Response of Plant and Soil N, P, and N:P Stoichiometry to N Addition in China: A Meta-Analysis
Abstract
1. Introduction
2. Results
2.1. The Effects of N Addition on N, P, and N:P
2.2. Influence of N Input Rate and Experimental Duration on the Effects Size
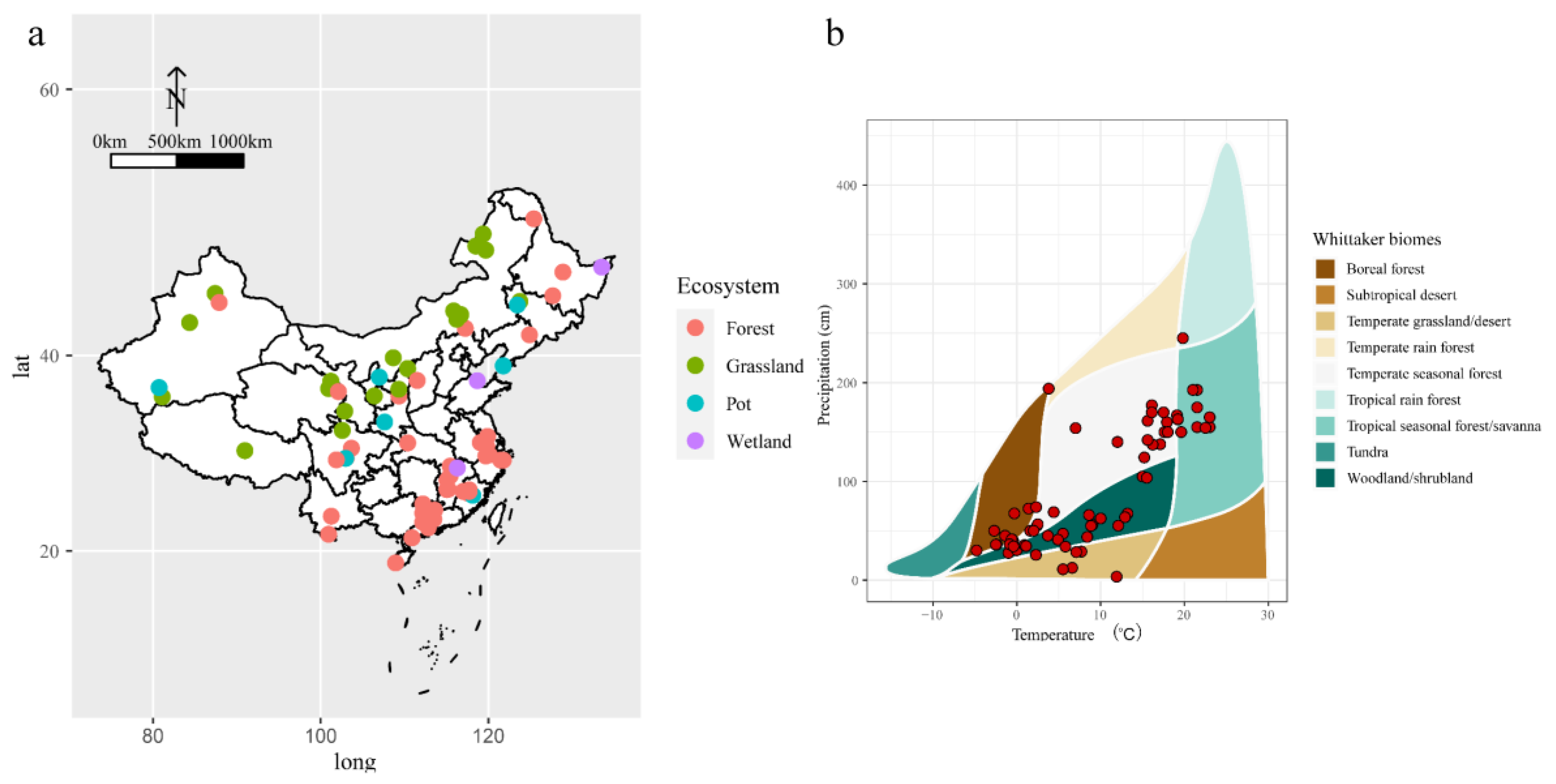
2.3. Influence of Ecosystem Types and Geographical Factors on Effect Size
3. Discussion
3.1. Effects of N Addition on [N], [P], and N:P Ratio of Plant and Soil in China
3.2. Main Factors Influencing the Response of These under N Addition
3.3. Implications and Guidelines for Future Work
4. Materials and Methods
4.1. Data Collection
4.2. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.C.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ma, X.F.; Gong, Y.M.; Li, K.H.; Han, W.X.; Liu, X.J. Responses and drivers of leaf nutrients and resorption to nitrogen enrichment across northern China’s grasslands: A meta-analysis. Catena 2021, 199, 105110. [Google Scholar] [CrossRef]
- Dentener, F.; Drevet, J.; Lamarque, J.F.; Bey, I.; Eickhout, B.; Fiore, A.M.; Hauglustaine, D.; Horowitz, L.W.; Krol, M.; Kulshrestha, U.C.; et al. Nitrogen and sulfur deposition on regional and global scales: A multimodel evaluation. Glob. Biogeochem. Cycles 2006, 20. [Google Scholar] [CrossRef]
- Liu, X.J.; Duan, L.; Mo, J.M.; Du, E.Z.; Shen, J.L.; Lu, X.K.; Zhang, Y.; Zhou, X.B.; He, C.N.; Zhang, F.S. Nitrogen deposition and its ecological impact in China: An overview. Environ. Pollut. 2011, 159, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.R.; Jia, Y.L.; He, N.P.; Zhu, J.X.; Chen, Z.; Wang, Q.F.; Piao, S.L.; Liu, X.J.; He, H.L.; Guo, X.B.; et al. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Fu, W.; Wu, H.; Zhao, A.; Hao, Z.; Chen, B. Ecological impacts of nitrogen deposition on terrestrial ecosystems: Research progresses and prospects. Chin. J. Plant Ecol. 2020, 44, 475–493. [Google Scholar] [CrossRef]
- Tischer, A.; Werisch, M.; Dobbelin, F.; Camenzind, T.; Rillig, M.C.; Potthast, K.; Hamer, U. Above- and belowground linkages of a nitrogen and phosphorus co-limited tropicalmountain pasture system-responses to nutrient enrichment. Plant Soil 2015, 391, 333–352. [Google Scholar] [CrossRef]
- Stevens, C.J. How long do ecosystems take to recover from atmospheric nitrogen deposition? Biol. Conserv. 2016, 200, 160–167. [Google Scholar] [CrossRef]
- Muller, M.; Oelmann, Y.; Schickhoff, U.; Bohner, J.; Scholten, T. Himalayan treeline soil and foliar C:N:P stoichiometry indicate nutrient shortage with elevation. Geoderma 2017, 291, 21–32. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef]
- Humbert, J.Y.; Dwyer, J.M.; Andrey, A.; Arlettaz, R. Impacts of nitrogen addition on plant biodiversity in mountain grasslands depend on dose, application duration and climate: A systematic review. Glob. Chang. Biol. 2016, 22, 110–120. [Google Scholar] [CrossRef]
- Garten; Charles, T. Correlations between concentrations of elements in plants. Nature 1976, 261, 686–688. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Hongwei, X.; Qing, Q.; Guanwen, L.; Guobin, L.; Violette, G.; Coen, J.R.; Sha, X. Impact of nitrogen addition on plant-soil-enzyme C–N–P stoichiometry and microbial nutrient limitation. Soil Biol. Biochem. 2022, 170, 108714. [Google Scholar]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Koerselman Willem Meuleman Arthur, F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Wang, C.T.; Sun, Y.; Chen, H.Y.H.; Ruan, H.H. Effects of elevated CO2 on the C:N stoichiometry of plants, soils, and microorganisms in terrestrial ecosystems. Catena 2021, 201, 105219. [Google Scholar] [CrossRef]
- Gusewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Feng, C.Y.; Zheng, C.Y.; Tian, D. Impacts of nitrogen addition on plant phosphorus content in forest ecosystems and the underlying mechanisms. Chin. J. Plant Ecol. 2019, 43, 185–196. [Google Scholar] [CrossRef]
- Liu, X.C.; Lamb, E.G.; Zhang, S.T. Nitrogen addition impacts on soil microbial stoichiometry are driven by changes in plant resource stoichiometry not by the composition of main microbial groups in an alpine meadow. Biol. Fertil. Soils 2020, 56, 261–271. [Google Scholar] [CrossRef]
- Hong, J.-T.; Wu, J.-B.; Wang, X.-D. Effects of global climate change on the C, N, and P stoichiometry of terrestrial plants. Chin. J. Appl. Ecol. 2013, 24, 2658–2665. [Google Scholar]
- Zheng, Z.M.; Lu, J.; Su, Y.Q.; Yang, Q.S.; Lin, Y.H.; Liu, H.M.; Yang, J.; Huang, H.; Wang, X.H. Differential effects of N and P additions on foliar stoichiometry between species and community levels in a subtropical forest in eastern China. Ecol. Indic. 2020, 117, 106537. [Google Scholar] [CrossRef]
- Shi, B.; Ling, X.; Cui, H.; Song, W.; Sun, W. Response of nutrient resorption of Leymus chinensis to nitrogen and phosphorus addition in a meadow steppe of northeast China. Plant Biol. 2020, 22, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.Z.; Yang, G.S.; Mao, R. Responses of leaf nitrogen and phosphorus allocation patterns to nutrient additions in a temperate freshwater wetland. Ecol. Indic. 2020, 110, 105949. [Google Scholar] [CrossRef]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, C.T.; Chen, H.Y.H.; Ruan, H.H. Responses of C:N stoichiometry in plants, soil, and microorganisms to nitrogen addition. Plant Soil 2020, 456, 277–287. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H.Y.H.; Jin, L.; Wang, C.T.; Zhang, R.T.; Ruan, H.H.; Yang, J.Y. Drought stress induced increase of fungi:bacteria ratio in a poplar plantation. Catena 2020, 193, 104607. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef]
- Redding, M.R.; Shorten, P.R.; Lewis, R.; Pratt, C.; Paungfoo-Lonhienne, C.; Hill, J. Soil N availability, rather than N deposition, controls indirect N2O emissions. Soil Biol. Biochem. 2016, 95, 288–298. [Google Scholar] [CrossRef]
- Tian, D.; Du, E.Z.; Jiang, L.; Ma, S.H.; Zeng, W.J.; Zou, A.L.; Feng, C.Y.; Xu, L.C.; Xing, A.J.; Wang, W.; et al. Responses of forest ecosystems to increasing N deposition in China: A critical review. Environ. Pollut. 2018, 243, 75–86. [Google Scholar] [CrossRef]
- Li, Y.; Niu, S.L.; Yu, G.R. Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: A meta-analysis. Glob. Chang. Biol. 2016, 22, 934–943. [Google Scholar] [CrossRef]
- Yang, D.X.; Song, L.; Jin, G.Z. The soil C:N:P stoichiometry is more sensitive than the leaf C:N:P stoichiometry to nitrogen addition: A four-year nitrogen addition experiment in a Pinus koraiensis plantation. Plant Soil 2019, 442, 183–198. [Google Scholar] [CrossRef]
- Yang, Y.H.; Luo, Y.Q.; Lu, M.; Schadel, C.; Han, W.X. Terrestrial C:N stoichiometry in response to elevated CO2 and N addition: A synthesis of two meta-analyses. Plant Soil 2011, 343, 393–400. [Google Scholar] [CrossRef]
- Xu, S.; Sardans, J.; Zhang, J.L.; Penuelas, J. Variations in foliar carbon:nitrogen and nitrogen:phosphorus ratios under global change: A meta-analysis of experimental field studies. Sci. Rep. 2020, 10, 12156. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Matson, P.A.; Vitousek, P.; Chapin, M.C. Principles of Terrestrial Ecosystem Ecology; Springer: New York, NY, USA, 2012. [Google Scholar]
- Walker, T.W.; Syers, J.K. The fate of phosphorus during pedogenesis. Geoderma 1976, 15, 1–19. [Google Scholar] [CrossRef]
- Tiessen, H.; Cuevas, E.; Chacon, P. The role of soil organic matter in sustaining soil fertility. Nature 1994, 371, 783–785. [Google Scholar] [CrossRef]
- Lu, X.K.; Mo, J.M.; Gilliam, F.S.; Fang, H.; Zhu, F.F.; Fang, Y.T.; Zhang, W.; Huang, J. Nitrogen Addition Shapes Soil Phosphorus Availability in Two Reforested Tropical Forests in Southern China. Biotropica 2012, 44, 302–311. [Google Scholar] [CrossRef]
- Mo, J.M.; Brown, S.; Xue, J.H.; Fang, Y.T.; Li, Z.; Li, D.J.; Dong, S.F. Response of nutrient dynamics of decomposing pine (Pinus massoniana) needles to simulated N deposition in a disturbed and a rehabilitated forest in tropical China. Ecol. Res. 2007, 22, 649–658. [Google Scholar] [CrossRef]
- Liu, G.; Fu, B. Characteristics and distributions of degraded ecological types in China. Acta Ecol. Sin. 2000, 1, 13–19. [Google Scholar]
- Elser, J.J.; Sterner, R.W.; Gorokhova, E. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.T.; Yang, J.Y.; Liao, J.H.; Chen, H.Y.H.; Ruan, H.H. Elevated CO2 shifts soil microbial communities from K- to r-strategists. Glob. Ecol. Biogeogr. 2021, 30, 961–972. [Google Scholar] [CrossRef]
- Tian, D.; Yan, Z.; Fang, J. Plant stoichiometry: A research frontier in ecology. Chin. J. Nat. 2018, 4, 235–241. [Google Scholar]
- Magill, A.H.; Aber, J.D.; Berntson, G.M.; Mcdowell, W.H.; Nadelhoffer, K.J.; Melillo, J.M.; Steudler, P. Long-Term Nitrogen Additions and Nitrogen Saturation in Two Temperate Forests. Ecosystems 2000, 3, 238–253. [Google Scholar] [CrossRef]
- Yin, L.; Shu Cai, Z.; Wenjuan, H. Effects of simulated nitrogen deposition on soil acid phosphomonoesterase activity and soil available phosphorus content in subtropical forests in Dinghushan Mountain. Chin. J. Appl. Ecol. 2011, 22, 631–636. [Google Scholar]
- Chen, M.; Chen, H.; Mao, Q.; Zhu, X.; Mo, J. Effect of nitrogen deposition on the soil phosphorus cycle in forest ecosystems: A review. Acta Ecol. Sin. 2016, 36, 4965–4976. [Google Scholar]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef]
- Miller, B.D.; Carter, K.R.; Reed, S.C.; Wood, T.E.; Cavaleri, M.A. Only sun-lit leaves of the uppermost canopy exceed both air temperature and photosynthetic thermal optima in a wet tropical forest. Agric. For. Meteorol. 2021, 301, 108347. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.T.; Chen, H.Y.H.; Luo, X.S.; Qiu, N.A.W.; Ruan, H.H.; Waring, B.G. Asymmetric responses of terrestrial C:N:P stoichiometry to precipitation change. Glob. Ecol. Biogeogr. 2021, 30, 1724–1735. [Google Scholar] [CrossRef]
- Tessier, J.T.; Raynal, D.J. Use of nitrogen to phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J. Appl. Ecol. 2003, 40, 523–534. [Google Scholar] [CrossRef]
- Jia, B.R. Litter decomposition and its underlying mechanisms. Chin. J. Plant Ecol. 2019, 43, 648–657. [Google Scholar] [CrossRef]
- Burda, B.U.; O’Connor, E.A.; Webber, E.M.; Redmond, N.; Perdue, L.A. Estimating data from figures with a Web-based program: Considerations for a systematic review. Res. Synth. Methods 2017, 8, 258–262. [Google Scholar] [CrossRef]
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]

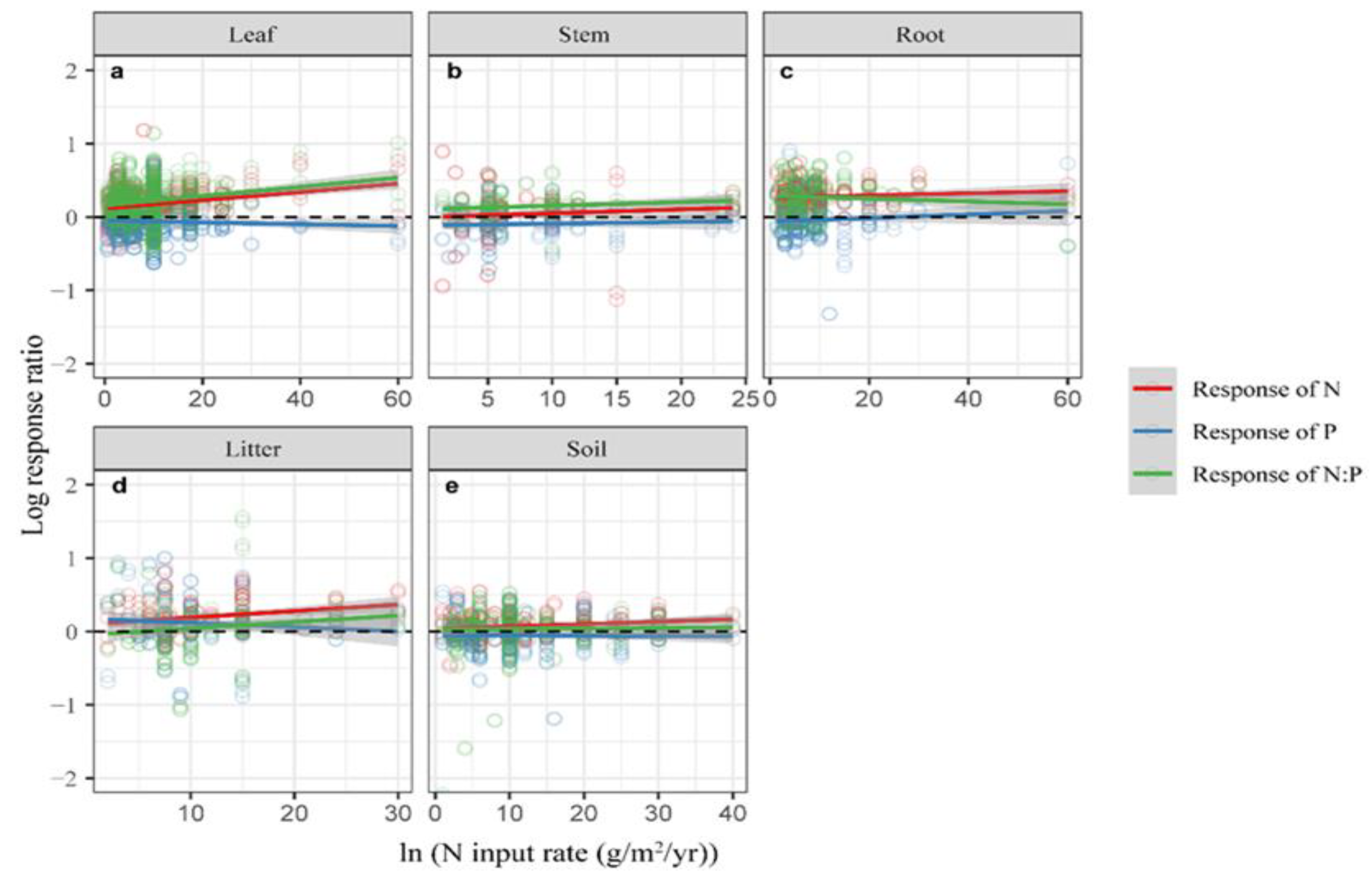



| N In Put (N) | Experimental Duration (ED) | |||||
|---|---|---|---|---|---|---|
| N | P | N:P | N | P | N:P | |
| Leaf | <0.01 | 0.66 | <0.01 | 0.09 | <0.01 | 0.02 |
| Stem | 0.34 | 0.71 | 0.11 | 0.68 | 0.62 | 0.80 |
| Root | 0.15 | 0.2 | 0.98 | 0.51 | 0.66 | 0.58 |
| Litter | <0.01 | 0.21 | 0.60 | 0.84 | 0.55 | 0.61 |
| Soil | 0.01 | 0.21 | 0.45 | 0.84 | 0.78 | 0.93 |
| N | P | N:P | ||||
|---|---|---|---|---|---|---|
| df | P | df | P | df | P | |
| Leaf | 42.74 | 0.05 | 42.36 | 0.40 | 45.77 | 0.38 |
| Steam | 5.75 | 0.47 | 5.57 | 0.53 | 4.49 | 0.85 |
| Root | 11.33 | 0.56 | 12.50 | 0.47 | 11.44 | 0.66 |
| Litter | 7.19 | 0.20 | 12.03 | 0.38 | 11.32 | 0.64 |
| Soil | 19.82 | 0.32 | 19.76 | 0.47 | 20.68 | 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zhang, W.; Ge, X.; Zheng, X.; Zhou, X.; Ding, H.; Zhang, A. Response of Plant and Soil N, P, and N:P Stoichiometry to N Addition in China: A Meta-Analysis. Plants 2023, 12, 2104. https://doi.org/10.3390/plants12112104
Chen S, Zhang W, Ge X, Zheng X, Zhou X, Ding H, Zhang A. Response of Plant and Soil N, P, and N:P Stoichiometry to N Addition in China: A Meta-Analysis. Plants. 2023; 12(11):2104. https://doi.org/10.3390/plants12112104
Chicago/Turabian StyleChen, Shuifei, Wenwen Zhang, Xiaomin Ge, Xiao Zheng, Xu Zhou, Hui Ding, and Aiguo Zhang. 2023. "Response of Plant and Soil N, P, and N:P Stoichiometry to N Addition in China: A Meta-Analysis" Plants 12, no. 11: 2104. https://doi.org/10.3390/plants12112104
APA StyleChen, S., Zhang, W., Ge, X., Zheng, X., Zhou, X., Ding, H., & Zhang, A. (2023). Response of Plant and Soil N, P, and N:P Stoichiometry to N Addition in China: A Meta-Analysis. Plants, 12(11), 2104. https://doi.org/10.3390/plants12112104






