CaWRKY50 Acts as a Negative Regulator in Response to Colletotrichum scovillei Infection in Pepper
Abstract
1. Introduction
2. Results
2.1. Characterization of CaWRKY50
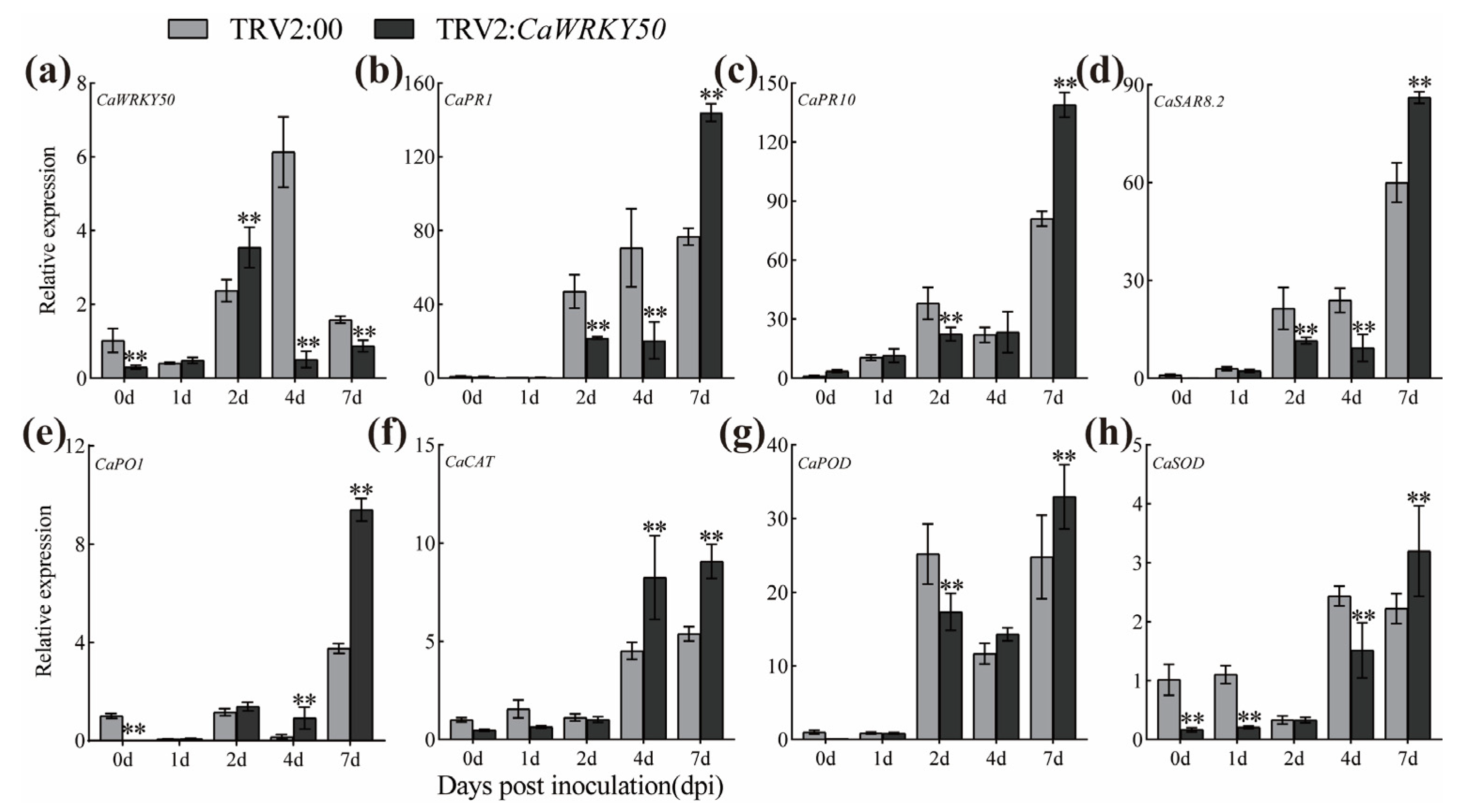
2.2. CaWRKY50 Silencing Improves Pepper Resistance to C. scovillei
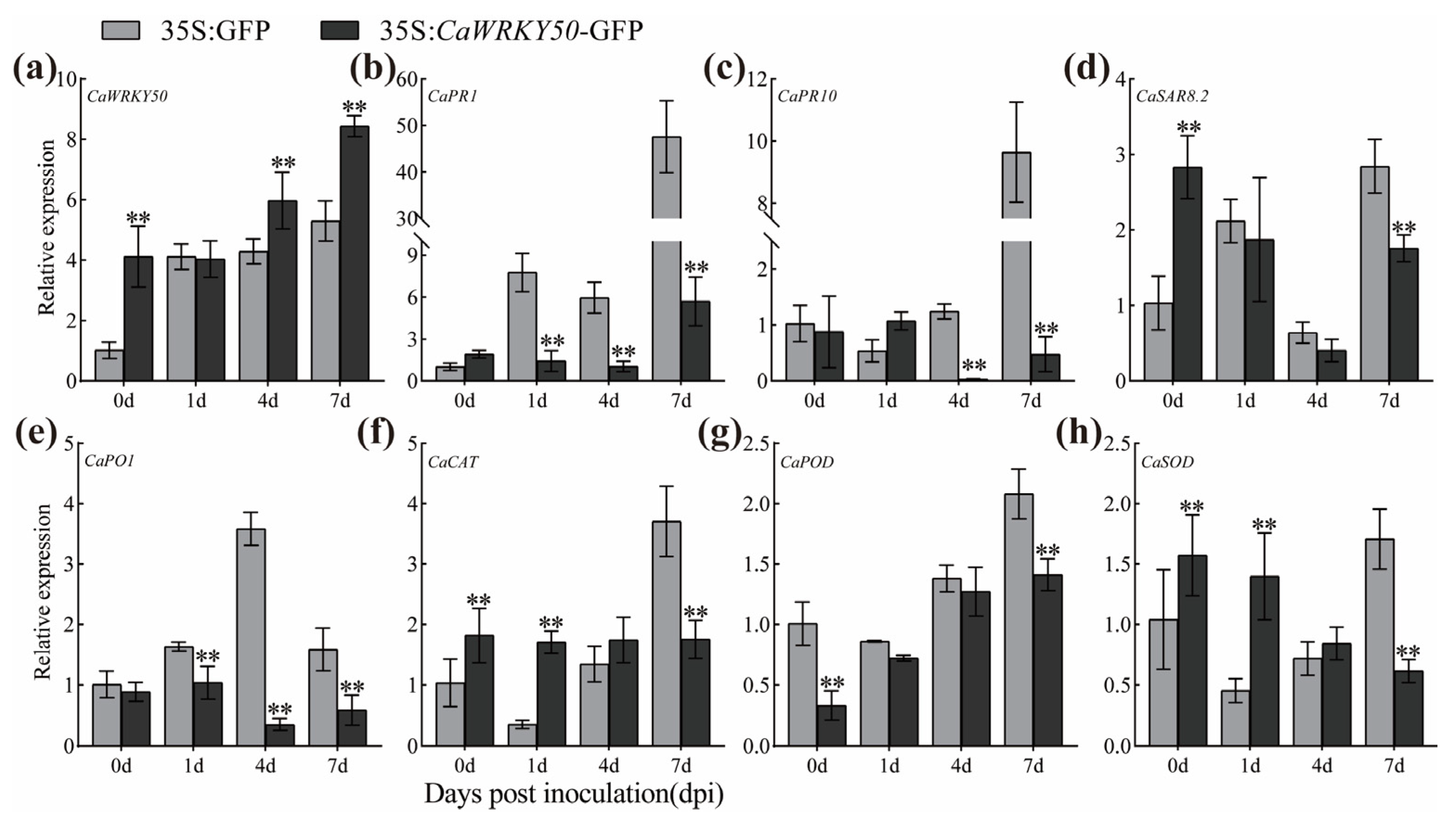
2.3. Transient CaWRKY50 Overexpression Enhances Susceptibility to C. scovillei in Pepper
2.4. Overexpression of CaWRKY50 in Tomato Decreases Resistance to C. scovillei
2.5. CaWRKY50 Specifically Binds the Promoter of CaEDS1 and CaSAMT1
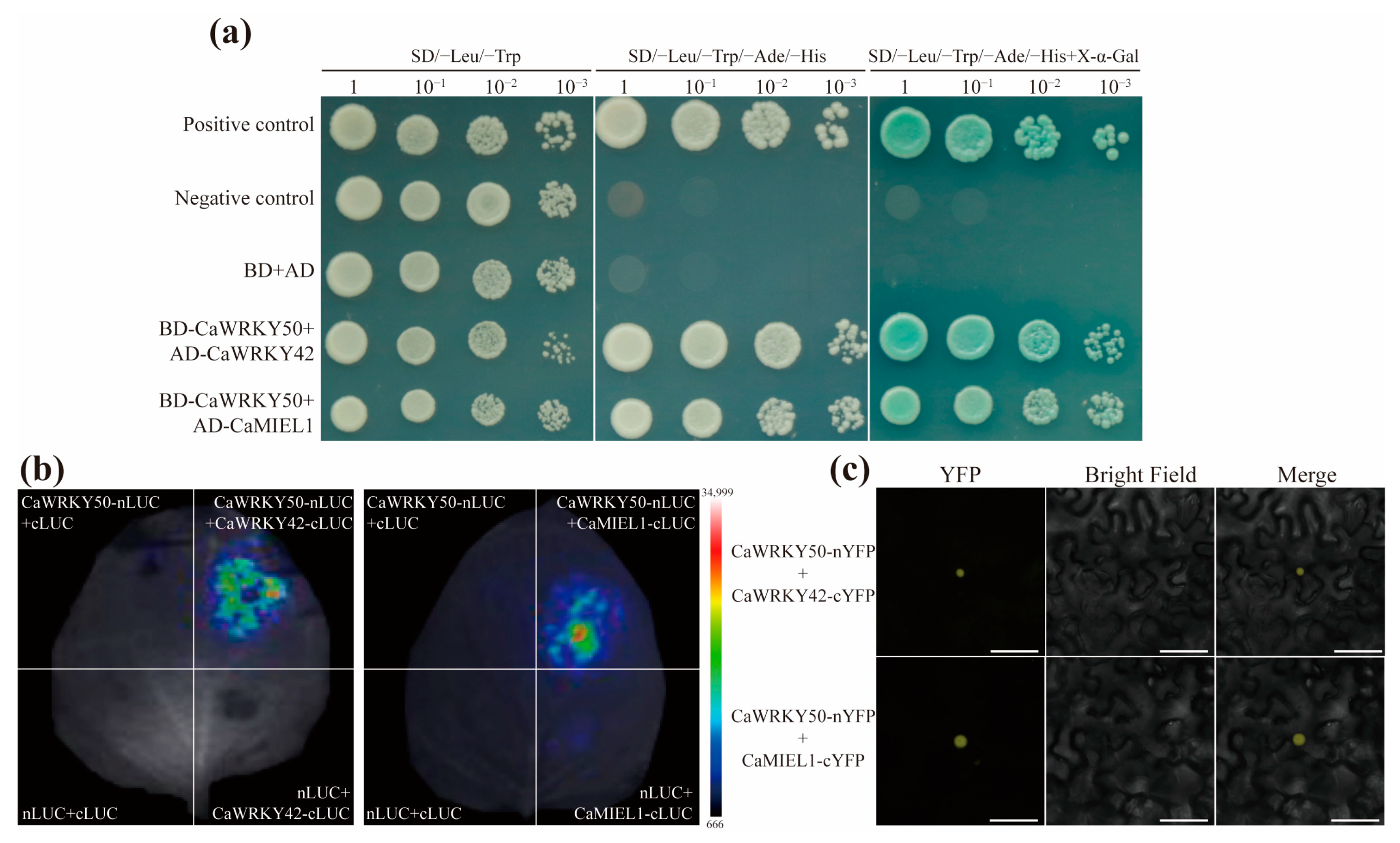
2.6. CaWRKY50 Interacts with CaWRKY42 and CaMIEL1
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Pathogen Inoculation, and Phytohormone Treatment
4.2. RNA Extraction and qRT-PCR Analysis
4.3. Phylogenetic Analysis and Subcellular Localization
4.4. Transcriptional Activity Assays in Yeast
4.5. Virus-Induced Gene Silencing (VIGS)
4.6. Transient and Transgenic Overexpression
4.7. Measurement of Physiological Indicators
4.8. Protein Interaction Assays
4.9. Yeast One-Hybrid (Y1H) Assay
4.10. GUS Activity Analysis and Dual Luciferase Reporter Assay
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qin, C.; Yu, C.; Shen, Y.; Fang, X.; Chen, L.; Min, J.; Cheng, J.; Zhao, S.; Xu, M.; Luo, Y.; et al. Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc. Natl. Acad. Sci. USA 2014, 111, 5135–5140. [Google Scholar] [CrossRef] [PubMed]
- de Silva, D.D.; Groenewald, J.Z.; Crous, P.W.; Ades, P.K.; Nasruddin, A.; Mongkolporn, O.; Taylor, P.W.J. Identification, prevalence and pathogenicity of Colletotrichum species causing anthracnose of Capsicum annuum in Asia. IMA Fungus 2019, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.H.; Fu, T.; Shin, J.H.; Song, Y.W.; Jang, D.C.; Kim, K.S. The small GTPase CsRAC1 is important for fungal development and pepper anthracnose in Colletotrichum scovillei. Plant Pathol. J. 2021, 37, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.; Zhang, Z.; Cao, Y.; Yu, H.; Ma, W.; Zhang, B.; Wang, R.; Gao, J.; Wang, L. Fine mapping of the major anthracnose resistance QTL AnR(GO)5 in Capsicum chinense ‘PBC932’. BMC Plant Biol. 2020, 20, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yu, X.; Chen, W.; Zhuang, W.; Wang, S.; Sun, C.; Cao, L.; Zhou, T.; Qu, S. MdWRKY75e enhances resistance to Alternaria alternata in Malus domestica. Hortic. Res. 2021, 8, 225. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Zhou, M.; Yoo, H.; Pruneda-Paz, J.L.; Spivey, N.W.; Kay, S.A.; Dong, X. Spatial and temporal regulation of biosynthesis of the plant immune signal salicylic acid. Proc. Natl. Acad. Sci. USA 2015, 112, 9166–9173. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Tieman, D.; Zeigler, M.; Schmelz, E.; Taylor, M.G.; Rushing, S.; Jones, J.B.; Klee, H.J. Functional analysis of a tomato salicylic acid methyl transferase and its role in synthesis of the flavor volatile methyl salicylate. Plant J. 2010, 62, 113–123. [Google Scholar] [CrossRef]
- Ament, K.; Krasikov, V.; Allmann, S.; Rep, M.; Takken, F.L.; Schuurink, R.C. Methyl salicylate production in tomato affects biotic interactions. Plant J. 2010, 62, 124–134. [Google Scholar] [CrossRef]
- Deng, B.; Wang, W.; Ruan, C.; Deng, L.; Yao, S.; Zeng, K. Involvement of CsWRKY70 in salicylic acid-induced citrus fruit resistance against Penicillium digitatum. Hortic. Res. 2020, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Chen, J.; Chang, M.; Chen, H.; Hall, K.; Korin, J.; Liu, F.; Wang, D.; Fu, Z.Q. Pandemonium breaks out: Disruption of salicylic acid-mediated defense by plant pathogens. Mol. Plant 2018, 11, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jeong, R.D.; Venugopal, S.C.; Lapchyk, L.; Navarre, D.; Kachroo, A.; Kachroo, P. SAG101 forms a ternary complex with EDS1 and PAD4 and is required for resistance signaling against turnip crinkle virus. PLoS Pathog. 2011, 7, e1002318. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Lai, Z.; Fan, B.; Chen, Z. Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell 2008, 20, 2357–2371. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, X.; Liu, H.; Wang, Y.; Nocker, S.; Tu, M.; Fang, J.; Guo, J.; Li, Z.; Wang, X. Overexpression of VqWRKY31 enhances powdery mildew resistance in grapevine by promoting salicylic acid signaling and specific metabolite synthesis. Hortic. Res. 2022, 9, uhab064. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Wang, C.; Zheng, X.; Hu, Z.; Zhu, Y.; Zhao, Y.; Jiang, A.; Zhang, H.; Shi, K.; Bai, Y.; et al. MKK4-MPK3-WRKY17-mediated salicylic acid degradation increases susceptibility to Glomerella leaf spot in apple. Plant Physiol. 2021, 186, 1202–1219. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zheng, W.; Li, J.; Liu, P.; Zhong, K.; Jin, P.; Xu, M.; Yang, J.; Chen, J. NbWRKY40 Positively regulates the response of Nicotiana benthamiana to tomato mosaic virus via salicylic acid signaling. Front. Plant Sci. 2020, 11, 603518. [Google Scholar] [CrossRef]
- Yang, S.; Cai, W.; Shen, L.; Cao, J.; Liu, C.; Hu, J.; Guan, D.; He, S. A CaCDPK29-CaWRKY27b module promotes CaWRKY40-mediated thermotolerance and immunity to Ralstonia solanacearum in pepper. New Phytol. 2022, 233, 1843–1863. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Shi, L.P.; Yang, S.; Qiu, S.S.; Ma, X.L.; Cai, J.S.; Guan, D.Y.; Wang, Z.H.; He, S.L. A conserved double-W box in the promoter of CaWRKY40 mediates autoregulation during response to pathogen attack and heat stress in pepper. Mol. Plant Pathol. 2021, 22, 3–18. [Google Scholar] [CrossRef]
- Dang, F.F.; Wang, Y.N.; Yu, L.; Eulgem, T.; Lai, Y.; Liu, Z.Q.; Wang, X.; Qiu, A.L.; Zhang, T.X.; Lin, J.; et al. CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant Cell Environ. 2013, 36, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Wang, Y.; She, J.; Lei, Y.; Liu, Z.; Eulgem, T.; Lai, Y.; Lin, J.; Yu, L.; Lei, D.; et al. Overexpression of CaWRKY27, a subgroup IIe WRKY transcription factor of Capsicum annuum, positively regulates tobacco resistance to Ralstonia solanacearum infection. Physiol. Plant. 2014, 150, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yang, S.; Yan, Y.; Xiao, Z.; Cheng, J.; Wu, J.; Qiu, A.; Lai, Y.; Mou, S.; Guan, D.; et al. CaWRKY6 transcriptionally activates CaWRKY40, regulates Ralstonia solanacearum resistance, and confers high-temperature and high-humidity tolerance in pepper. J. Exp. Bot. 2015, 66, 3163–3174. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Li, X.; Weng, Y.; Liu, Z.; Ashraf, M.F.; Noman, A.; Yang, S.; Ifnan, M.; Qiu, S.; Yang, Y.; et al. CaWRKY22 acts as a positive regulator in pepper response to Ralstonia solanacearum by constituting networks with CaWRKY6, CaWRKY27, CaWRKY40, and CaWRKY58. Int. J. Mol. Sci. 2018, 19, 1426. [Google Scholar] [CrossRef]
- Dang, F.; Lin, J.; Chen, Y.; Li, G.X.; Guan, D.; Zheng, S.J.; He, S. A feedback loop between CaWRKY41 and H2O2 coordinates the response to Ralstonia solanacearum and excess cadmium in pepper. J. Exp. Bot. 2019, 70, 1581–1595. [Google Scholar] [CrossRef]
- Hussain, A.; Khan, M.I.; Albaqami, M.; Mahpara, S.; Noorka, I.R.; Ahmed, M.A.A.; Aljuaid, B.S.; El-Shehawi, A.M.; Liu, Z.; Farooq, S.; et al. CaWRKY30 Positively Regulates Pepper Immunity by Targeting CaWRKY40 against Ralstonia solanacearum Inoculation through Modulating Defense-Related Genes. Int. J. Mol. Sci. 2021, 22, 12091. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Cai, W.; Liu, C.; Hu, J.; Shen, L.; Huang, X.; Guan, D.; He, S. CaWRKY28 Cys249 is required for interaction with CaWRKY40 in the regulation of pepper immunity to Ralstonia solanacearum. Mol. Plant. Microbe Interact. 2021, 34, 733–745. [Google Scholar] [CrossRef]
- Wang, Y.; Dang, F.; Liu, Z.; Wang, X.; Eulgem, T.; Lai, Y.; Yu, L.; She, J.; Shi, Y.; Lin, J.; et al. CaWRKY58, encoding a group I WRKY transcription factor of Capsicum annuum, negatively regulates resistance to Ralstonia solanacearum infection. Mol. Plant Pathol. 2013, 14, 131–144. [Google Scholar] [CrossRef]
- Ifnan Khan, M.; Zhang, Y.; Liu, Z.; Hu, J.; Liu, C.; Yang, S.; Hussain, A.; Furqan Ashraf, M.; Noman, A.; Shen, L. CaWRKY40b in pepper acts as a negative regulator in response to Ralstonia solanacearum by directly modulating defense genes including CaWRKY40. Int. J. Mol. Sci. 2018, 19, 1403. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, F.; Zhu, C.; Li, X.; Dai, X.; Yang, B.; Zou, X.; Ma, Y. Identification, expression, alternative splicing and functional analysis of pepper WRKY gene family in response to biotic and abiotic stresses. PLoS ONE 2019, 14, e0219775. [Google Scholar] [CrossRef]
- Chen, S.; Ding, Y.; Tian, H.; Wang, S.; Zhang, Y. WRKY54 and WRKY70 positively regulate SARD1 and CBP60g expression in plant immunity. Plant Signal. Behav. 2021, 16, 1932142. [Google Scholar] [CrossRef]
- Besseau, S.; Li, J.; Palva, E.T. WRKY54 and WRKY70 co-operate as negative regulators of leaf senescence in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 2667–2679. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xi, H.; Dai, Z.; Lecourieux, F.; Yuan, L.; Liu, X.; Patra, B.; Wei, Y.; Li, S.; Wang, L. VvWRKY8 represses stilbene synthase genes through direct interaction with VvMYB14 to control resveratrol biosynthesis in grapevine. J. Exp. Bot. 2019, 70, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Dai, W.; Zhang, C.; Wang, Y.; Wu, M.; Zhao, Y.; Ma, Q.; Xiang, Y.; Cheng, B. The maize WRKY transcription factor ZmWRKY17 negatively regulates salt stress tolerance in transgenic Arabidopsis plants. Planta 2017, 246, 1215–1231. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Tian, A.G.; Zou, H.F.; Xie, Z.M.; Lei, G.; Huang, J.; Wang, C.M.; Wang, H.W.; Zhang, J.S.; Chen, S.Y. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol. J. 2008, 6, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, W.; Zhou, R.; Wang, L.; Wang, X.; Wang, Q.; Feng, Z.; Li, Y.; Qiu, D.; He, G.; et al. The Brachypodium distachyon BdWRKY36 gene confers tolerance to drought stress in transgenic tobacco plants. Plant Cell Rep. 2015, 34, 23–35. [Google Scholar] [CrossRef]
- Jiao, S.Z.; Guo, C.; Yao, W.K.; Zhang, N.B.; Zhang, J.Y.; Xu, W.R. An Amur grape VaHsfC1 is involved in multiple abiotic stresses. Sci. Hortic. 2022, 295, 110785. [Google Scholar] [CrossRef]
- Dumanovic, J.; Nepovimova, E.; Natic, M.; Kuca, K.; Jacevic, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2020, 11, 552969. [Google Scholar] [CrossRef]
- Zhang, H.X.; Feng, X.H.; Ali, M.; Jin, J.H.; Wei, A.M.; Khattak, A.M.; Gong, Z.H. Identification of pepper CaSBP08 gene in defense response against Phytophthora capsici infection. Front. Plant Sci. 2020, 11, 183. [Google Scholar] [CrossRef]
- Ma, X.; Gai, W.X.; Li, Y.; Yu, Y.N.; Ali, M.; Gong, Z.H. The CBL-interacting protein kinase CaCIPK13 positively regulates defence mechanisms against cold stress in pepper. J. Exp. Bot. 2022, 73, 1655–1667. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Gai, W.X.; Li, C.; Gong, Z.H. The CaCIPK3 gene positively regulates drought tolerance in pepper. Hortic. Res. 2021, 8, 216. [Google Scholar] [CrossRef]
- Loake, G.; Grant, M. Salicylic acid in plant defence—The players and protagonists. Curr. Opin. Plant Biol. 2007, 10, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Feng, X.H.; Jin, J.H.; Khan, A.; Guo, W.L.; Du, X.H.; Gong, Z.H. CaSBP11 Participates in the Defense Response of Pepper to Phytophthora capsici through Regulating the Expression of Defense-Related Genes. Int. J. Mol. Sci. 2020, 21, 9065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Ali, M.; Feng, X.H.; Jin, J.H.; Huang, L.J.; Khan, A.; Lv, J.G.; Gao, S.Y.; Luo, D.X.; Gong, Z.H. A Novel Transcription Factor CaSBP12 Gene Negatively Regulates the Defense Response against Phytophthora capsici in Pepper (Capsicum annuum L.). Int. J. Mol. Sci. 2018, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, X.; Zhang, Q.; Chai, S.; Yin, W.; Gao, M.; Li, Z.; Wang, X. The transcription factors VaERF16 and VaMYB306 interact to enhance resistance of grapevine to Botrytis cinerea infection. Mol. Plant Pathol. 2022, 23, 1415–1432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, Y.; Li, X.; Li, Y. CYSTEINE-RICH RECEPTOR-LIKE KINASE5 (CRK5) and CRK22 regulate the response to Verticillium dahliae toxins. Plant Physiol. 2022, 190, 714–731. [Google Scholar] [CrossRef]
- Zhou, M.; Lu, Y.; Bethke, G.; Harrison, B.T.; Hatsugai, N.; Katagiri, F.; Glazebrook, J. WRKY70 prevents axenic activation of plant immunity by direct repression of SARD1. New Phytol. 2018, 217, 700–712. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Bai, T.; Zhang, M.; Jia, Y.L.; Shen, D.Y.; Zhang, M.X.; Dou, D.L. A Phytophthora capsici effector suppresses plant immunity via interaction with EDS1. Mol. Plant Pathol. 2020, 21, 502–511. [Google Scholar] [CrossRef]
- Tang, H.; Bi, H.; Liu, B.; Lou, S.; Song, Y.; Tong, S.; Chen, N.; Jiang, Y.; Liu, J.; Liu, H. WRKY33 interacts with WRKY12 protein to up-regulate RAP2.2 during submergence induced hypoxia response in Arabidopsis thaliana. New Phytol. 2021, 229, 106–125. [Google Scholar] [CrossRef]
- Zhou, R.; Dong, Y.; Liu, X.; Feng, S.; Wang, C.; Ma, X.; Liu, J.; Liang, Q.; Bao, Y.; Xu, S.; et al. JrWRKY21 interacts with JrPTI5L to activate the expression of JrPR5L for resistance to Colletotrichum gloeosporioides in walnut. Plant J. 2022, 111, 1152–1166. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, W.; Li, C.; Qiao, H.; Song, S.; Yang, R.; Sun, L.; Ma, J.; Ma, X.; Wang, S. SlVQ15 interacts with jasmonate-ZIM domain proteins and SlWRKY31 to regulate defense response in tomato. Plant Physiol. 2022, 190, 828–842. [Google Scholar] [CrossRef]
- Marino, D.; Froidure, S.; Canonne, J.; Ben Khaled, S.; Khafif, M.; Pouzet, C.; Jauneau, A.; Roby, D.; Rivas, S. Arabidopsis ubiquitin ligase MIEL1 mediates degradation of the transcription factor MYB30 weakening plant defence. Nat. Commun. 2013, 4, 1476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, X.; Gai, W.; Xiao, L.; Gong, Z. First Report of Colletotrichum gloeosporioides Causing Anthracnose on Pepper in Shaanxi Province, China. Plant Dis. 2021, 105, 2242. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Mao, S.L.; Zhang, Z.H.; Palloix, A.; Wang, L.H.; Zhang, B.X. Resistances to anthracnose (Colletotrichum acutatum) of Capsicum mature green and ripe fruit are controlled by a major dominant cluster of QTLs on chromosome P5. Sci. Hortic. 2015, 181, 81–88. [Google Scholar] [CrossRef]
- Zou, J.; Li, N.; Hu, N.; Tang, N.; Cao, H.; Liu, Y.; Chen, J.; Jian, W.; Gao, Y.; Yang, J.; et al. Co-silencing of ABA receptors (SlRCAR) reveals interactions between ABA and ethylene signaling during tomato fruit ripening. Hortic. Res. 2022, 9, uhac057. [Google Scholar] [CrossRef]
- Tian, S.L.; Li, L.; Chai, W.G.; Shah, S.N.; Gong, Z.H. Effects of silencing key genes in the capsanthin biosynthetic pathway on fruit color of detached pepper fruits. BMC Plant Biol. 2014, 14, 314. [Google Scholar] [CrossRef]
- Wang, W.; Li, T.; Chen, Q.; Deng, B.; Deng, L.; Zeng, K. Transcription factor CsWRKY65 participates in the establishment of disease resistance of citrus fruits to Penicillium digitatum. J. Agric. Food Chem. 2021, 69, 5671–5682. [Google Scholar] [CrossRef]
- Sun, H.J.; Uchii, S.; Watanabe, S.; Ezura, H. A highly efficient transformation protocol for Micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol. 2006, 47, 426–431. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Shang, Y.; Lin, H.; Wang, Y.; Cai, R.; Tang, X.; Zhou, J.M. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 2008, 146, 368–376. [Google Scholar] [CrossRef]
- Walter, M.; Chaban, C.; Schutze, K.; Batistic, O.; Weckermann, K.; Nake, C.; Blazevic, D.; Grefen, C.; Schumacher, K.; Oecking, C.; et al. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 2004, 40, 428–438. [Google Scholar] [CrossRef]
- Wang, L.; Tang, W.; Hu, Y.; Zhang, Y.; Sun, J.; Guo, X.; Lu, H.; Yang, Y.; Fang, C.; Niu, X.; et al. A MYB/bHLH complex regulates tissue-specific anthocyanin biosynthesis in the inner pericarp of red-centered kiwifruit Actinidia chinensis cv. Hongyang. Plant J. 2019, 99, 359–378. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ma, X.; Xiao, L.-D.; Yu, Y.-N.; Yan, H.-L.; Gong, Z.-H. CaWRKY50 Acts as a Negative Regulator in Response to Colletotrichum scovillei Infection in Pepper. Plants 2023, 12, 1962. https://doi.org/10.3390/plants12101962
Li Y, Ma X, Xiao L-D, Yu Y-N, Yan H-L, Gong Z-H. CaWRKY50 Acts as a Negative Regulator in Response to Colletotrichum scovillei Infection in Pepper. Plants. 2023; 12(10):1962. https://doi.org/10.3390/plants12101962
Chicago/Turabian StyleLi, Yang, Xiao Ma, Luo-Dan Xiao, Ya-Nan Yu, Hui-Ling Yan, and Zhen-Hui Gong. 2023. "CaWRKY50 Acts as a Negative Regulator in Response to Colletotrichum scovillei Infection in Pepper" Plants 12, no. 10: 1962. https://doi.org/10.3390/plants12101962
APA StyleLi, Y., Ma, X., Xiao, L.-D., Yu, Y.-N., Yan, H.-L., & Gong, Z.-H. (2023). CaWRKY50 Acts as a Negative Regulator in Response to Colletotrichum scovillei Infection in Pepper. Plants, 12(10), 1962. https://doi.org/10.3390/plants12101962






