Type of Anion Largely Determines Salinity Tolerance in Four Rumex Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Establishment, Cultivation and Treatments
2.3. Plant Harvest and Measurements
2.4. Data Analysis
3. Results
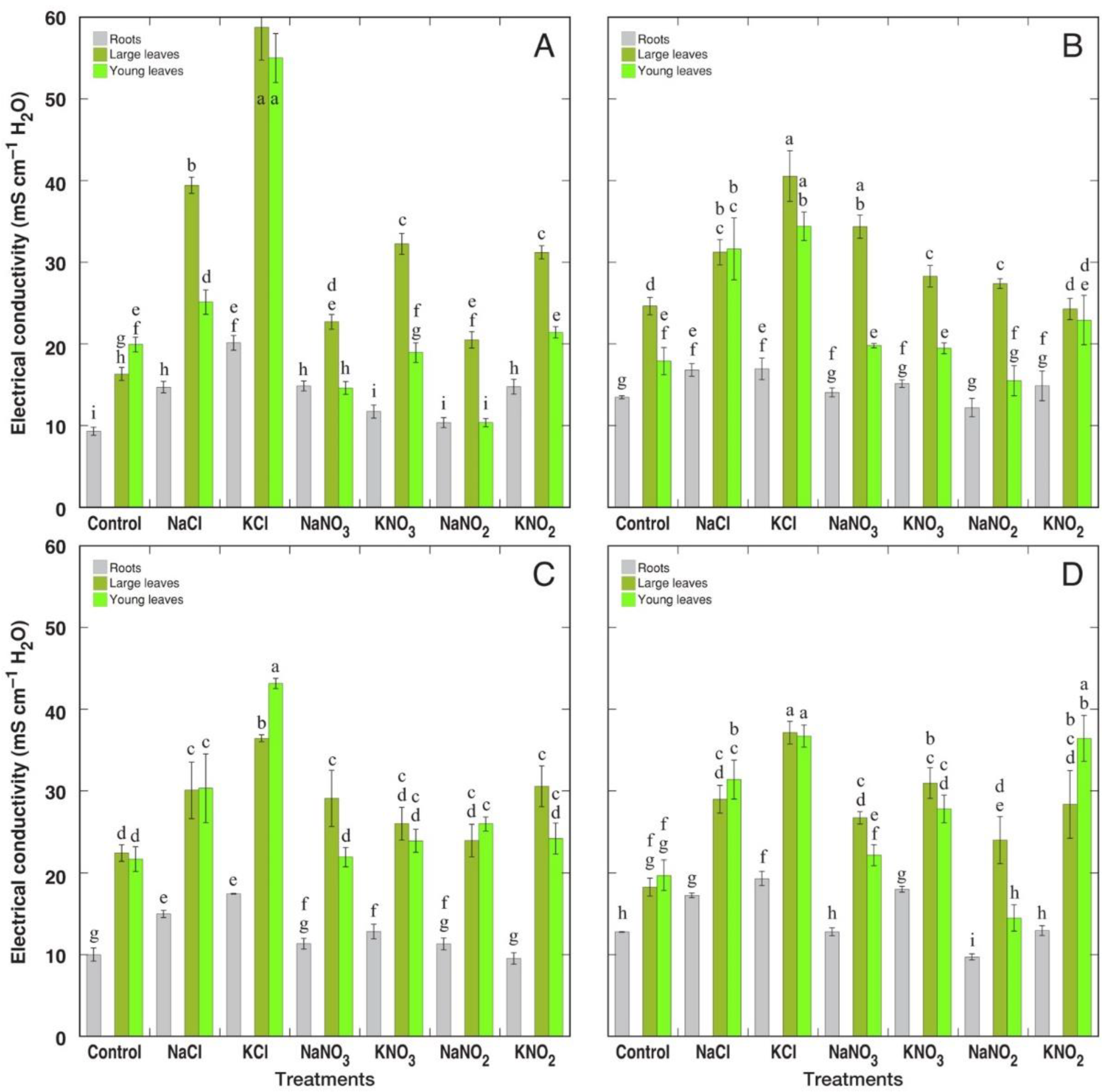
3.1. Morphological and Physiological Effects
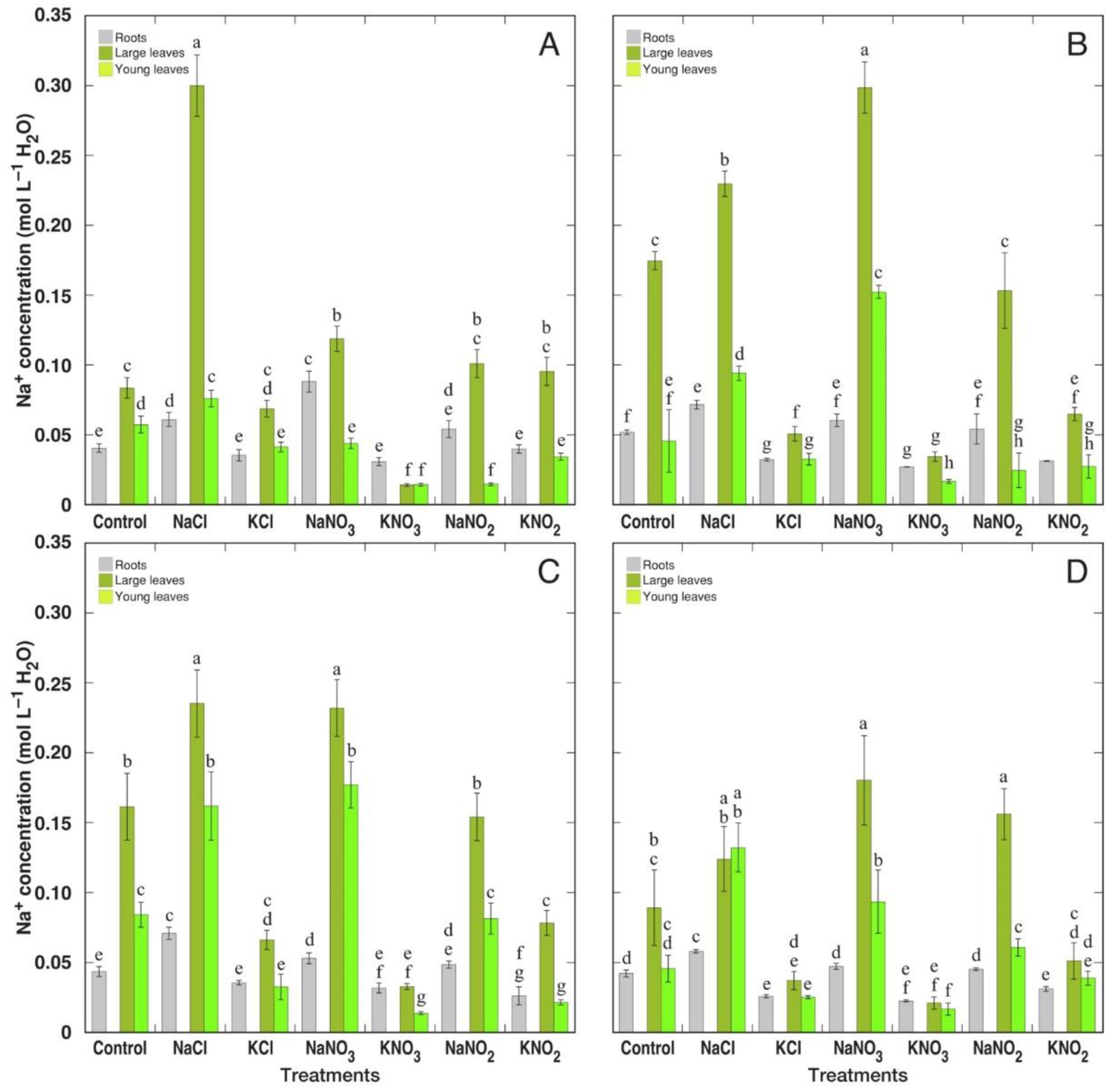
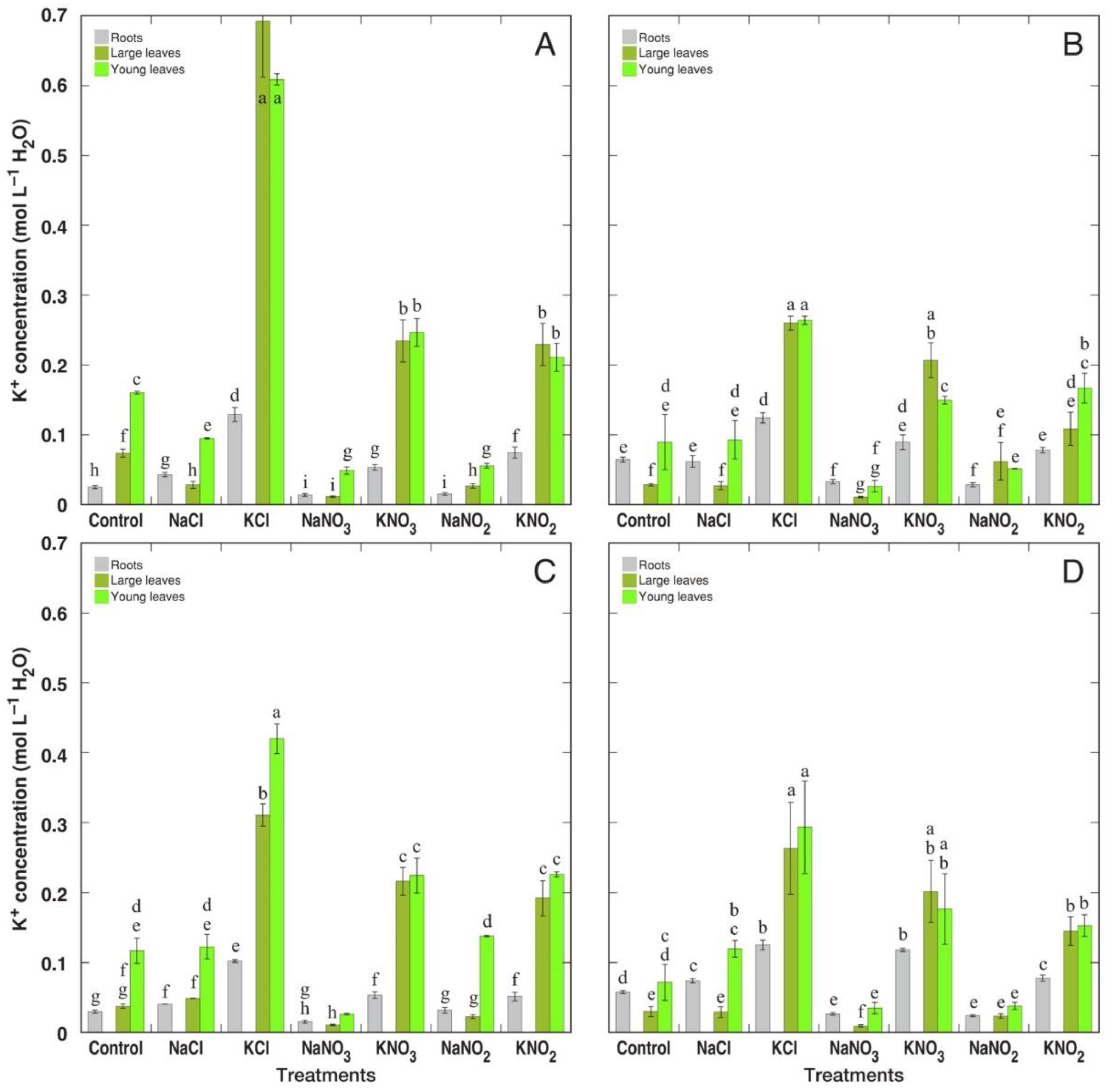
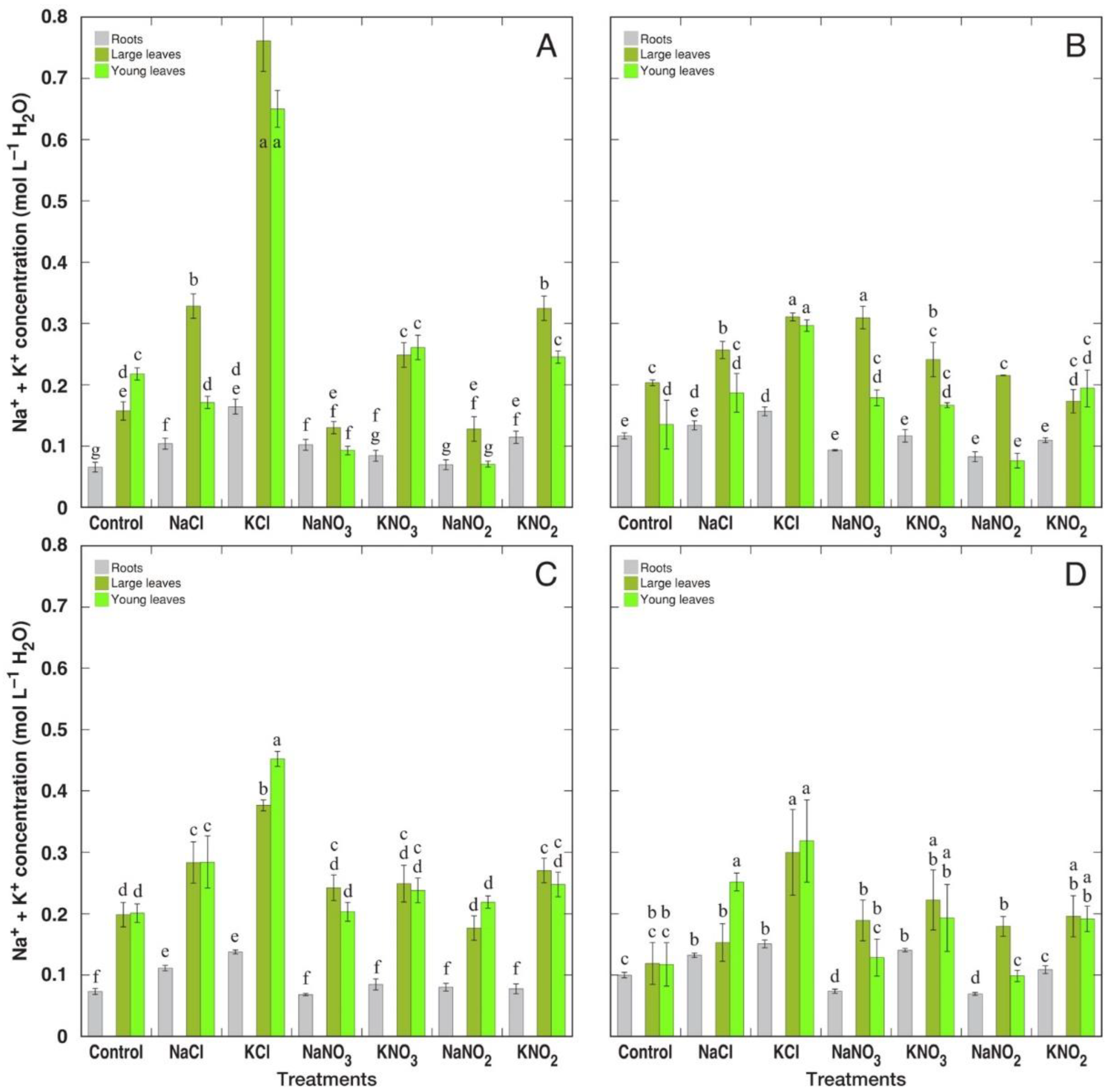
3.2. Ion Accumulation
3.3. Overall Effect Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Samsone, I.; Ievinsh, G. Different plant species accumulate various concentration of Na+ in a sea-affected coastal wetland during a vegetation season. Environ. Exp. Biol. 2018, 16, 117–127. [Google Scholar]
- Ievinsh, G.; Ieviņa, S.; Andersone-Ozola, U.; Samsone, I. Leaf sodium, potassium and electrolyte accumulation capacity of plant species from salt-affected coastal habitats of the Baltic Sea: Towards a definition of Na hyperaccumulation. Flora 2021, 274, 151748. [Google Scholar] [CrossRef]
- Shumway, S.W.; Bertness, M.D. Patch size effects on marsh secondary succession mechanisms. Ecology 1994, 75, 564–568. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Coskun, D.; Schulze, L.M.; Wong, J.R.; Britto, D.T. Sodium as nutrient and toxicant. Plant Soil 2013, 369, 1–23. [Google Scholar] [CrossRef]
- Ondrasek, G.; Rengel, Z. Environmental salinization processes: Detection, implications & solutions. Sci. Total Environ. 2021, 754, 142432. [Google Scholar]
- Belkheiri, O.; Mulas, M. The effects of salt stress on growth, water relations and ion accumulation in two halophyte Atriplex species. Environ. Exp. Bot. 2013, 86, 17–28. [Google Scholar] [CrossRef]
- Ramos, J.; López, M.J.; Benlloch, M. Effect of NaCl and KCl salts on the growth and solute accumulation of the halophyte Atriplex nummularia. Plant Soil. 2004, 259, 163–169. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Han, B.; Wang, B.; Guo, A.; Zheng, D.; Liu, C.; Chang, L.; Peng, M.; Wang, X. Sodium instead of potassium and chloride is an important macronutrient to improve leaf succulence and shoot development for halophyte Sesuvium portulacastrum. Plant Physiol. Biochem. 2012, 51, 53–62. [Google Scholar] [CrossRef]
- Purmale, L.; Jēkabsone, A.; Andersone-Ozola, U.; Ievinsh, G. Salinity tolerance, ion accumulation potential and osmotic adjustment in vitro and in planta of different Armeria maritima accessions from a dry coastal meadow. Plants 2022, 11, 2570. [Google Scholar] [CrossRef] [PubMed]
- Ievinsh, G.; Andersone-Ozola, U.; Jēkabsone, A. Similar responses of relatively salt-tolerant plants to Na and K during chloride salinity: Comparison of growth, water content and ion accumulation. Life 2022, 12, 1577. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, V.P.; Prasad, S.M. Nitrogen modifies NaCl toxicity in eggplant seedlings: Assessment of chlorophyll a fluorescence, antioxidative response and proline metabolism. Biocatal. Agric. Biotechnol. 2016, 7, 76–86. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Li, W.; Li, C.; Duan, D.; Tadano, T. Interactive effects of sodium chloride and nitrogen on growth and ion accumulation of a halophyte. Commun. Soil. Sci. Plant Anal. 2004, 15–16, 211–2123. [Google Scholar] [CrossRef]
- Yuan, J.-F.; Feng, G.; Ma, H.-Y.; Tian, C.-Y. Effect of nitrate on root development and nitrogen uptake of Suaeda physophora under NaCl salinity. Pedosphere 2010, 20, 536–544. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef]
- Ushakova, S.A.; Kovaleva, N.P.; Tikhomirova, N.A.; Gribovskaya, I.V.; Kolmakova, A.A. Effect of photosynthetically active radiation, salinization, and type of nitrogen nutrition on growth of Salicornia europaea. Russ. J. Plant Physiol. 2006, 53, 785–793. [Google Scholar] [CrossRef]
- van der Sman, A.J.M.; Joosten, N.N.; Blom, C.W.P.M. Flooding regimes and life-history characteristics of short-lived species in river forelands. J. Ecol. 1993, 81, 121–130. [Google Scholar] [CrossRef]
- van der Sman, A.J.M.; Blom, C.W.P.M.; Barendse, G.W.M. Flooding resistance and shoot elongation in relation to developmental stage and environmental conditions in Rumex maritimus L. and Rumex palustris Sm. New Phytol. 1993, 125, 73–84. [Google Scholar] [CrossRef]
- Sager, L.; Clerc, C. Factors influencing the distribution of Hydrocharis morsus-ranae L. and Rumex hydrolapathum Huds. in a mowed low-lying marshland, Réserve de Cheyres, lac de Neuchâtel, Switzerland. Hydrobiologia 2006, 570, 223–229. [Google Scholar] [CrossRef]
- Ievinsh, G.; Dišlere, E.; Karlsons, A.; Osvalde, A.; Vikmane, M. Physiological responses of wetland species Rumex hydrolapathum to increased concentration of biogenous heavy metals Zn and Mn in substrate. Proc. Latv. Acad. Sci. B 2020, 76, 278–288. [Google Scholar] [CrossRef]
- Jehlik, V.; Sádlo, J.; Dostálek, J.; Jarolimová, V.; Klimeŝ, L. Chorology and ecology of Rumex confertus Willd. in the Czech Republic. Bot. Lithuan. 2001, 7, 235–244. [Google Scholar]
- Hill, M.O.; Ellenberg, H.H. Ellenberg’s Indicator Values for British Plants. ECOFACT Research Report; Technical Annex; Institute of Terrestrial Ecology: Wallingford, UK, 1999; Volume 2. [Google Scholar]
- Tyler, T.; Herbertsson, L.; Olofsson, J.; Olsson, P.A. Ecological indicator and traits values for Swedish vascular plants. Ecol. Indic. 2021, 120, 106923. [Google Scholar] [CrossRef]
- Crawford, N.M. Nitrate: Nutrient and signal for plant growth. Plant Cell 1995, 7, 859–868. [Google Scholar] [PubMed]
- Geilfus, C.-M. Chloride: From nutrient to toxicant. Plant Cell Physiol. 2018, 59, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.B.; Moura, J.J.G. How biology handles nitrite. Chem. Rev. 2014, 114, 5273–5357. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Collins, K.D.; Neilson, G.W.; Enderby, J.E. Ions in water: Characterizing the forces that control chemical processes and biological structure. Biophys. Chem. 2007, 128, 95–104. [Google Scholar] [CrossRef]
- Collins, K.D. Why continuum electrostatics theories cannot explain biological structure, polyelectrolytes or ionic strength effects in ion–protein interactions. Biophys. Chem. 2012, 167, 43–59. [Google Scholar] [CrossRef]
- Leigh, R.A.; Wyn Jones, R.G. A hypothesis relating critical potassium concentrations for growth to the distribution and function of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Percey, W.J.; Shabala, L.; Wu, Q.; Su, N.; Breadmore, M.C.; Guijt, R.M.; Bose, J.; Shabala, S. Potassium retention in leaf mesophyll as an element of salinity tissue tolerance in halophytes. Plant Physiol. Biochem. 2016, 109, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Poolman, B.; Boersma, A.J. Ionic strength sensing in living cells. ACS Chem. Biol. 2017, 12, 2510–2514. [Google Scholar] [CrossRef] [PubMed]
- Dolling, P.J.; Ritchie, G.S.P. Estimates of soil solution ionic strength and the determination of pH in West Australian soils. Austr. J. Soil Res. 1985, 23, 309–314. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis for cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Lv, S.; Nie, L.; Fan, P.; Wang, X.; Jiang, D.; Chen, X.; Li, Y. Sodium plays a more important role than potassium and chloride in growth of Salicornia europaea. Acta Physiol. Plant. 2012, 34, 503–513. [Google Scholar] [CrossRef]
- Yao, S.; Chen, S.; Xu, D.; Lan, H. Plant growth and responses of antioxidants of Chenopodium album to long-term NaCl and KCl stress. Plant Growth Reg. 2010, 60, 115–125. [Google Scholar] [CrossRef]
- Glenn, E.; Pfister, R.; Brown, J.J.; Thompson, T.L.; O’Leary, J. Na and K accumulation and salt tolerance of Atriplex canescens (Chenopodiaceae) genotypes. Am. J. Bot. 1996, 83, 997–1005. [Google Scholar] [CrossRef]
- Reich, M.; Aghajanzadeh, T.; Helm, J.; Parmar, S.; Hawkesford, M.J.; De Kok, L.J. Chloride and sulfate salinity differently affect biomass, mineral nutrient composition and expression of sulfate transport and assimilation genes in Brassica rapa. Plant Soil 2017, 411, 310–332. [Google Scholar] [CrossRef]
- Křištálová, V.; Hejcman, M.; Červená, K.; Pavlů, V. Effect of nitrogen and phosphorus availability on the emergence, growth and over-wintering of Rumex crispus and Rumex obtusifolius. Grass Forage Sci. 2011, 66, 361–369. [Google Scholar] [CrossRef]
- Song, J.; Wang, B. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 2015, 115, 541–553. [Google Scholar] [CrossRef]
- Yuan, F.; Xu, X.; Leng, B.; Wang, B. Beneficial effects of salinity on halophyte growth: Morphology, cells, and genes. Open Life Sci. 2019, 14, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Ievinsh, G.; Andersone-Ozola, U.; Sieriņa, A. Growth and physiological performance of hydroponically-grown ornamental indoor plants in relation to their potential use in botanical biofilters: Effect of mineral nutrient availability. Proc. Latv. Acad. Sci. B 2022, 76, 278–288. [Google Scholar]
- Ievinsh, G.; Andersone-Ozola, U.; Zeipiņa, S. Comparison of the effects of compost and vermicompost soil amendments in organic production of four herb species. Biol. Agric. Hortic. 2020, 36, 257–282. [Google Scholar] [CrossRef]
- Riley, W.J.; Ortiz-Monasterio, I.; Matson, P.A. Nitrogen leaching and soil nitrate, nitrite, and ammonium levels under irrigated wheat in Northern Mexico. Nutr. Cycl. Agroecosyst. 2001, 61, 223–236. [Google Scholar] [CrossRef]
- Morot-Gaudry-Talarmain, Y.; Rockel, P.; Moureaux, T.; Quillere, I.; Leydecker, M.T.; Kaiser, W.M.; Morot-Gaudry, J.F. Nitrite accumulation and nitric oxide emission in relation to cellular signaling in nitrite reductase antisense tobacco. Planta 2002, 215, 708–715. [Google Scholar]
- Hachiya, T.; Ueda, N.; Kitagawa, M.; Hanke, G.; Suzuki, A.; Hase, T.; Sakakibara, H. Arabidopsis root-type ferredoxin:NADP(H) oxidoreductase 2 is involved in detoxification of nitrite in roots. Plant Cell Physiol. 2016, 57, 2440–2450. [Google Scholar] [CrossRef]
- Ezzine, M.; Debouba, M.; Ghorbel, M.H.; Gouia, H. Ion uptake and structural modifications induced by nitrogen source in tomato (Solanum lycopersicum Mill. Cv. Ibiza F1). C. R. Biol. 2011, 334, 526–534. [Google Scholar] [CrossRef]
- Ezzine, M.; Ghorbel, M.H. Physiological and biochemical responses resulting from nitrite accumulation in tomato (Lycopersicon esculentum Mill. cv. Ibiza F1). J. Plant Physiol. 2006, 163, 1032–1039. [Google Scholar] [CrossRef]
- Kotur, Z.; Siddiqi, Y.M.; Glass, A.D.M. Characterization of nitrite uptake in Arabidopsis thaliana: Evidence for a nitrite-specific transporter. New Phytol. 2013, 200, 201–210. [Google Scholar] [CrossRef]
- Samater, A.H.; Van Cleemput, O.; Ertebo, T. Influence of the presence of nitrite and nitrate in soil on maize biomass production, nitrogen immobilization and nitrogen recovery. Biol. Fertil. Soils 1998, 27, 211–218. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, D.; Hu, Z.; Zhang, A.; Li, F.; Zhang, J.; Wu, Y.; Tang, Y. Tolerance and physiological responses of sweet flag (Acorus calamus L.) under nitrite stress during wastewater treatment. Ecol. Eng. 2018, 122, 107–111. [Google Scholar] [CrossRef]

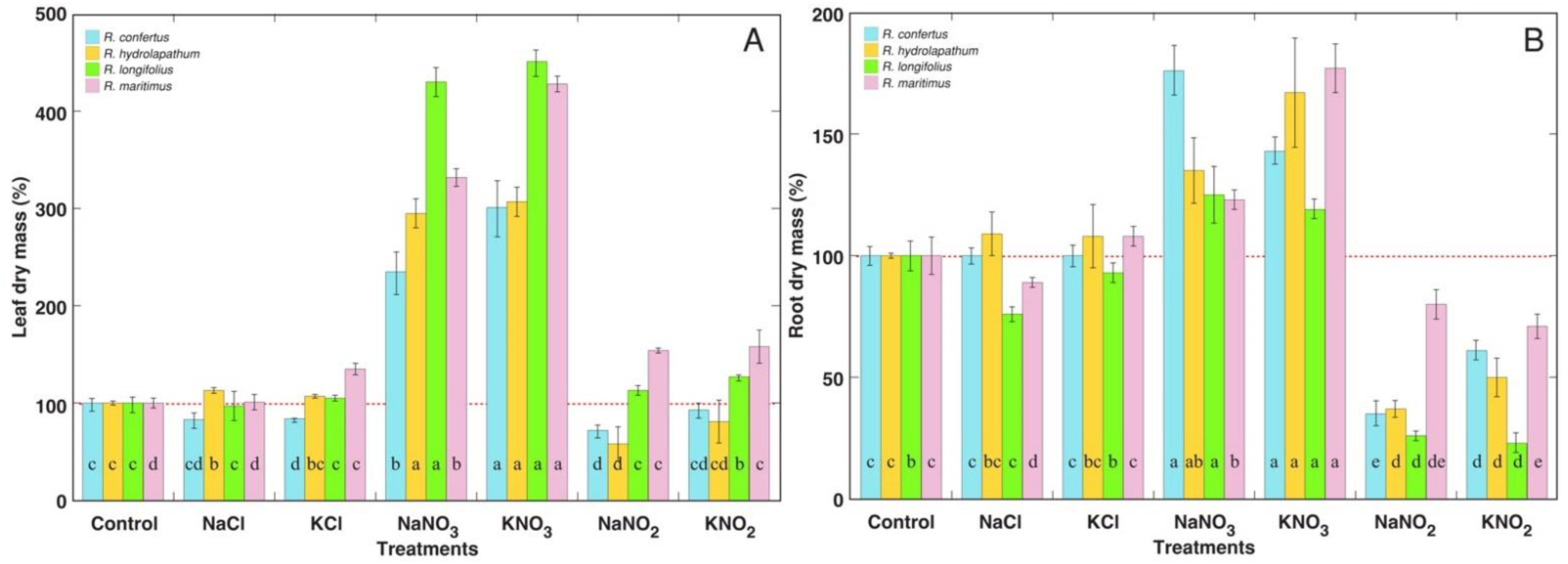
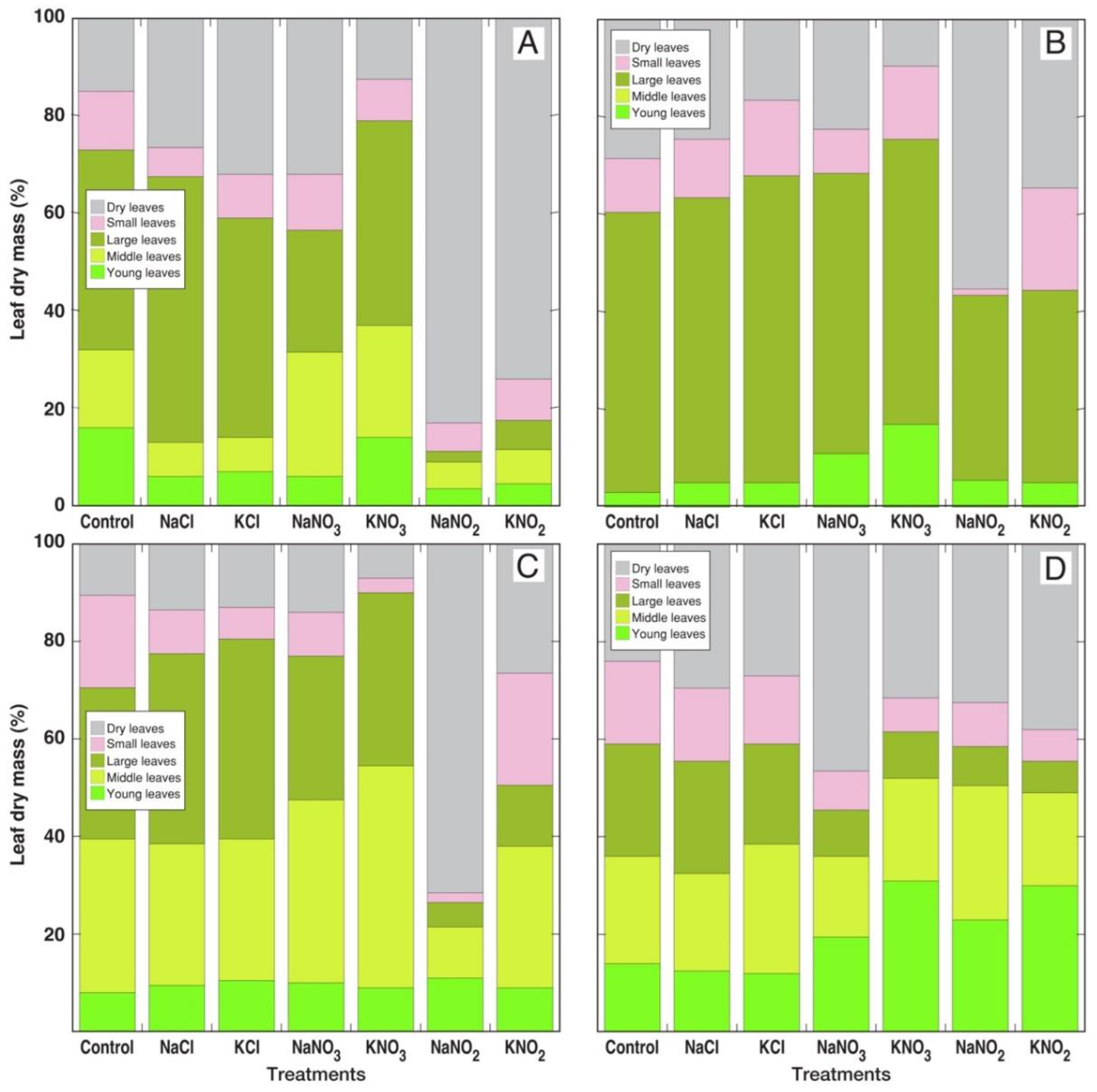

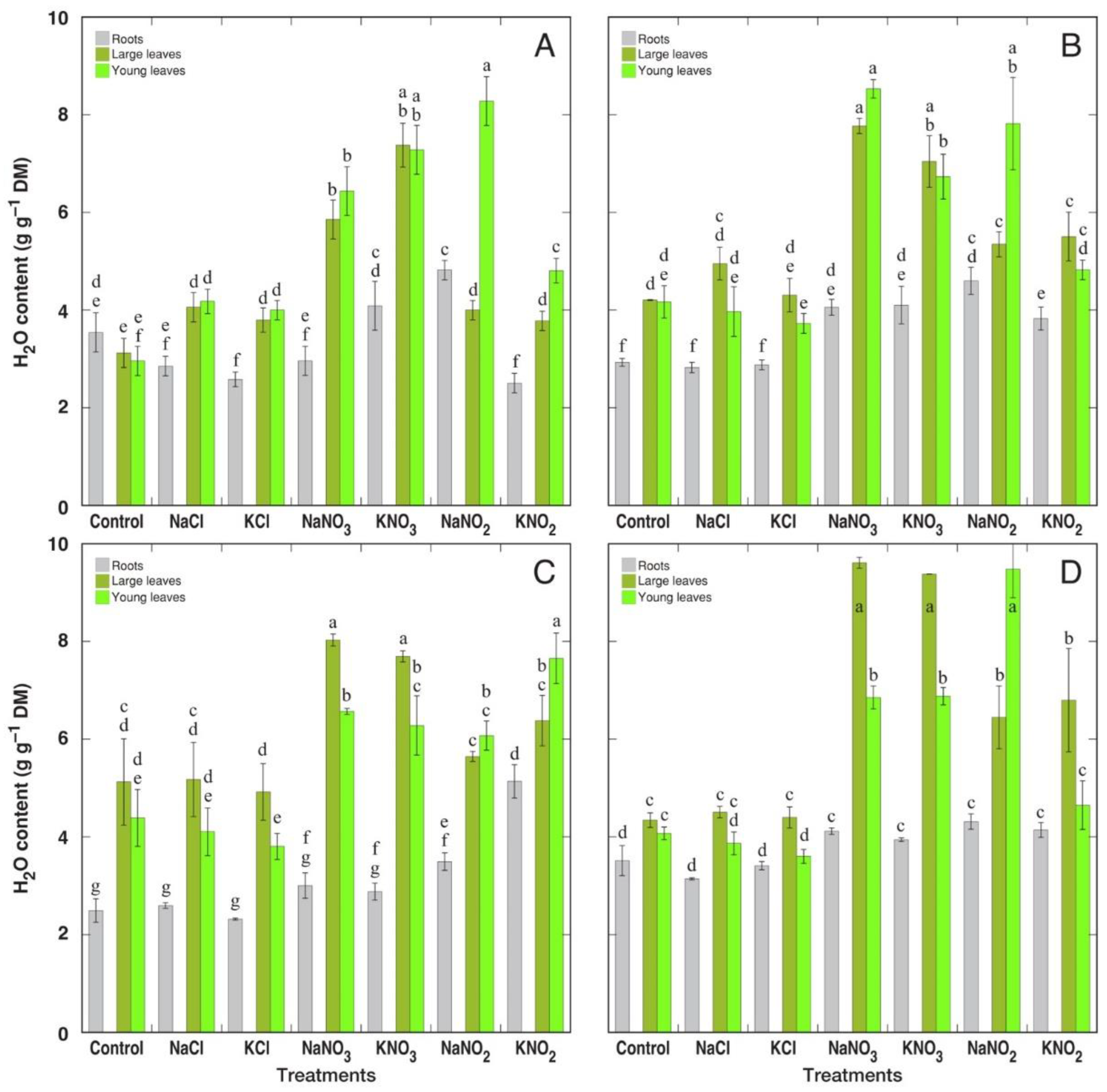
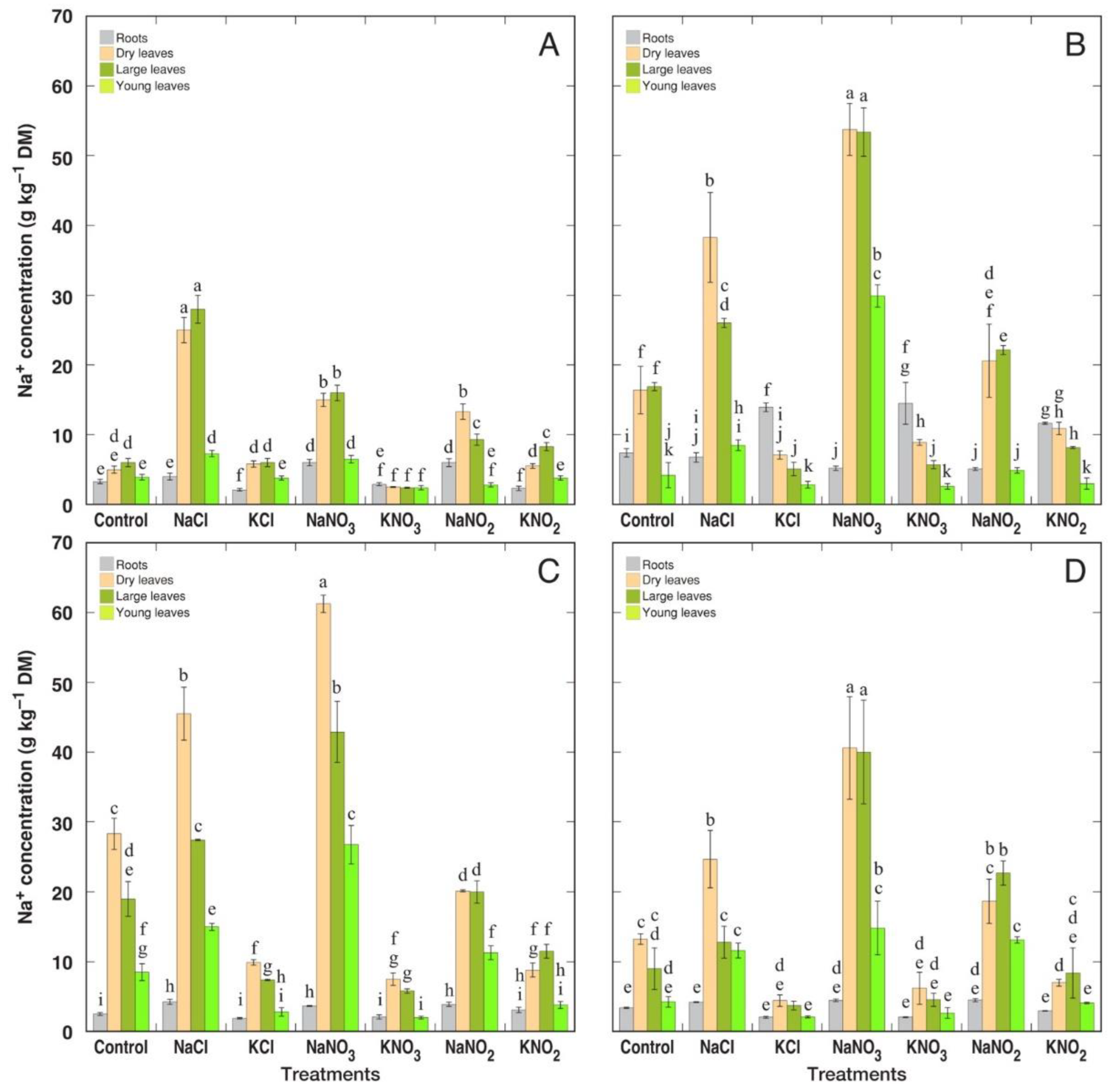

| Species | Salt | Proportion of Dry Leaves in Leaf Biomass (%) | Number of Leaves (%) | H2O Content in Large Leaves (%) | Chlorophyll Concentration in Large Leaves (%) | EC in Large Leaves (%) |
|---|---|---|---|---|---|---|
| R. confertus | NaCl | +77 | −43 | +30 | −9 | +142 |
| KCl | +113 | −63 | +21 | −12 | +260 | |
| NaNO3 | +113 | +93 | +88 | +5 | +39 | |
| KNO3 | 0 | +132 | +137 | +5 | +98 | |
| NaNO2 | +453 | +82 | +28 | −13 | +26 | |
| KNO2 | +393 | 0 | +21 | −19 | +91 | |
| R. hydrolapathum | NaCl | 0 | 0 | 0 | +4 | +27 |
| KCl | +43 | 0 | 0 | −8 | +64 | |
| NaNO3 | −22 | +102 | +85 | +26 | +39 | |
| KNO3 | −67 | +73 | +68 | +36 | +15 | |
| NaNO2 | +90 | 0 | +27 | +14 | +11 | |
| KNO2 | 0 | 0 | +31 | +22 | 0 | |
| R. longifolius | NaCl | 0 | 0 | 0 | −9 | +34 |
| KCl | 0 | 0 | 0 | −11 | +54 | |
| NaNO3 | 0 | +167 | +57 | −3 | +30 | |
| KNO3 | −33 | +146 | +50 | −5 | 0 | |
| NaNO2 | +581 | +54 | 0 | +14 | 0 | |
| KNO2 | +152 | +84 | 0 | +22 | +36 | |
| R. maritimus | NaCl | 0 | 0 | 0 | 0 | +59 |
| KCl | 0 | 0 | 0 | 0 | +103 | |
| NaNO3 | +94 | +100 | +121 | +13 | +46 | |
| KNO3 | +31 | +113 | +116 | +13 | +70 | |
| NaNO2 | +35 | +63 | +49 | +13 | +31 | |
| KNO2 | +58 | +65 | +57 | +14 | +55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landorfa-Svalbe, Z.; Andersone-Ozola, U.; Ievinsh, G. Type of Anion Largely Determines Salinity Tolerance in Four Rumex Species. Plants 2023, 12, 92. https://doi.org/10.3390/plants12010092
Landorfa-Svalbe Z, Andersone-Ozola U, Ievinsh G. Type of Anion Largely Determines Salinity Tolerance in Four Rumex Species. Plants. 2023; 12(1):92. https://doi.org/10.3390/plants12010092
Chicago/Turabian StyleLandorfa-Svalbe, Zaiga, Una Andersone-Ozola, and Gederts Ievinsh. 2023. "Type of Anion Largely Determines Salinity Tolerance in Four Rumex Species" Plants 12, no. 1: 92. https://doi.org/10.3390/plants12010092
APA StyleLandorfa-Svalbe, Z., Andersone-Ozola, U., & Ievinsh, G. (2023). Type of Anion Largely Determines Salinity Tolerance in Four Rumex Species. Plants, 12(1), 92. https://doi.org/10.3390/plants12010092








