Evaluation of Cowpea Landraces under a Mediterranean Climate
Abstract
1. Introduction
2. Results
2.1. Plant Phenological and Agronomical Traits
2.2. Correlations among Studied Traits
2.3. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Growth Conditions
4.3. Phenological and Yield Related Traits
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martos-Fuentes, M.; Fernández, J.A.; Ochoa, J.; Carvalho, M.; Carnide, V.; Rosa, E.A.; Pereira, G.; Barcelos, C.; Bebeli, P.J.; Egea-Gilabert, C. Genotype by environment interactions in cowpea (Vigna unguiculata L. Walp.) grown in the Iberian Peninsula. Crop Pasture Sci. 2017, 68, 924–931. [Google Scholar] [CrossRef]
- Mbuma, N.W.; Gerrano, A.S.; Lebaka, N.; Mofokeng, A.; Labuschagne, M. The evaluation of a southern African cowpea germplasm collection for seed yield and yield components. Crop Sci. 2020, 61, 466–489. [Google Scholar] [CrossRef]
- Nassir, A.L.; Olayiwola, M.O.; Olangunju, S.O.; Adewusi, K.M.; Jinabu, S.S. Genotype × environment analysis of cowpea grain production in the forest and derived savannah cultivation ecologies. Agro-Sci. 2021, 20, 20–24. [Google Scholar] [CrossRef]
- Goa, Y.; Mohhammed, H.; Worku, W.; Urage, E. Genotype by environment interaction and yield stability of cowpea (Vigna unguiculata (L.) Walp.) genotypes in moisture limited areas of Southern Ethiopia. Heliyon 2022, 8, e09013. [Google Scholar] [CrossRef]
- Gumede, M.T.; Gerrano, A.S.; Modi, A.T.; Thungo, Z. Influence of genotype and environment on grain yield among cowpea (Vigna unguiculata (L.) Walp.) genotypes under dry land farming system. Acta Agric. Scand.—B Soil Plant Sci. 2022, 72, 709–719. [Google Scholar] [CrossRef]
- Panchta, R.; Arya, S.; Singh, D.P.; Satral; Preeti; Kumar, R. Genetic variability and association studies in cowpea [Vigna unguiculata (L.) Walp.] for seed yield and related traits. Forage Res. 2020, 46, 232–235. [Google Scholar]
- Rolim, R.R.; do Nascimento, N.F.F.; Nascimento, M.F.; de Araujo, H.F.P. Genotype x environment interaction and stability in landraces of cowpea under dryland conditions. Rev. Caatinga Mossoró 2023, 36, 339–348. [Google Scholar]
- Pejić, B.; Maĉkić, K.; Mikić, A.; Ćupina, B.; Pekşen, E.; Krstić, D.; Antanasović, S. Effect of water stress on the yield of cowpea (Vigna unguiculata L. Walp.) in temperate climatic conditions. J. Cont. Agric. 2013, 62, 168–176. [Google Scholar]
- Toudou Daouda, A.K.; Sanoussi, A.; Maȃrouhi, I.M.; Falalou, H.; Yacoubou, B. Effect of water deficit at different stages of development on the yield components of cowpea (Vigna unguiculata L. Walp.) genotypes. Afr. J. Biotechnol. 2018, 17, 279–287. [Google Scholar] [CrossRef]
- Moreira, R.; Nunes, C.; Pais, I.; Semedo, J.; Guimarães, J.B.; Simões, F.; Veloso, M.M.; Scotti-Campos, P. How to Improve Already Improved Cowpea-Terminal Drought. Biol. Life Sci. Forum 2022, 11, 45. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavits, Z. Cultivar Evaluation and Mega-Environment Investigation Based on the GGE Biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Egbadzor, K.F.; Danquah, E.Y.; Ofori, K.; Yeboah, M.; Offei, S.K. Diversity in 118 Cowpea (Vigna unguiculata (L.) Walp.) accessions assessed with 16 morphological traits. Int. J. Plant Breed. Genet. 2014, 8, 13–24. [Google Scholar] [CrossRef][Green Version]
- Ishikawa, H.; Drabo, I.; Joseph, B.B.; Muranaka, S.; Fatokun, C.; Boukar, O. Characteristics of farmers’ selection criteria for cowpea (Vigna unguiculata) varieties differ between north and south regions of Burkina Faso. Exp. Agric. 2020, 56, 94–103. [Google Scholar] [CrossRef]
- Mohammed, S.B.; Dzidzienyo, D.K.; Umar, M.L.; Ishiyaku, M.F.; Tongoona, P.B.; Gracen, V. Appraisal of cowpea cropping systems and farmers’ perceptions of production constraints and preferences in the dry savannah areas of Nigeria. CABI Agric. Biosci. 2021, 2, 25. [Google Scholar] [CrossRef]
- Casañas, F.; Simó, J.; Casals, J.; Prohens, J. Toward an Evolved Concept of Landrace. Front. Plant Sci. 2017, 8, 145. [Google Scholar] [CrossRef]
- Tavoularis, K. Average Crop Yields in Greece; Ministry of Rural Development and Food, Agricultural Policy and Documentation Directorate Department of Agricultural Statistics: Athens, Greece, 2012; pp. 1–11.
- Aliyu, A.M.; Lawal, O.O.; Wahab, A.A.; Ibrahim, U.Y. Evaluation of Advanced Breeding Lines of Cowpea (Vigna unguiculata L. Walp.) for High Seed Yield under Farmers’ Field Conditions. Plant Breed. Biotech. 2019, 7, 12–23. [Google Scholar] [CrossRef]
- Ajayi, A.T.; Gbadamosi, A.E. Genetic variability, character association and yield potentials of twenty five accessions of cowpea (Vigna unguiculata L. Walp.). J. Pure Appl. Agric. 2020, 5, 1–16. [Google Scholar]
- Manivannan, N.; Bharathi Kumar, K.; Mahalingam, A.; Ramakrishnan, P. Stability analysis for seed yield in cowpea genotypes (Vigna unguiculata (L.) Walp.). Electron. J. Plant Breed. 2019, 10, 1246–1249. [Google Scholar] [CrossRef]
- Abdou, S. Evaluation of cowpea [Vigna unguiculata (L) Walp.] lines for high grain and fodder yields in the dry season of Niger republic. Heliyon 2022, 8, e09147. [Google Scholar] [CrossRef]
- Monteagudo, A.; Casas, A.M.; Cantalapiedra, C.P.; Contreras-Moreira, B.; Pilar Gracia, M.; Igartua, E. Harnessing novel diversity from landraces to improve an elite barley variety. Front. Plant Sci. 2019, 10, 434. [Google Scholar] [CrossRef]
- Shimelis, H.; Shiringani, R. Variance components and heritabilities of yield and agronomic traits among cowpea genotypes. Euphytica 2010, 176, 383–389. [Google Scholar] [CrossRef]
- Devi, S.M.; Jayamani, P. Genetic variability, heritability, genetic advance studies in cowpea germplasm [Vigna unguiculata (L.) Walp.]. Electron. J. Plant Breed. 2018, 9, 476–481. [Google Scholar] [CrossRef]
- Mofokeng, M.A.; Mashilo, J.; Rantso, P.; Shimelis, H. Genetic variation and genetic advance in cowpea based on yield and yield-related traits. Acta Agric. Scand.—B Soil Plant Sci. 2020, 70, 381–391. [Google Scholar] [CrossRef]
- Araméndiz-Tatis, H.; Cardona-Ayala, C.; Espitia-Camacho, M. Heritability, genetic gain, and correlations in cowpea beans (Vigna unguiculata [L.] (Walp.). Rev. Colomb. Cienc. Hortic. 2021, 15, e12321. [Google Scholar] [CrossRef]
- Singh, A.; Singh, M.K.; Arya, M.; Jaiswal, A.; Tripathi, K.; Chaturvedi, S.K. Assessment of genetic variability among agro-morphological traits in cowpea (Vigna unguiculata L.) in the Bundelkhand region of Uttar Pradesh. J. Pharm. Innov. 2022, 11, 689–695. [Google Scholar]
- Gerrano, A.S.; van Rensburg, W.S.J.; Kutu, F.R. Agronomic evaluation and identification of potential cowpea (Vigna unguiculata L. Walp.) genotypes in South Africa. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2019, 69, 295–303. [Google Scholar] [CrossRef]
- Ceritoğlu, M.; Erman, M. Determination of Some Agronomic Traits and Their Correlation with Yield Components in Cowpea. SJAFS 2020, 34, 154–161. [Google Scholar] [CrossRef]
- Meena, H.K.; Krishna, R.K.; Bhuri, S. Character Associations between Seed Yield and Its Components Traits in Cowpea [Vigna unguiculata (L.) Walp.]. Indian J. Agric. Res. 2015, 49, 567–570. [Google Scholar] [CrossRef]
- Walle, T.; Mekbib, F.; Amsalu, B.; Gedil, M. Genetic Diversity of Ethiopian Cowpea [Vigna unguiculata (L) Walp.] Genotypes Using Multivariate Analyses. Ethiop. J. Agric. Sci. 2019, 29, 89–104. [Google Scholar]
- Edematie, V.E.; Fatokun, C.; Boukar, O.; Adetimirin, V.O.; Kumar, P.L. Inheritance of Pod Length and Other Yield Components in Two Cowpea and Yard-Long Bean Crosses. Agronomy 2021, 11, 682. [Google Scholar] [CrossRef]
- Kouam, E.B.; Ngompe-Deffo, T.; Anoumma, M.; Pasquet, R.S. Preliminary study on character associations, phenotypic and genotypic divergence for yield and related quantitative traits among cowpea landraces (Vigna unguiculata) from the Western Highland Region of Cameroon. Open Agric. 2018, 3, 84–97. [Google Scholar] [CrossRef]
- Nkhoma, N.; Shimelis, H.; Laing, M.D.; Shayanowako, A.; Mathew, I. Assessing the genetic diversity of cowpea [Vigna unguiculata (L.) Walp.] germplasm collections using phenotypic traits and SNP markers. BMC Genet. 2020, 21, 110. [Google Scholar] [CrossRef]
- Sharma, A.; Mishra, S.P.; Gour, L. Heritable relationship and variability of yield and yield determinants in cow pea. Int. J. Chem. Stud. 2019, 7, 3605–3611. [Google Scholar]
- Putri, P.H.; Nugrahaeni, N. Cowpea [Vigna unguiculata (L.) Walp.] Yield Variance and Supported Character. In Advances in Biological Sciences Research vo. 14., Proceedings of the 3rd KOBI Congress, International and National Conferences, Bengkulu, Indonesia, 24–25 November 2020; Springer Nature: Berlin/Heidelberg, Germany, 2020; Volume 14, pp. 65–71. [Google Scholar]
- Owusu, E.Y.; Karikari, B.; Kusi, F.; Haruna, M.; Amoah, R.A.; Attamah, P.; Adazebra, G.; Sie, E.K.; Issahaku, M. Genetic variability, heritability and correlation analysis among maturity and yield traits in Cowpea (Vigna unguiculata (L) Walp.) in Northern Ghana. Heliyon 2021, 7, e07890. [Google Scholar] [CrossRef]
- Tyagi, P.C.; Nirmal, K.; Agarwal, M.C. Genetic variability and association of component characters for seed yield in cowpea [Vigna unguiculata (L.) Walp.]. Legume Res. 2000, 23, 92–96. [Google Scholar]
- Gerrano, A.S.; Thungo, Z.G.; Mavengahama, S. Phenotypic description of elite cowpea (Vigna ungiculata L. Walp.) genotypes grown in drought-prone environments using agronomic traits. Heliyon 2022, 8, e08855. [Google Scholar] [CrossRef]
- Adhikari, U.; Nejadhashemi, A.P.; Woznicki, S.A. Climate change and eastern Africa: A review of impact on major crops. Food Energy Secur. 2015, 4, 110–132. [Google Scholar] [CrossRef]
- Ferrero, R.; Lima, M.; Gonzalez-Andujar, J.L. Crop production structure and stability under climate change in South America. Ann. Appl. Biol. 2017, 172, 65–73. [Google Scholar] [CrossRef]
- Warsame, A.A.; Sheik-Ali, I.A.; Jama, O.M.; Hassan, A.A.; Barre, G.M. Assessing the effects of climate change and political instability on sorghum production: Empirical evidence from Somalia. J. Clean. Prod. 2022, 360, 131893. [Google Scholar] [CrossRef]
- Kapazoglou, A.; Gerakari, M.; Lazaridi, E.; Kleftogianni, K.; Sarri, E.; Tani, E.; Bebeli, P.J. Crop Wild Relatives: A Valuable Source of Tolerance to Various Abiotic Stresses. Plants 2023, 12, 328. [Google Scholar] [CrossRef]
- Zeven, A.C. Landraces: A review of definitions and classifications. Euphytica 1998, 104, 127–139. [Google Scholar] [CrossRef]
- Ficiciyan, A.; Loos, J.; Sievers-Glotzbach, S.; Tscharntke, T. More than Yield: Ecosystem Services of Traditional versus Modern Crop Varieties Revisited. Sustainability 2018, 10, 2834. [Google Scholar] [CrossRef]
- Bocci, R.; Bussi, B.; Petitti, M.; Franciolini, R.; Altavilla, V.; Galluzzi, G.; Di Luzio, P.; Migliorini, P.; Spagnolo, S.; Floriddia, R.; et al. Yield, yield stability and farmers’ preferences of evolutionary populations of bread wheat: A dynamic solution to climate change. Eur. J. Agron. 2020, 121, 126156. [Google Scholar] [CrossRef]
- Hadou El Hadj, D.; Tellah, S.; Goumeida, K.; Aitouakli, S.; Tifest, C.; Ammi, N.; Ratet, P.; Pulvento, C.; Sellami, M.H. Evaluation of Adaptability of Different Faba Bean Landraces under Mediterranean Field Conditions of Central-Northern Algeria. Agronomy 2022, 12, 1660. [Google Scholar] [CrossRef]
- Al-Abdallat, A.M.; Karadsheh, A.; Hadadd, N.I.; Akash, M.W.; Ceccarelli, S.; Baum, M.; Hasan, M.; Jighly, A.; Abu Elenein, J.M. Assessment of genetic diversity and yield performance in Jordanian barley (Hordeum vulgare L.) landraces grown under Rainfed conditions. BMC Plant Biol. 2017, 17, 191. [Google Scholar] [CrossRef]
- Marone, D.; Russo, M.A.; Mores, A.; Ficco, D.B.M.; Laidò, G.; Mastrangelo, A.M.; Borrelli, G.M. Importance of Landraces in Cereal Breeding for Stress Tolerance. Plants 2021, 10, 1267. [Google Scholar] [CrossRef]
- Sari, G.N.; Saptadi, D.; Kuswanto, K. The Yield Stability and Adaptability of Bambara Groundnut at Three Locations. AGRIVITA J. Agric. Sci. 2022, 44, 130–138. [Google Scholar] [CrossRef]
- Carvalho, M.; Bebeli, P.J.; Pereira, G.; Castro, I.; Egea-Gillabert, C.; Matos, M.; Lazaridi, E.; Duarte, I.; Lino-Neto, T.; Ntatsi, G.; et al. European cowpea landraces for a more sustainable agriculture system and novel foods. JSFA 2017, 97, 4399–4407. [Google Scholar] [CrossRef]
- Lazaridi, E.; Ntatsi, G.; Fernández, J.A.; Karapanos, I.; Carnide, V.; Savvas, D.; Bebeli, P.J. Phenotypic diversity and evaluation of fresh pods of cowpea landraces from Southern Europe. J. Sci. Food Agric. 2017, 97, 4326–4333. [Google Scholar] [CrossRef]
- Rivas, R.; Falcão, H.M.; Ribeiro, R.V.; Machado, E.C.; Pimentel, C.; Santos, M.G. Drought tolerance in cowpea species is driven by less sensitivity of leaf gas exchange to water deficit and rapid recovery of photosynthesis after rehydration. S. Afr. J. Bot. 2016, 103, 101–107. [Google Scholar] [CrossRef]
- Gnankambary, K.; Sawadogo, N.; Diéni, Z.; Batieno, T.B.J.; Tinegré, J.B.D.S.; Sawadogo, M.; Quédraogo, T.J. Assessment of Cowpea (Vigna unguiculata (L.) Walp.) Mutant Lines for Drought Tolerance. Hindawi Int. J. Agron. 2020, 2020, 8823498. [Google Scholar] [CrossRef]
- Lazaridi, E.; Bebeli, P.J. Cowpea constraints and breeding in Europe. Plants 2023, 12, 1339. [Google Scholar] [CrossRef]
- de Souza, C.L.C.; de Almeida Lopez, A.C.; Gomes, R.L.F.; de Moura Rocha, M.; Silva, E.M. Variability and correlations in cowpea populations for green-grain production. CBAB 2007, 7, 262–269. [Google Scholar] [CrossRef]
- Lazaridi, E.; Ntatsi, G.; Savvas, D.; Bebeli, P.J. Diversity in cowpea (Vigna unguiculata (L.) Walp.) local populations from Greece. Genet. Resour. Crop Evol. 2016, 64, 1529–1551. [Google Scholar] [CrossRef]
- Bertoldo, J.G.; Coimbra, J.L.M.; Guidolin, A.F.; de Andrade, L.R.B.; Nodari, R.O. Agronomic potential of genebank landrace elite accessions for common bean genetic breeding. Sci. Agric. 2014, 71, 120–125. [Google Scholar] [CrossRef]
- Hedge, V.S.; Mishra, S.K. Landraces of cowpea, Vigna unguiculata (L.) Walp., as potential sources of genes for unique characters in breeding. Genet. Resour. Crop Evol. 2009, 56, 615–627. [Google Scholar] [CrossRef]
- Carvalho, M.; Carnide, V.; Sobreira, C.; Castro, I.; Coutinho, J.; Barros, A.; Rosa, E. Cowpea Immature Pods and Grains Evaluation: An Opportunity for Different Food Sources. Plants 2022, 11, 2079. [Google Scholar] [CrossRef]
- Santos, R.; Carvalho, M.; Rosa, E.; Carnide, V.; Castro, I. Root and Agro-Morphological Traits Performance in Cowpea under Drought Stress. Agronomy 2020, 10, 1604. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Ceccarelli, S.; Blair, M.W.; Upadhyaya, H.D.; Are, A.K.; Ortiz, R. Landrace Germplasm for Improving Yield and Abiotic Stress Adaptation. Trends Plant Sci. 2016, 21, 31–42. [Google Scholar] [CrossRef]
- Caproni, L.; Raggi, L.; Ceccarelli, S.; Negri, V.; Carboni, A. In-Depth Characterization of Common Bean Diversity Discloses Its Breeding Potential for Sustainable Agriculture. Sustainability 2019, 11, 5443. [Google Scholar] [CrossRef]
- Dadson, R.B.; Hashem, F.M.; Javaid, I.; Joshi, J.; Allen, A.L.; Devine, T.E. Effect of Water Stress on the Yield of Cowpea (Vigna unguiculata L. Walp.) Genotypes in the Delmarva Region of the United States. J. Agron. Crop Sci. 2005, 191, 210–217. [Google Scholar] [CrossRef]
- IPCC Climate Change. Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Reisinger, A., Eds.; IPCC: Geneva, Switzerland, 2007; p. 104. [Google Scholar]
- Ehlers, J.D.; Hall, A.E. Heat tolerance of contrasting cowpea lines in short and long days. Field Crop Res. 1998, 55, 11–21. [Google Scholar] [CrossRef]
- Hall, A.E. Phenotyping cowpeas for adaptation to drought. Front. Physiol. 2012, 3, 155. [Google Scholar] [CrossRef]
- Reis, E.R.; Drinkwater, L.E. Cultivar mixtures: A meta-analysis of the effect of intraspecific diversity on crop yield. Ecological Applications. ESA 2017, 28, 62–77. [Google Scholar] [CrossRef]
- Tratwal, A.; Bocianowski, J. Cultivar mixtures as part of integrated protection of spring barley. JPDP 2018, 125, 41–50. [Google Scholar] [CrossRef]
- Wuest, S.E.; Reter, R.; Niklaus, P.A. Ecological and evolutionary approaches to improving crop variety mixtures. Nat. Ecol. Evol. 2021, 5, 1068–1077. [Google Scholar] [CrossRef]
- Belko, N.; Cisse, N.; Diop, N.N.; Zombre, G.; Thiaw, S.; Muranaka, S.; Ehlers, J.D. Selection for post-flowering drought resistance in short and medium duration Cowpeas using stress tolerance indices. Crop Sci. 2014, 54, 25–33. [Google Scholar] [CrossRef]
- Perrino, P.; Laghetti, G.; Spagnoletti Zeuli, P.L.; Monti, L.M. Diversification of cowpea in the Mediterranean and other centres of cultivation. Genet. Resour. Crop Evol. 1993, 40, 121–132. [Google Scholar] [CrossRef]
- Manggoel, W.; Uguru, M.I.; Ndam, O.N.; Dasbak, M.A. Genetic variability, correlation and path coefficient analysis of some yield components of ten cowpea [Vigna unguiculata (L.) Walp.] accessions. J. Plant Breed. Crop Sci. 2012, 4, 80–86. [Google Scholar] [CrossRef]
- Ajayi, A.T.; Adekola, M.O.; Taiwo, B.H.; Azuh, V.O. Character expression and differences in yield potential of ten genotypes of Cowpea (Vigna unguiculata (L.) Walp.). Int. J. Plant Res. 2014, 4, 63–71. [Google Scholar] [CrossRef]
- Gepts, P. A comparison between crop domestication, classical plant breeding and genetic engineering. Crop Sci. 2002, 42, 1780–1790. [Google Scholar] [CrossRef]
- de Freitas, T.G.G.; Silva, P.S.L.; Dovale, J.C.; Silva, I.N.; da Silva, E.M. Grain yield and path analysis in the evaluation of cowpea landraces. Rev. Caatinga Mossoró. 2019, 32, 302–311. [Google Scholar] [CrossRef]
- Kindie, Y.; Tesso, B.; Amsalu, B. Genotype X Environment Interaction and Yield Stability in Early- Maturing Cowpea (Vigna unguiculata (L.) Walp.) Landraces in Ethiopia. Adv. Agric. 2021, 2021, 3786945. [Google Scholar] [CrossRef]
- Horn, L.N.; Shimelis, H. Production constraints and breeding approaches for cowpea improvement for drought prone agro-ecologies in Sub-Saharan Africa. Ann. Agric. Sci. 2020, 65, 83–91. [Google Scholar] [CrossRef]
- Terzopoulos, P.J. Collection, Registration and Evaluation of Genetic Material Established in Our Country in the Species Vicia faba L. Ph.D. Thesis, Agricultural University of Athens, Athens, Greece, 2002. (In Greek). [Google Scholar]
- Ullah, H.; Subthain, H.; Khalil, I.H.; Khan, W.U.; Jamal, Y.; Alam, M. Stress selection indices an acceptable tool to screen superior wheat genotypes under irrigated and rain-fed conditions. Pak. J. Bot. 2014, 46, 627–638. [Google Scholar]
- Ghaed-rahimi, L.; Heidari, B.; Dadkhodaie, A. Construction and efficiency of selection indices in wheat (Triticum aestivum L.) under drought stress and well-irrigated conditions. Plant Breed. Biotechnol. 2017, 5, 78–87. [Google Scholar] [CrossRef]
- Bebeli, P.J.; Thanopoulos, R. The Plant Wealth of Lemnos Island—A Source of Prosperity for Local Society; MedINA: Athens, Greece, 2020; pp. 81, 193. (In Greek) [Google Scholar]
- Goenaga, R.; Gillaspie, A.G., Jr.; Quiles, A. Field Screening of Cowpea Genotypes for Alkaline Soil Tolerance. Hort. Sci. 2010, 45, 1639–1642. [Google Scholar] [CrossRef]
- Tampakaki, A.P.; Fotiadis, C.T.; Ntatsi, G.; Savvas, D. Phylogenetic multilocus sequence analysis of indigenous slow-growing rhizobia nodulating cowpea (Vigna unguiculata L.) in Greece. Syst. Appl. Microbiol. 2017, 40, 179–189. [Google Scholar] [CrossRef]
- da Costa, E.M.; de Carvalho, T.S.; Guimarães, A.A.; Leão, A.C.R.; Cruz, L.M.; de Baura, V.A.; Lebbe, L.; Willems, A.; de Souza Moreira, F.M. Classification of the inoculant strain of cowpea UFLA03-84 and of other strains from soils of the Amazon region as Bradyrhizobium viridifuturi (symbiovar tropici). Braz. J. Microbiol. 2019, 50, 335–345. [Google Scholar] [CrossRef]
- Angelotti, F.; Barbosa, L.G.; Barros, J.R.A.; Santos, C.A.F. Cowpea (Vigna unguiculata) development under different temperatures and carbon dioxide concentrations. Rev. Pesqui. Agropecu. Trop. 2020, 50, 1–7. [Google Scholar] [CrossRef]
- Barros, J.R.A.; Guimarães, M.J.M.; e Silva, R.M.; Rêgo, M.T.C.; de Melo, N.F.; de Melo Chaves, A.R.; Angelloti, F. Selection of cowpea cultivars for high temperature tolerance: Physiological, biochemical and yield aspects. Physiol. Mol. Biol. Plants 2021, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Herawati, R.; Lestaru, A.P.; Nurmegawati; Ganefianti, D.W.; Romeida, A. Comparative study on the stability and adaptability of different models to develop a high-yield inbred line from landrace rice varieties. Ann. Agric. Sci. 2021, 66, 184–192. [Google Scholar] [CrossRef]
- Mahdy, R.E.; Ashehri, D.; Alatawi, H.A.; Al-Amrah, H.; Mahdy, E.E. Direct and Indirect Selection for Grain Yield and Grain Weight in Late Generations of Bread Wheat under Drought Stress and Normal Irrigation Environments. Plants 2022, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, B.; Tbaei-Aghdaei, S.R.; Assareh, M.H.; Darvish, F. Evaluation of Stability Parameters for Discrimination of Stable, Adaptable and High Flower Yielding Landraces of Rosa damascena. J. Agr. Sci. Tech. 2011, 13, 99–110. [Google Scholar]
- Abdelghany, A.M.; Zhang, S.; Azam, M.; Shaibu, A.S.; Feng, Y.; Qi, J.; Li, J.; Li, Y.; Tian, Y.; Hong, H.; et al. Exploring the Phenotypic Stability of Soybean Seed Compositions Using Multi-Trait Stability Index Approach. Agronomy 2021, 11, 2200. [Google Scholar] [CrossRef]


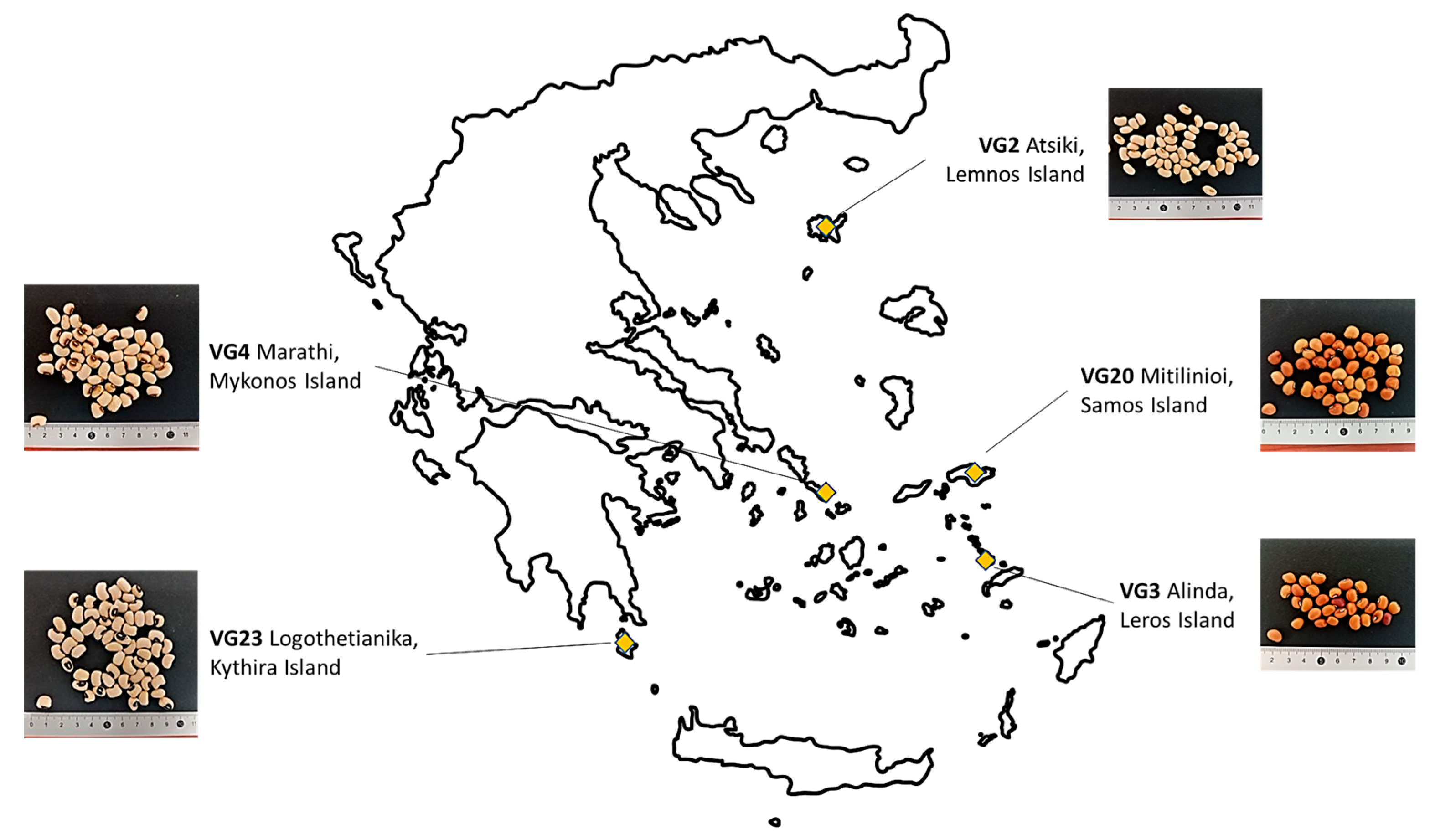
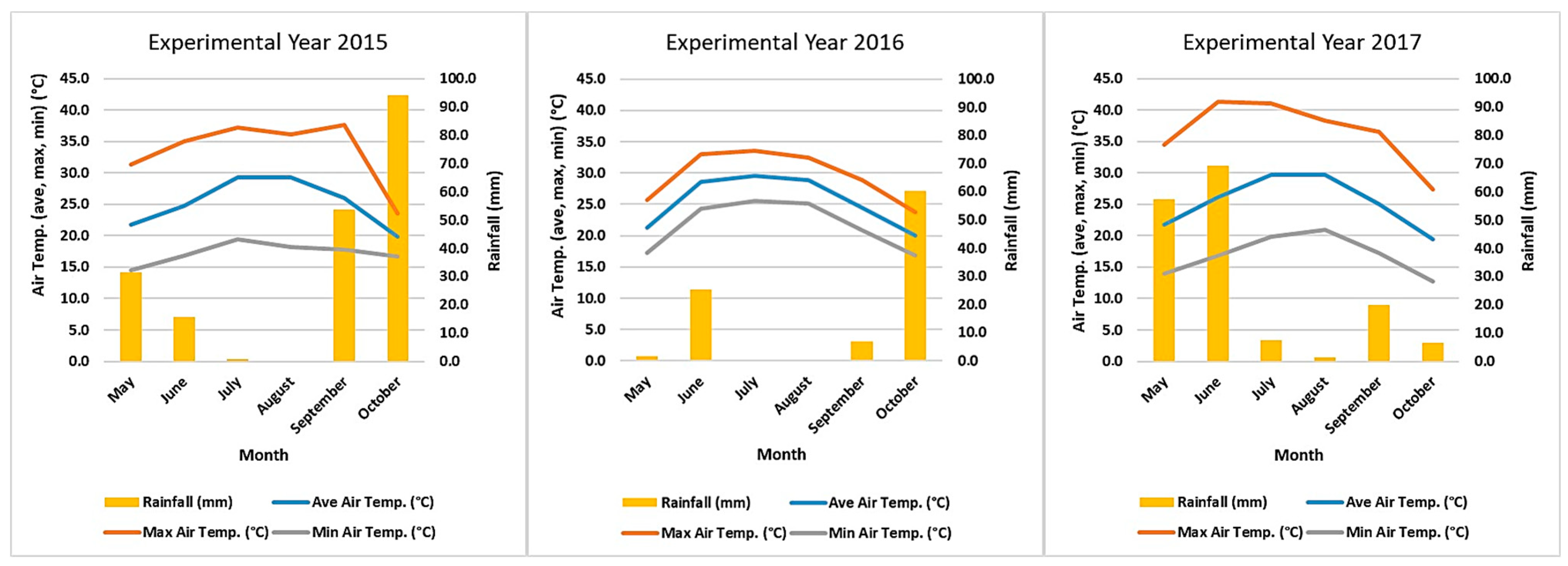
| Experimental Year | Accession | DFL | DMAT | FDUR |
|---|---|---|---|---|
| 2015 | IT97K-499-35 | 68.00 ± 2.00 b–f | 83.66 ± 1.33 | 95.67 ± 0.88 a |
| VG2 | 60.67 ± 2.60 f | 80.33 ± 3.18 | 58.00 ± 1.73 ef | |
| VG3 | 68.67 ± 0.88 b–f | 94.00 ± 2.08 | 64.67 ± 0.66 d | |
| VG4 | 62.33 ± 1.76 de | 85.00 ± 2.08 | 61.67 ± 2.40 de | |
| VG20 | 67.33 ± 2.33 b–f | 96.67 ± 2.40 | 63.00 ± 4.51 e | |
| VG23 | 62.33 ± 1.20 de | 91.67 ± 9.17 | 58.67 ± 1.20 ef | |
| 2016 | IT97K-499-35 | 72.67 ± 1.45 a–d | 87.00 ± 4.04 | 74.33 ± 0.33 cd |
| VG2 | 64.58 ± 1.53 c–f | 76.25 ± 2.25 | 50.00 ± 1.00 fg | |
| VG3 | 75.17 ± 1.59 ab | 85.42 ± 1.42 | 41.67 ± 2.85 gh | |
| VG4 | 60.50 ± 0.76 f | 76.25 ± 3.78 | 25.75 ± 2.27 i | |
| VG20 | 75.08 ± 1.58 ab | 86.33 ± 2.33 | 45.33 ± 1.45 gh | |
| VG23 | 60.17 ± 1.30 f | 75.17 ± 2.46 | 38.00 ± 1.00 h | |
| 2017 | IT97K-499-35 | 80.00 ± 1.73 a | 98.33 ± 2.73 | 89.66 ± 3.28 ab |
| VG2 | 65.33 ± 0.67 b–f | 90.00 ± 2.00 | 81.33 ± 0.88 bc | |
| VG3 | 74.00 ± 2.31 abc | 95.00 ± 2.52 | 81.33 ± 0.88 bc | |
| VG4 | 64.67 ± 3.28 c–f | 90.33 ± 2.91 | 83.00 ± 2.65 bc | |
| VG20 | 71.33 ± 2.40 a–e | 92.00 ± 1.15 | 84.00 ± 1.73 bc | |
| VG23 | 63.67 ± 2.19 def | 84.33 ± 1.20 | 82.00 ± 2.00 bc | |
| Main effects | ||||
| 2015 | 64.88 ± 1.01 b | 88.56 ± 2.05 a | 66.94 ± 3.26 b | |
| 2016 | 68.03 ± 1.65 a | 81.07 ± 1.60 b | 45.85 ± 3.63 c | |
| 2017 | 69.84 ± 1.62 a | 91.67 ± 1.30 a | 83.83 ± 0.99 a | |
| IT97K-499-35 | 73.56 ± 1.95 a | 89.67 ± 2.66 ab | 86.55 ± 3.33 a | |
| VG2 | 63.53 ± 1.15 b | 82.19 ± 2.40 b | 63.11 ± 4.74 b | |
| VG3 | 72.61 ± 1.31 a | 91.47 ± 1.83 a | 63.11 ± 6.05 b | |
| VG4 | 62.50 ± 1.25 b | 83.86 ± 2.54 ab | 56.81 ± 8.44 c | |
| VG20 | 71.25 ± 1.55 a | 91.67 ± 1.81 a | 64.11 ± 5.77 b | |
| VG23 | 62.06 ± 0.96 b | 83.72 ± 3.65 ab | 59.56 ± 6.40 bc | |
| Statistical Significance | ||||
| Experimental Year | *** | *** | *** | |
| Accession | *** | ** | *** | |
| Exp. Year × Accession | * | ns | *** | |
| Experimental Year | Accession | PH (cm) | NPOD | PODL (cm) | SPOD | SEEDW (g) | NSEED | 100 SW (g) |
|---|---|---|---|---|---|---|---|---|
| 2015 | IT97K-499-35 | 34.39 ± 8.11 b–d | 19.58 ± 6.72 | 12.20 ± 0.51 | 9.23 ± 1.63 a–c | 14.94 ± 4.00 | 129.27 ± 48.64 | 15.96 ± 0.07 cd |
| VG2 | 25.08 ± 3.41 b–d | 14.78 ± 2.18 | 9.31 ± 0.70 | 5.61 ± 0.59 c | 11.56 ± 2.78 | 88.17 ± 17.28 | 14.05 ± 0.42 de | |
| VG3 | 45.33 ± 6.85 b | 12.31 ± 1.23 | 10.59 ± 0.14 | 5.27 ± 0.19 c | 12.38 ± 2.11 | 63.06 ± 7.28 | 18.76 ± 0.94 b | |
| VG4 | 37.08 ± 2.80 b–d | 16.75 ± 2.84 | 11.81 ± 0.17 | 8.14 ± 0.76 a–c | 17.36 ± 2.38 | 143.58 ± 29.07 | 22.49 ± 0.55 a | |
| VG20 | 26.49 ± 0.99 b–d | 13.50 ± 3.53 | 12.50 ± 0.55 | 6.70 ± 0.34 bc | 14.95 ± 4.84 | 93.33 ± 29.60 | 15.97 ± 0.85 cd | |
| VG23 | 70.94 ± 3.40 a | 18.56 ± 0.75 | 14.48 ± 0.17 | 9.82 ± 0.40 a–c | 21.17 ± 2.18 | 171.67 ± 12.51 | 17.20 ± 0.36 bc | |
| 2016 | IT97K-499-35 | 22.96 ± 3.22 cd | 8.83 ± 1.52 | 15.20 ± 0.93 | 12.65 ± 0.32 ab | 10.42 ± 1.60 | 34.84 ± 0.91 | 15.74 ± 0.26 c–e |
| VG2 | 19.82 ± 0.93 d | 12.81 ± 1.52 | 8.98 ± 3.31 | 8.07 ± 2.78 a–c | 8.15 ± 1.00 | 52.10 ± 8.74 | 13.19 ± 0.72 e | |
| VG3 | 27.70 ± 5.68 b–d | 12.81 ± 0.69 | 8.98 ± 3.31 | 14.39 ± 0.98 a | 13.05 ± 4.93 | 28.21 ± 5.75 | 15.39 ± 0.19 c–e | |
| VG4 | 23.92 ± 0.74 cd | 7.89 ± 0.93 | 5.33 ± 1.68 | 5.05 ± 1.65 c | 7.79 ± 1.43 | 44.08 ± 8.52 | 24.79 ± 1.19 a | |
| VG20 | 29.13 ± 2.30 b–d | 8.69 ± 3.05 | 15.97 ± 4.05 | 11.01 ± 1.03 a–c | 13.46 ± 5.43 | 26.59 ± 4.79 | 16.68 ± 0.70 b–d | |
| VG23 | 41.44 ± 6.80 b–d | 9.92 ± 2.89 | 15.73 ± 3.53 | 10.67 ± 1.52 a–c | 13.81 ± 4.83 | 30.70 ± 471 | 17.14 ± 0.40 bc | |
| 2017 | IT97K-499-35 | 25.49 ± 1.57 b–d | 15.72 ± 2.21 | 13.71 ± 0.96 | 7.59 ± 0.46 bc | 14.54 ± 2.89 | 108.28 ± 22.37 | 15.95 ± 0.17 c–e |
| VG2 | 29.48 ± 3.58 b–d | 10.97 ± 1.64 | 11.78 ± 0.19 | 5.87 ± 0.13 c | 13.66 ± 1.72 | 59.47 ± 8.03 | 14.14 ± 0.16 de | |
| VG3 | 35.61 ± 1.21 b | 21.32 ± 6.84 | 12.42 ± 1.61 | 6.56 ± 1.66 bc | 21.06 ± 8.59 | 132.49 ± 51.09 | 19.03 ± 0.14 b | |
| VG4 | 35.12 ± 1.57 b–d | 13.62 ± 4.00 | 11.52 ± 2.08 | 5.46 ± 1.82 c | 13.84 ± 8.18 | 82.47 ± 46.27 | 22.56 ± 0.08 a | |
| VG20 | 32.60 ± 1.85 b–d | 15.71 ± 2.85 | 12.13 ± 1.40 | 6.54 ± 1.03 bc | 14.15 ± 2.61 | 97.54 ± 25.99 | 15.12 ± 0.18 c–e | |
| VG23 | 30.61 ± 3.58 b–d | 13.66 ± 4.05 | 13.48 ± 1.08 | 7.79 ± 1.00 bc | 18.40 ± 6.38 | 103.71 ± 40.96 | 15.76 ± 0.12 c–e | |
| Main effects | ||||||||
| 2015 | 39.89 ± 4.11a | 15.91 ± 1.35 a | 11.81 ± 0.42 | 7.46 ± 0.50 b | 15.39 ± 1.35 | 114.85 ± 13.03 a | 17.41 ± 0.68 | |
| 2016 | 27.50 ± 2.17b | 9.92 ± 0.85 b | 13.25 ± 1.53 | 10.31 ± 0.91 a | 11.11 ± 1.40 | 36.08 ± 3.07 b | 17.16 ± 0.91 | |
| 2017 | 31.49 ± 1.18b | 15.17 ± 1.57 a | 12.51 ± 0.50 | 6.63 ± 0.45 b | 15.94 ± 2.09 | 97.33 ± 13.43 a | 17.09 ± 0.70 | |
| IT97K-499-35 | 27.62 ± 3.09bc | 14.71 ± 2.61 | 13.70 ± 0.60 a | 9.82 ± 0.90 a | 13.30 ± 1.66 | 90.80 ± 21.07 | 15.88 ± 0.10 c | |
| VG2 | 24.79 ± 2.01c | 12.85 ± 0.98 | 10.02 ± 1.07 a | 6.51 ± 0.91 bc | 11.12 ± 1.27 | 66.58 ± 8.18 | 13.80 ± 0.29 d | |
| VG3 | 36.21 ± 3.64b | 15.01 ± 2.67 | 13.76 ± 1.64 a | 8.74 ± 1.53 a–c | 15.50 ± 3.24 | 74.58 ± 21.44 | 17.73 ± 0.65 b | |
| VG4 | 32.04 ± 2.26bc | 12.75 ± 1.94 | 9.55 ± 1.31 b | 6.22 ± 0.89 c | 13.00 ± 2.86 | 102.02 ± 23.85 | 23.28 ± 0.53 a | |
| VG20 | 29.41 ± 1.26bc | 12.63 ± 1.89 | 13.53 ± 1.39 a | 8.08 ± 0.85 a–c | 14.19 ± 2.24 | 72.49 ± 16.22 | 15.92 ± 0.39 c | |
| VG23 | 47.66 ± 6.50a | 14.04 ± 1.92 | 14.56 ± 1.12 a | 9.43 ± 0.69 ab | 17.79 ± 2.62 | 90.04 ± 21.56 | 16.70 ± 0.28 bc | |
| Statistical Significance | ||||||||
| Experimental Year | *** | * | ns | *** | ns | *** | ns | |
| Accession | *** | ns | * | * | ns | ns | *** | |
| Exp. Year × Accession | *** | ns | ns | * | ns | ns | ** | |
| Experimental Year | DFL | FDUR | DMAT | PH | NPOD | PODL | SPOD | SEEDW | NSEED | 100 SW | SY |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 6.60% | 20.68% | 9.84% | 43.68% | 36.03% | 14.99% | 28.57% | 37.17% | 48.12% | 16.65% | 37.18% |
| 2016 | 10.26% | 33.63% | 8.38% | 33.49% | 36.52% | 48.92% | 37.61% | 53.74% | 36.06% | 22.54% | 53.75% |
| 2017 | 9.85% | 5.03% | 6.01% | 15.95% | 43.85% | 17.13% | 28.95% | 55.65% | 58.53% | 17.30% | 55.65% |
| Total CV% | 9.47% | 30.11% | 9.54% | 38.36% | 44.01% | 32.24% | 38.84% | 50.93% | 68.75% | 18.65% | 50.94% |
| Accession | DFL | FDUR | DMAT | PH | NPOD | PODL | SPOD | SEEDW | NSEED | 100 SW | SY |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IT97K-499-35 | 7.96% | 11.53% | 8.89% | 33.56% | 53.33% | 13.11% | 27.37% | 37.49% | 69.61% | 1.87% | 37.50% |
| VG2 | 5.43% | 22.54% | 8.75% | 24.36% | 22.88% | 32.10% | 41.91% | 34.31% | 36.85% | 6.26% | 34.32% |
| VG3 | 5.42% | 28.78% | 6.02% | 30.13% | 53.43% | 35.80% | 52.50% | 62.68% | 86.24% | 10.98% | 62.70% |
| VG4 | 6.01% | 44.58% | 9.10% | 21.16% | 45.60% | 41.13% | 42.75% | 65.99% | 71.83% | 6.90% | 65.99% |
| VG20 | 6.52% | 27.02% | 5.93% | 12.85% | 44.81% | 30.81% | 31.55% | 47.40% | 67.14% | 7.40% | 47.40% |
| VG23 | 4.64% | 32.22% | 13.08% | 40.88% | 40.95% | 22.99% | 21.83% | 44.23% | 70.13% | 5.12% | 44.22% |
| Trait | DFL | DMAT | PH | NPOD | PODL | SPOD | SEEDW | NSEED | 100 SW | SY |
|---|---|---|---|---|---|---|---|---|---|---|
| DFL | 0.351 | 0.512 | −0.262 | 0.075 | 0.349 | 0.299 | 0.126 | −0.036 | −0.251 | 0.126 |
| FDUR | 0.506 | 0.004 | 0.348 | 0.048 | −0.202 | 0.209 | 0.386 | −0.224 | 0.209 | |
| DMAT | 0.191 | 0.205 | 0.228 | −0.110 | 0.265 | 0.244 | −0.024 | 0.265 | ||
| PH | 0.353 | 0.232 | −0.002 | 0.377 | 0.421 | 0.158 | 0.377 | |||
| NPOD | 0.250 | −0.001 | 0.830 | 0.880 | −0.011 | 0.830 | ||||
| PODL | 0.768 | 0.540 | 0.080 | −0.282 | 0.534 | |||||
| SPOD | 0.240 | −0.080 | −0.281 | 0.240 | ||||||
| SEEDW | 0.774 | 0.044 | 1.000 | |||||||
| NSEED | 0.103 | 0.774 | ||||||||
| 100 SW | 0.044 |
| Trait | PC1 (41.58%) | PC2 (22.77%) | PC3 (15.53%) |
|---|---|---|---|
| Days to 50% flowering | −0.117 | 0.433 | 0.767 |
| Flowering duration | 0.363 | −0.207 | 0.780 |
| Days to 50% pod maturation | 0.415 | −0.008 | 0.728 |
| Plant height | 0.799 | 0.125 | −0.315 |
| Number of pods per plant | 0.776 | −0.234 | 0.355 |
| Pod length | 0.226 | 0.930 | 0.194 |
| Number of seeds per pod | −0.058 | 0.933 | −0.100 |
| Seed weight per plant | 0.939 | 0.156 | 0.173 |
| Number of seeds per plant | 0.864 | −0.324 | 0.190 |
| Hundred-seed weight | 0.181 | −0.399 | −0.430 |
| Seed yield (kg ha−1) | 0.939 | 0.156 | 0.173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazaridi, E.; Bebeli, P.J. Evaluation of Cowpea Landraces under a Mediterranean Climate. Plants 2023, 12, 1947. https://doi.org/10.3390/plants12101947
Lazaridi E, Bebeli PJ. Evaluation of Cowpea Landraces under a Mediterranean Climate. Plants. 2023; 12(10):1947. https://doi.org/10.3390/plants12101947
Chicago/Turabian StyleLazaridi, Efstathia, and Penelope J. Bebeli. 2023. "Evaluation of Cowpea Landraces under a Mediterranean Climate" Plants 12, no. 10: 1947. https://doi.org/10.3390/plants12101947
APA StyleLazaridi, E., & Bebeli, P. J. (2023). Evaluation of Cowpea Landraces under a Mediterranean Climate. Plants, 12(10), 1947. https://doi.org/10.3390/plants12101947







