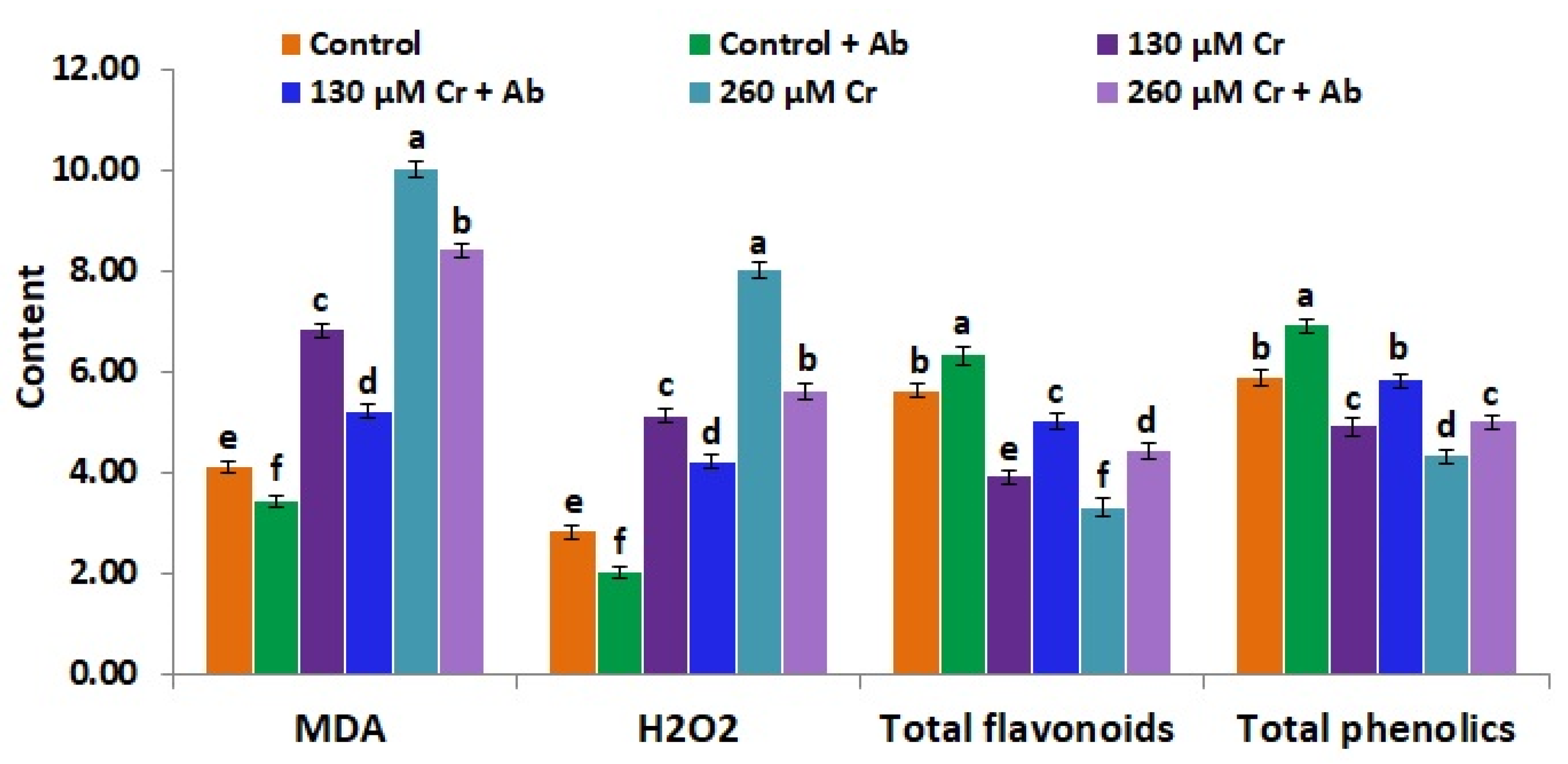
3.8. A. Brasilense EMCC1454 Reduces Oxidative Stress Biomarkers and Improves the Contents of Flavonoids and Phenolics of Chickpea under Chromium Stress
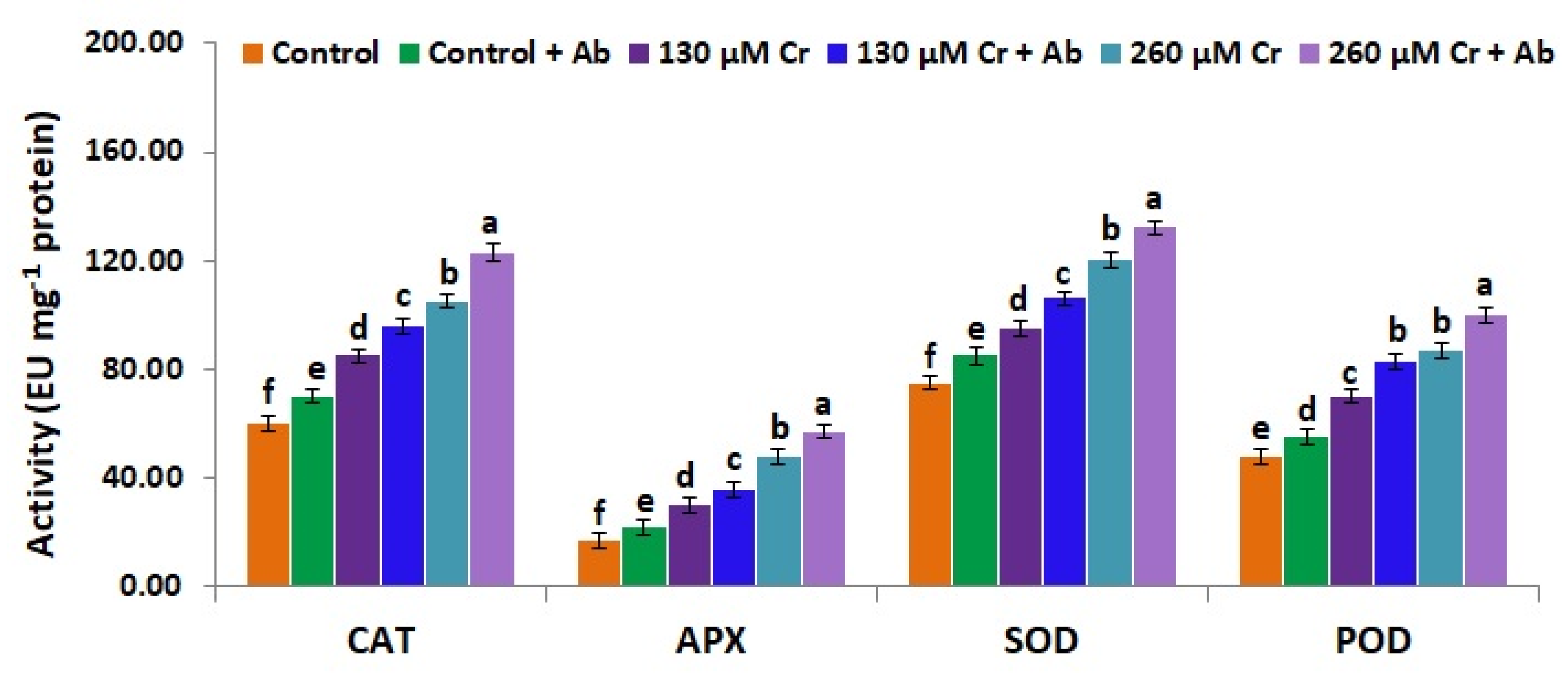
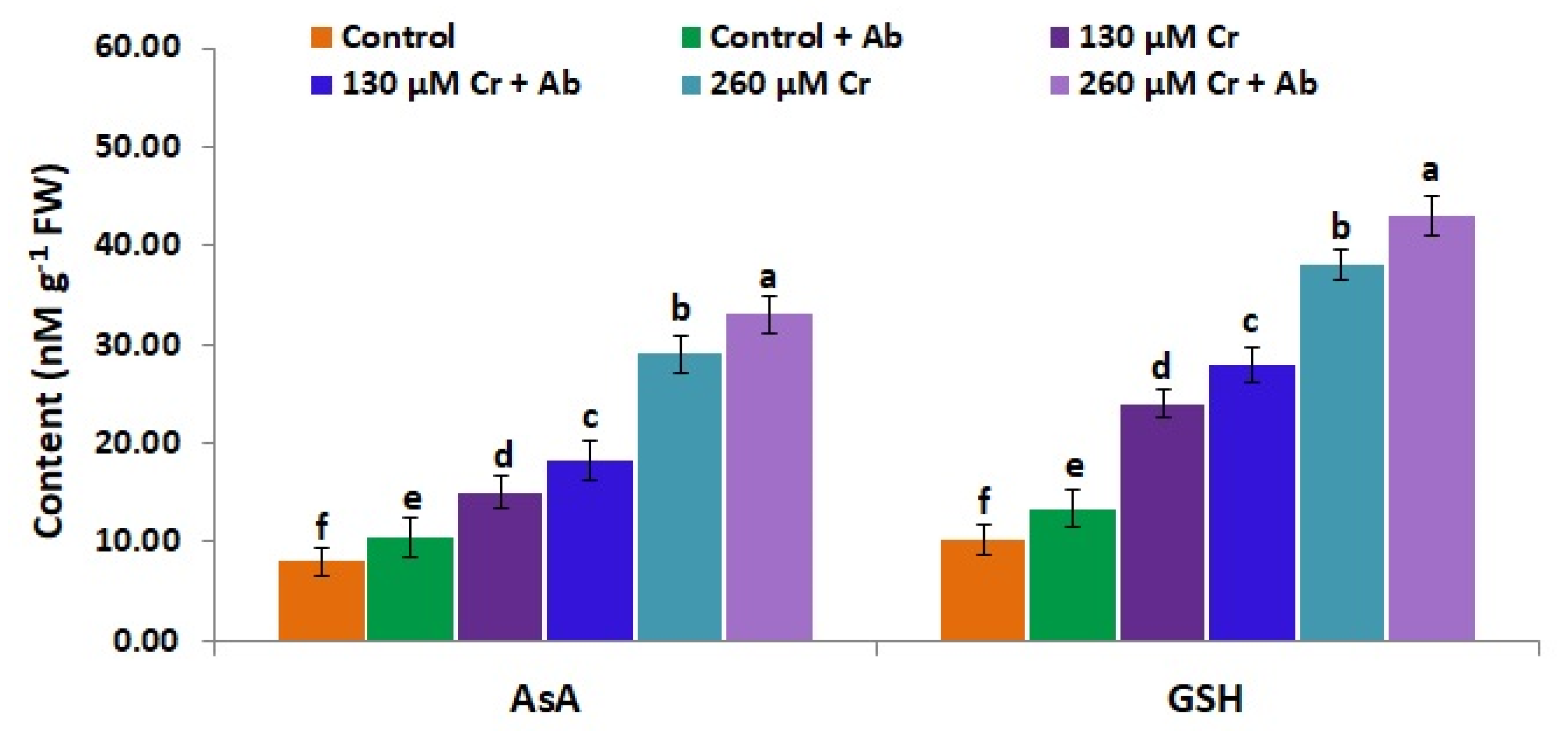
3.9. A. Brasilense EMCC1454 Induces Levels of Enzymatic and Non-Enzymatic Antioxidants of chickpea Plants under Chromium Stress
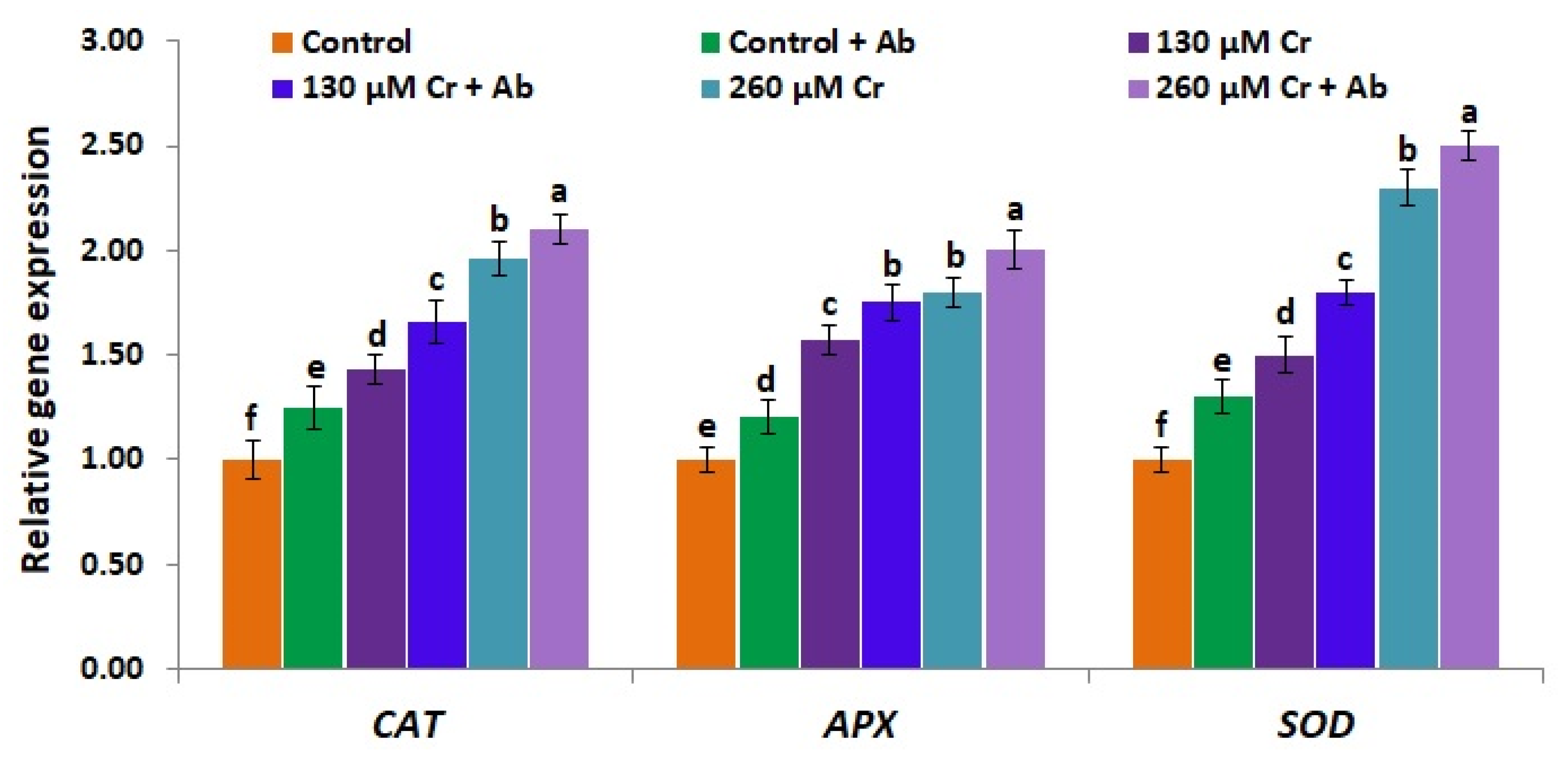
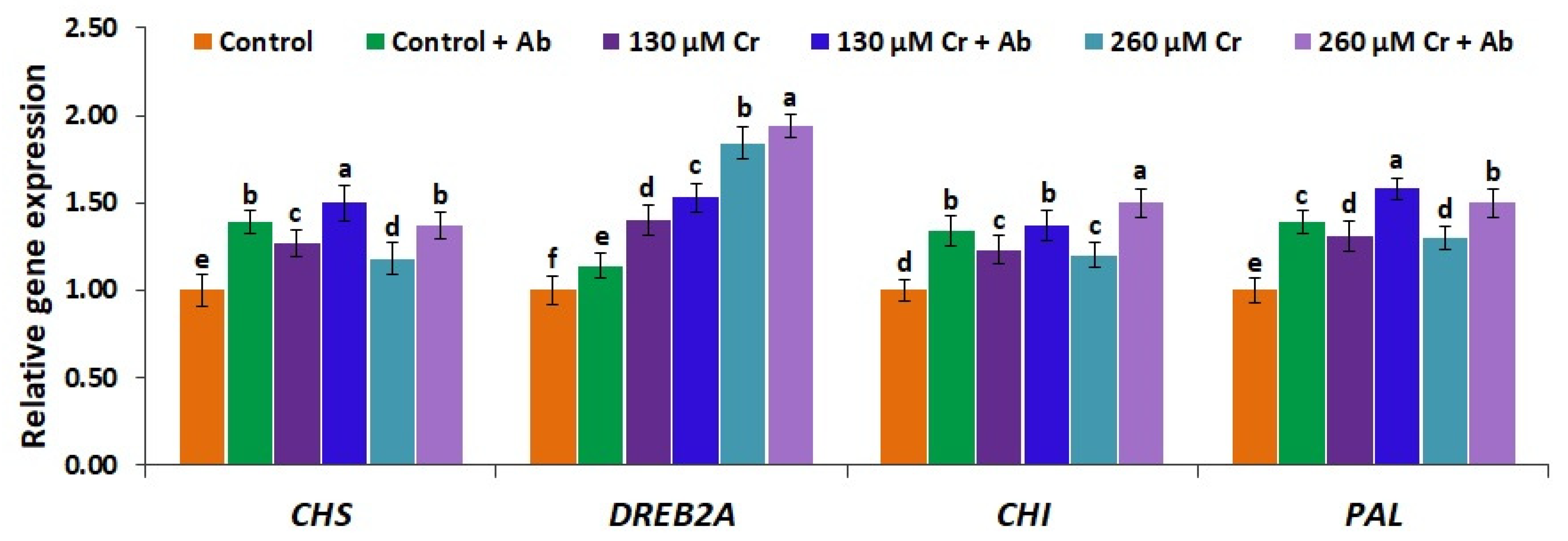
3.10. A. Brasilense EMCC1454 Induces the Expression of Antioxidant Genes and Genes Mediating Tolerance to Chromium Toxicity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Esawi, M.A.; Elkelish, A.; Soliman, M.; Elansary, H.O.; Zaid, A.; Wani, S.H. Serratia marcescens BM1 Enhances Cadmium Stress Tolerance and Phytoremediation Potential of Soybean Through Modulation of Osmolytes, Leaf Gas Exchange, Antioxidant Machinery, and Stress-Responsive Genes Expression. Antioxidants 2020, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Noor, I.; Sohail, H.; Sun, J.; Nawaz, M.A.; Li, G.; Hasanuzzaman, M.; Liu, J. Heavy metal and metalloid toxicity in horticultural plants: Tolerance mechanism and remediation strategies. Chemosphere 2022, 303, 135196. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Mir, R.A.; Tyagi, A.; Manzar, N.; Kashyap, A.S.; Mushtaq, M.; Raina, A.; Park, S.; Sharma, S.; Mir, Z.A.; et al. Chromium Toxicity in Plants: Signaling, Mitigation, and Future Perspectives. Plants 2023, 12, 1502. [Google Scholar] [CrossRef]
- Diwan, H.; Khan, I.; Ahmad, A.; Iqbal, M. Induction of phytochelatins and antioxidant defence system in Brassica juncea and Vigna radiata in response to chromium treatments. Plant Growth Regul. 2010, 61, 97–107. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Kumar, P.; Tokas, J.; Singal, H.R. Amelioration of Chromium VI Toxicity in Sorghum (Sorghum bicolor L.) using Glycine Betaine. Sci. Rep. 2019, 9, 16020. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium Bioaccumulation and Its Impacts on Plants: An Overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, N.L.; Singh, C.K.; Sarkar, S.K.; Singh, I.; Dotaniya, M.L. Effect of chromium (VI) toxicity on morpho-physiological characteristics, yield, and yield components of two chickpea (Cicer arietinum L.) varieties. PLoS ONE 2020, 15, e0243032. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Das, H.; Meena, V.D. Assessment of chromium efficacy on germination, root elongation, and coleoptile growth of wheat (Triticum aestivum L.) at different growth periods. Environ. Monit. Assess. 2014, 186, 2957–2963. [Google Scholar] [CrossRef]
- Gill, R.A.; Zhang, N.; Ali, B.; Farooq, M.A.; Xu, J.; Gill, M.B.; Mao, B.; Zhou, W. Role of exogenous salicylic acid in regulating physio-morphic and molecular changes under chromium toxicity in black-and yellow-seeded Brassica napus L. Environ. Sci. Pollut. Res. 2016, 23, 20483–20496. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ali, S.; Abid, M.; Rizwan, M.; Ali, B.; Tanveer, A.; Ahmad, I.; Azam, M.; Ghani, M.A. Glycinebetaine alleviates the chromium toxicity in Brassica oleracea L. by suppressing oxidative stress and modulating the plant morphology and photosynthetic attributes. Environ. Sci. Pollut. Res. 2020, 27, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Santos, C.; Azevedo, R.; Moutinho-Pereira, J.; Correia, C.; Dias, M.C. Chromium (VI) induces toxicity at different photosynthetic levels in pea. Plant Physiol. Biochem. 2012, 53, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Dheeba, B.; Sampathkumar, P.; Kannan, K. Fertilizers and Mixed Crop Cultivation of Chromium Tolerant and Sensitive Plants under Chromium Toxicity. J. Toxicol. 2015, 2015, 367217. [Google Scholar] [CrossRef]
- Kundu, D.; Dey, S.; Raychaudhuri, S.S. Chromium (VI)—Induced stress response in the plant Plantago ovata Forsk In Vitro. Genes Environ. 2018, 40, 21. [Google Scholar] [CrossRef] [PubMed]
- Vardharajula, S.; Zulfikar, A.S.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp., effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Inter. 2011, 6, 1–14. [Google Scholar]
- Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphylla Forssk. J. Plant Interact. 2014, 9, 802–810. [Google Scholar] [CrossRef]
- Kumar, Y.; Zhang, L.; Panigrahi, P.; Dholakia, B.B.; Dewangan, V.; Chavan, S.G.; Kunjir, S.M.; Wu, X.; Li, N.; Rajmohanan, P.R.; et al. Fusarium oxysporum mediates systems metabolic reprogramming of chickpea roots as revealed by a combination of proteomics and metabolomics. Plant Biotechnol. J. 2016, 14, 1589–1603. [Google Scholar] [CrossRef]
- Parsottambhai, S.K. Molecular and Biochemical Characterization of Microbial Antagonism in Chickpea (Cicer arietinum L.) against Wilt (Fusarium oxysporum f. sp. ciceri) Infection. Ph.D. Thesis, Navsari Agricultural University, Navsari, India, 2017. [Google Scholar]
- Hungria, M.; Campo, R.J.; Souza, E.M.; Pedrosa, F.O. Inoculation with selected strains of Azospirillum brasilense and A. lipoferum improves yields of maize and wheat in Brazil. Plant Soil 2010, 331, 413–425. [Google Scholar] [CrossRef]
- Shi, P.; Zhu, K.; Zhang, Y.; Chai, T. Growth and Cadmium Accumulation of Solanum nigrum L. Seedling were Enhanced by Heavy Metal-Tolerant Strains of Pseudomonas aeruginosa. Water Air Soil Pollut. 2016, 227, 459. [Google Scholar] [CrossRef]
- Fukami, J.; Ollero, F.J.; Megías, M.; Hungria, M. Phytohormones and induction of plant-stress tolerance and defense genes by seed and foliar inoculation with Azospirillum brasilense cells and metabolites promote maize growth. AMB Express 2017, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Lv, X.; Jia, H.; Hua, L.; Xu, H.; Zhou, R.; Zhao, J.; Ren, X.; Guo, J. Effects of salicylic acid, Fe (II) and plant growth-promoting bacteria on Cd accumulation and toxicity alleviation of Cd tolerant and sensitive tomato genotypes. J. Environ. Manag. 2018, 214, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.M.; Lee, I.J. Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ. Exp. Bot. 2017, 136, 68–77. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A. Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ. Exp. Bot. 2019, 159, 55–65. [Google Scholar] [CrossRef]
- Rodriguez, H.; Gonzalez, T.; Goire, I.; Bashan, Y. Gluconic acid production and phosphate solubilization by the plant growth-promoting bacterium Azospirillum spp. Naturwissenschaften 2004, 91, 552–555. [Google Scholar] [CrossRef]
- Khalid, M.; Bilal, M.; Hassani, D.; Hmn, I.; Wang, H.; Huang, D. Mitigation of salt stress in white clover (Trifolium repens) by Azospirillum brasilense and its inoculation effect. Bot. Stud. 2017, 58, 5. [Google Scholar] [CrossRef]
- Zaheer, M.S.; Ali, H.H.; Erinle, K.O.; Wani, S.H.; Okon, O.G.; Nadeem, M.A.; Nawaz, M.; Bodlah, M.A.; Waqas, M.M.; Iqbal, J.; et al. Inoculation of Azospirillum brasilense and exogenous application of trans-zeatin riboside alleviates arsenic induced physiological damages in wheat (Triticum aestivum). Environ. Sci. Pollut. Res. 2022, 29, 33909–33919. [Google Scholar] [CrossRef]
- Agami, R.A.; Ghramh, H.A.; Hashem, M. Seed inoculation with Azospirillum lipoferum alleviates the adverse effects of drought stress on wheat plants. J. Appl. Bot. Food Qual. 2017, 90, 165–173. [Google Scholar]
- Hamaoui, B.; Abbadi, J.M.; Burdman, S.; Rashid, A.; Sarig, S.; Okon, Y. Effects of inoculation with Azospirillum brasilense on chickpeas (Cicer arietinum) and faba beans (Vicia faba) under different growth conditions. Agronomie 2001, 21, 553–560. [Google Scholar] [CrossRef]
- Milán-Carrillo, J.; Valdéz-Alarcón, C.; Gutiérrez-Dorado, R.; Cárdenas-Valenzuela, O.G.; Mora-Escobedo, R.; Garzón-Tiznado, J.A.; Reyes-Moreno, C. Nutritional properties of quality protein maize and chickpea extruded based weaning food. Plant Foods Hum. Nutr. 2007, 62, 31–37. [Google Scholar] [CrossRef]
- Rasool, S.; Abdel Latef, A.A.; Ahmad, P. Chickpea: Role and responses under abiotic and biotic stress. In Legumes under Environmental Stress: Yield, Improvement and Adaptations; Azooz, M.M., Ahmad, P., Eds.; John Wiley: Chichester, UK, 2015; pp. 67–79. [Google Scholar]
- Kotula, L.; Khan, H.A.; Quealy, J.; Turner, N.C.; Vadez, V.; Siddique, K.H.; Clode, P.L.; Colmer, T.D. Salt sensitivity in chickpea (Cicer arietinum L.): Ions in reproductive tissues and yield components in contrasting genotypes. Plant Cell Environ. 2015, 38, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Eswaramoorthy, R.; Iqbal, R.K.; Danish, S. Metal-tolerant and siderophore producing Pseudomonas fluorescence and Trichoderma spp. improved the growth, biochemical features and yield attributes of chickpea by lowering Cd uptake. Sci. Rep. 2023, 13, 4471. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, A.; Kumar, R.; Pandir, R.; Bhardwaj, P.; Wusirika, R.; Kumar, S. Pseudomonas citronellolis; a multi-metal resistant and potential plant growth promoter against arsenic (V) stress in chickpea. Plant Physiol. Biochem. 2019, 142, 179–192. [Google Scholar] [CrossRef]
- Weselowski, B.; Nathoo, N.; Eastman, A.W.; MacDonald, J.; Yuan, Z.-C. Isolation, identification and characterization of Paenibacillus polymyxa CR1 with potentials for biopesticide, biofertilization, biomass degradation and biofuel production. BMC Microbiol. 2016, 16, 244. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Hankin, L.; Anagnostakis, S.L. The use of solid media for detection of enzyme production by fungi. Mycologia 1975, 67, 597–607. [Google Scholar] [CrossRef]
- Harrigan, W.F.; McCance, M.E. Laboratory Methods in Food and Dairy Microbiology; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Sampson, M.N.; Gooday, G.W. Involvement of chitinases of Bacillus thuringiensis during pathogenesis in insects. Microbiology 1998, 144, 2189–2194. [Google Scholar] [CrossRef]
- Gohel, H.R.; Contractor, C.N.; Ghosh, S.K.; Braganza, V.J. A comparative study of various staining techniques for determination of extra cellular cellulase activity on Carboxy Methyl Cellulose (CMC) agar plates. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 261–266. [Google Scholar]
- Shultana, R.; Kee, Z.A.T.; Yusop, M.R.; Saud, H.M. Characterization of salt-tolerant plant growth-promoting rhizobacteria and the effect on growth and yield of saline-affected rice. PLoS ONE 2020, 15, e02385372020. [Google Scholar] [CrossRef]
- Rodríguez-Salazar, J.; Suarez, R.; Caballero-Mellado, J.; Iturriaga, G. Trehalose accumulation in Azospirillum brasilense improves drought tolerance and biomass in maize plants. FEMS Microbiol. Lett. 2009, 296, 52–59. [Google Scholar] [CrossRef]
- Honma, M.; Shimomura, T. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 1978, 42, 1825–1831. [Google Scholar]
- Chen, S.-L.; Tsai, M.-K.; Huang, Y.-M.; Huang, C.-H. Diversity and characterization of Azotobacter isolates obtained from rice rhizosphere soils in Taiwan. Ann. Microbiol. 2018, 68, 17–26. [Google Scholar] [CrossRef]
- Kim, S.; Li, X.H.; Hwang, H.J.; Lee, J.H. Thermoregulation of Pseudomonas aeruginosa biofilm formation. Appl. Environ. Microbiol. 2020, 86, e01584-20. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Plant Physiol. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Chen, G.; Asada, K. Inactivation of ascorbate peroxidase by thiols requires hydrogen peroxide. Plant Cell Physiol. 1992, 33, 117–123. [Google Scholar]
- Aebi, H. Catalase in vitro. Meth Enzymol. 1984, 105, 121–126. [Google Scholar]
- Dhindsa, R.S.; Plumb-Dhinsda, P.; Thorne, P.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alamri, S.A.; Ali, H.M.; Alayafi, A.A. Bacillus firmus (SW5) augments salt tolerance in soybean (Glycine max L.) by modulating root system architecture, antioxidant defense systems and stress-responsive genes expression. Plant Physiol. Biochem. 2018, 132, 375–384. [Google Scholar] [CrossRef]
- Srinivasan, R.M.J.N.; Chandrasekar, M.J.N.; Nanjan, M.J.; Suresh, B. Antioxidant activity of Caesalpinia digyna root. J. Ethnopharmacol. 2007, 113, 284–291. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties. Methods Soil. 1965, 9, 1149–1178. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Wolf, B. A comprehensive system of leaf analysis and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Dey, P.M. Oligosaccharides. In Methods in Plant Biochemistry, Carbohydrates; Dey, P.M., Ed.; Academic Press: London, UK, 1990; Volume 2, pp. 189–218. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, P.P.; Teare, J.D. Rapid determination of free proline of water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.; Grattan, S. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Holá, D.; Benešová, M.; Honnerová, J.; Hnilička, F.; Rothová, O.; Kočová, M.; Hniličková, H. The evaluation of photosynthetic parameters in maize inbred lines subjected to water deficiency: Can these parameters be used for the prediction of performance of hybrid progeny? Photosynthetica 2010, 4, 545–558. [Google Scholar] [CrossRef]
- Yamasaki, S.; Dillenburg, L.C. Measurements of leaf relative water content in Araucaria angustifolia. Rev. Bras. Fisiol. Veg. 1999, 11, 69–75. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alzahrani, S.M.; Ali, H.M.; Alayafi, A.A.; Ahmad, M. Serratia liquefaciens KM4 Improves Salt Stress Tolerance in Maize by Regulating Redox Potential, Ion Homeostasis, Leaf Gas Exchange and Stress-Related Gene Expression. Int. J. Mol. Sci. 2018, 19, 3310. [Google Scholar] [CrossRef]
- Rao, K.V.M.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeon pea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analyses: Automation and comparison with manual methods. Am. J. Enol. Viticult. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys 1978, 186, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Putter, J.; Becker, R. Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1974; p. 685. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Yu, C.W.; Murphy, T.M.; Lin, C.H. Hydrogen peroxide-induces chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 955–963. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd-Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef]
- Roorkiwal, M.; Nayak, S.N.; Thudi, M.; Upadhyaya, H.D.; Brunel, D.; Mournet, P.; This, D.; Sharma, P.C.; Varshney, R.K. Allele diversity for abiotic stress responsive candidate genes in chickpea reference set using gene based SNP markers. Front. Plant Sci. 2014, 5, 248. [Google Scholar] [CrossRef]
- Parray, A.P.; Jan, S.; Kamili, A.N.; Qadri, R.A.; Egamberdieva, D.; Ahmad, P. Current perspectives on plant growth promoting rhizobacteria. Plant Growth Regul. 2016, 35, 877–902. [Google Scholar] [CrossRef]
- Haque, M.M.; Biswas, M.S.; Mosharaf, M.K.; Haque, M.A.; Islam, M.S.; Nahar, K.; Islam, M.M.; Shozib, H.B.; Islam, M.M.; Elahi, F.-E. Halotolerant biofilm-producing rhizobacteria mitigate seawater-induced salt stress and promote growth of tomato. Sci. Rep. 2022, 12, 5599. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Prasad, M.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Çam, S.; Küçük, Ç.; Cevheri, C. The effect of salinity-resistant biofilm-forming Azotobacter spp. on salt tolerance in maize growth. Zemdirb.-Agric. 2022, 109, 349–358. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; Gonzalez-Mendoza, D.; Elbaalawy, A.M. Azospirillum brasilense and Saccharomyces cerevisiae as Alternative for Decrease the Effect of Salinity Stress in Tomato (Lycopersicon esculentum) Growth. Phyton 2022, 91, 21. [Google Scholar] [CrossRef]
- García, J.E.; Maroniche, G.; Creus, C.; Suárez-Rodríguez, R.; Ramirez-Trujillo, J.A.; Groppa, M.D. In vitro PGPR properties and osmotic tolerance of different Azospirillum native strains and their effects on growth of maize under drought stress. Microbiol. Res. 2017, 202, 21–29. [Google Scholar] [CrossRef]
- Ma, Y.; Prasad, M.N.; Rajkumar, M.; Freitas, H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Adv. 2011, 29, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Gupta, A.; Mohapatra, S. A comparative analysis of exopolysaccharide and phytohormone secretions by four drought-tolerant rhizobacterial strains and their impact on osmotic-stress mitigation in Arabidopsis thaliana. World J. Microb. Biotechnol. 2019, 35, 90. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chi, J. Effect of Cd-tolerant plant growth-promoting rhizobium on plant growth and Cd uptake by Lolium multiforum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil. Plant Soil 2014, 375, 205–214. [Google Scholar] [CrossRef]
- Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Radhakrishnan, R.; Al-Huqail, A.A.; Al-Otibi, F.O.N.; Malik, J.A.; Alharbi, R.I.; Egamberdieva, D. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J. Plant Interact. 2018, 13, 37–44. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Wu, G.; Njeri, K.V.; Shen, Q.; Zhang, N.; Zhang, R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant. 2016, 158, 34–44. [Google Scholar] [CrossRef]
- Qu, L.; Huang, Y.; Zhu, C.; Zeng, H.; Shen, C.; Liu, C.; Zhao, Y.M. Rhizobia inoculation enhances the soybean’s tolerance to salt stress. Plant Soil 2016, 400, 209–222. [Google Scholar] [CrossRef]
- Gupta, A.K.; Kaur, N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 2005, 30, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Doganlar, Z.B.; Demir, K.; Basak, H.; Gul, I. Effects of salt stress on pigment and total soluble protein contents of three different tomato cultivars. Afr. J. Agric. Res. 2010, 5, 2056–2065. [Google Scholar]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef]
- Abd_Allah, E.F.; Hashem, A.; Alqarawi, A.A.; Bahkali, A.H.; Alwhibi, M.S. Enhancing growth performance and systemic acquired resistance of medicinal plant Sesbania sesban (L.) Merr using arbuscular mycorrhizal fungi under salt stress. Saudi J. Biol. Sci. 2016, 22, 274–283. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Physiol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Mutlu, S.; Atici, Ö.; Nalbantoglu, B. Effects of salicylic acid and salinity on apoplastic antioxidant enzymes in two wheat cultivars differing in salt tolerance. Biol. Plant. 2009, 53, 334–338. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Al-Huqail, A.A.; Wirth, S.; Egamberdieva, D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Microbiol. 2016, 7, 1167. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C.H. Simultaneous measurement of foliar glutathione, γ-glutamylcysteine, and amino acids by high-performance liquid chromatography: Comparison with two other assay methods for glutathione. Anal. Biochem. 1998, 264, 98–110. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A.; Witczak, J.; Ahmad, M. Analysis of Genetic Variation and Enhancement of Salt Tolerance in French Pea (Pisum Sativum L.). Int. J. Mol. Sci. 2018, 19, 2433. [Google Scholar] [CrossRef]
- Sharif, R.; Raza, A.; Chen, P.; Li, Y.; El-Ballat, E.M.; Rauf, A.; Hano, C.; El-Esawi, M.A. HD-ZIP Gene Family: Potential Roles in Improving Plant Growth and Regulating Stress-Responsive Mechanisms in Plants. Genes 2021, 12, 1256. [Google Scholar] [CrossRef]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef] [PubMed]
| Nitrogen Fixation | Siderophore Formation | Phosphate Solubilization |
|---|---|---|
| + | + | + |
| Amylase | Protease | Chitinase | Cellulase |
|---|---|---|---|
| + | + | − | + |
| Cr (µM) | APX | CAT | POD | SOD |
|---|---|---|---|---|
| 0 | 0.06 ± 0.02 c | 0.04 ± 0.01 c | 0.08 ± 0.02 c | 0.03 ± 0.01 c |
| 130 | 0.08 ± 0.02 b | 0.07 ± 0.02 b | 0.11 ± 0.02 b | 0.06 ± 0.02 b |
| 260 | 0.11 ± 0.03 a | 0.10 ± 0.02 a | 0.15 ± 0.03 a | 0.08 ± 0.02 a |
| Cr (µM) | β-Carotene-Linoleic Acid (IC50, μg mL−1) | DPPH (IC50, μg mL−1) | Superoxide-Scavenging (SOS) Activity (%) |
|---|---|---|---|
| 0 | 0.46 ± 0.05 a | 0.41 ± 0.03 a | 32.44 ± 1.32 a |
| 130 | 0.42 ± 0.04 b | 0.37 ± 0.04 b | 27.61 ± 1.22 b |
| 260 | 0.37 ± 0.04 c | 0.32 ± 0.04 c | 23.48 ± 1.20 c |
| Cr (µM) | A. Brasilense EMCC1454 | Root Length (cm plant−1) | Shoot Length (cm plant−1) | Root Dry Weight (g plant−1) | Shoot Dry Weight (g plant−1) | Root DW/Shoot DW |
|---|---|---|---|---|---|---|
| 0 | −Ab (T1) | 21.32 ± 1.67 b | 35.67 ± 1.78 b | 8.03 ± 0.34 b | 8.91 ± 0.37 b | 0.90 ± 0.06 b |
| +Ab (T2) | 22.99 ± 1.43 a | 37.96 ± 1.43 a | 9.11 ± 0.29 a | 9.86 ± 0.31 a | 0.92 ± 0.09 a | |
| 130 | −Ab (T3) | 17.01 ± 1.28 d | 30.12 ± 1.82 d | 5.98 ± 0.22 d | 6.77 ± 0.28 d | 0.88 ± 0.06 c |
| +Ab (T4) | 19.13 ± 1.32 c | 33.25 ± 1.92 c | 6.97 ± 0.27 c | 7.72 ± 0.25 c | 0.90 ± 0.09 b | |
| 260 | −Ab (T5) | 10.81± 1.17 f | 24.82 ± 1.53 f | 3.59 ± 0.31 f | 5.02 ± 0.24 f | 0.72 ± 0.09 e |
| +Ab (T6) | 12.98± 1.11 e | 28.11 ± 1.61 e | 5.01 ± 0.33 e | 6.13 ± 0.32 e | 0.82 ± 0.07 d |
| Cr (µM) | A. Brasilense EMCC1454 | Cr | N | P | Ca | K |
|---|---|---|---|---|---|---|
| 0 | −Ab (T1) | 0.02 ± 0.01 d | 29.2 ± 0.10 b | 19.9 ± 0.93 b | 2.0 ± 0.09 b | 10.2 ± 0.31 b |
| +Ab (T2) | 0.01 ± 0.01 d | 32.8 ± 0.13 a | 21.9 ± 0.81 a | 2.3 ± 0.08 a | 11.6 ± 0.28 a | |
| 130 | −Ab (T3) | 0.25 ± 0.02 b | 24.1 ± 0.11 c | 15.0 ± 0.94 d | 1.4 ± 0.06 d | 8.1 ± 0.25 d |
| +Ab (T4) | 0.18 ± 0.01 c | 28.8 ± 0.12 b | 17.9 ± 0.93 c | 1.8 ± 0.09 c | 9.2 ± 0.22 c | |
| 260 | −Ab (T5) | 0.40 ± 0.01 a | 17.7 ± 0.14 e | 10.2 ± 0.88 f | 0.9 ± 0.07 f | 5.1 ± 0.21 f |
| +Ab (T6) | 0.26 ± 0.02 b | 20.6 ± 0.15 d | 13.0 ± 0.96 e | 1.2 ± 0.09 e | 6.9 ± 0.24 e |
| Cr (µM) | A. Brasilense EMCC1454 | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Carotenoid |
|---|---|---|---|---|---|
| 0 | −Ab (T1) | 1.44 ± 0.06 b | 0.53 ± 0.05 b | 1.97 ± 0.07 b | 0.36 ± 0.04 c |
| +Ab (T2) | 1.61 ± 0.05 a | 0.60 ± 0.03 a | 2.21 ± 0.09 a | 0.43 ± 0.06 b | |
| 130 | −Ab (T3) | 1.13 ± 0.06 d | 0.34 ± 0.04 c | 1.47 ± 0.05 d | 0.42 ± 0.04 b |
| +Ab (T4) | 1.30 ± 0.04 c | 0.52 ± 0.04 b | 1.81 ± 0.08 c | 0.51 ± 0.05 a | |
| 260 | −Ab (T5) | 0.86 ± 0.05 f | 0.27 ± 0.03 d | 1.13 ± 0.06 f | 0.43 ± 0.04 b |
| +Ab (T6) | 1.00 ± 0.05 e | 0.35 ± 0.05 c | 1.35 ± 0.08 e | 0.50 ± 0.06 a |
| Cr (µM) | A. Brasilense EMCC1454 | Pn (μmol m−2 s−1) | E (mmol m−2 s−1) | Gs (mol m−2 s−1) | RWC (%) |
|---|---|---|---|---|---|
| 0 | −Ab (T1) | 10.03 ± 0.39 b | 1.65 ± 0.11 b | 0.10 ± 0.02 b | 82.7 ± 2.91 b |
| +Ab (T2) | 11.10 ± 0.42 a | 1.74 ± 0.10 a | 0.12 ± 0.02 a | 85.1 ± 2.93 a | |
| 130 | −Ab (T3) | 8.01 ± 0.38 c | 1.41 ± 0.11 d | 0.06 ± 0.01 d | 64.4 ± 2.82 d |
| +Ab (T4) | 9.94 ± 0.41 b | 1.52 ± 0.10 c | 0.08 ± 0.02 c | 71.6 ± 2.77 c | |
| 260 | −Ab (T5) | 5.96 ± 0.30 e | 1.17 ± 0.10 f | 0.04 ± 0.02 e | 49.2 ± 2.91 e |
| +Ab (T6) | 7.02 ± 0.37 d | 1.28 ± 0.11 e | 0.06 ± 0.03 d | 63.9 ± 2.96 d |
| Cr (µM) | A. Brasilense EMCC1454 | Soluble Sugars (mg g−1 FW) | Proteins (mg g−1 of FW) | Proline (µg g−1 FW) | Glycine Betaine (µmol g−1 FW) |
|---|---|---|---|---|---|
| 0 | −Ab (T1) | 5.98 ± 0.57 e | 19.8 ± 1.32 e | 26.2 ± 1.88 f | 3.07 ± 0.22 f |
| +Ab (T2) | 6.81 ± 0.46 d | 20.8 ± 1.42 d | 33.3 ± 1.89 e | 5.63 ± 0.31 e | |
| 130 | −Ab (T3) | 6.92 ± 0.62 d | 22.5 ± 1.38 c | 44.5 ± 1.82 d | 9.68 ± 0.76 d |
| +Ab (T4) | 9.03 ± 0.59 c | 25.9 ± 1.53 b | 68.2 ± 1.94 c | 18.84 ± 1.02 c | |
| 260 | −Ab (T5) | 9.97 ± 0.66 b | 26.1 ± 1.44 b | 86.6 ± 3.32 b | 24.86 ± 1.41 b |
| +Ab (T6) | 11.02 ± 0.58 a | 31.2 ± 1.52 a | 108.9 ± 3.83 a | 32.37 ± 1.52 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Ballat, E.M.; Elsilk, S.E.; Ali, H.M.; Ali, H.E.; Hano, C.; El-Esawi, M.A. Metal-Resistant PGPR Strain Azospirillum brasilense EMCC1454 Enhances Growth and Chromium Stress Tolerance of Chickpea (Cicer arietinum L.) by Modulating Redox Potential, Osmolytes, Antioxidants, and Stress-Related Gene Expression. Plants 2023, 12, 2110. https://doi.org/10.3390/plants12112110
El-Ballat EM, Elsilk SE, Ali HM, Ali HE, Hano C, El-Esawi MA. Metal-Resistant PGPR Strain Azospirillum brasilense EMCC1454 Enhances Growth and Chromium Stress Tolerance of Chickpea (Cicer arietinum L.) by Modulating Redox Potential, Osmolytes, Antioxidants, and Stress-Related Gene Expression. Plants. 2023; 12(11):2110. https://doi.org/10.3390/plants12112110
Chicago/Turabian StyleEl-Ballat, Enas M., Sobhy E. Elsilk, Hayssam M. Ali, Hamada E. Ali, Christophe Hano, and Mohamed A. El-Esawi. 2023. "Metal-Resistant PGPR Strain Azospirillum brasilense EMCC1454 Enhances Growth and Chromium Stress Tolerance of Chickpea (Cicer arietinum L.) by Modulating Redox Potential, Osmolytes, Antioxidants, and Stress-Related Gene Expression" Plants 12, no. 11: 2110. https://doi.org/10.3390/plants12112110
APA StyleEl-Ballat, E. M., Elsilk, S. E., Ali, H. M., Ali, H. E., Hano, C., & El-Esawi, M. A. (2023). Metal-Resistant PGPR Strain Azospirillum brasilense EMCC1454 Enhances Growth and Chromium Stress Tolerance of Chickpea (Cicer arietinum L.) by Modulating Redox Potential, Osmolytes, Antioxidants, and Stress-Related Gene Expression. Plants, 12(11), 2110. https://doi.org/10.3390/plants12112110








