Contrasting Regulators of the Onset and End of the Seed Release Phenology of a Temperate Desert Shrub Nitraria tangutorum
Abstract
1. Introduction
2. Results
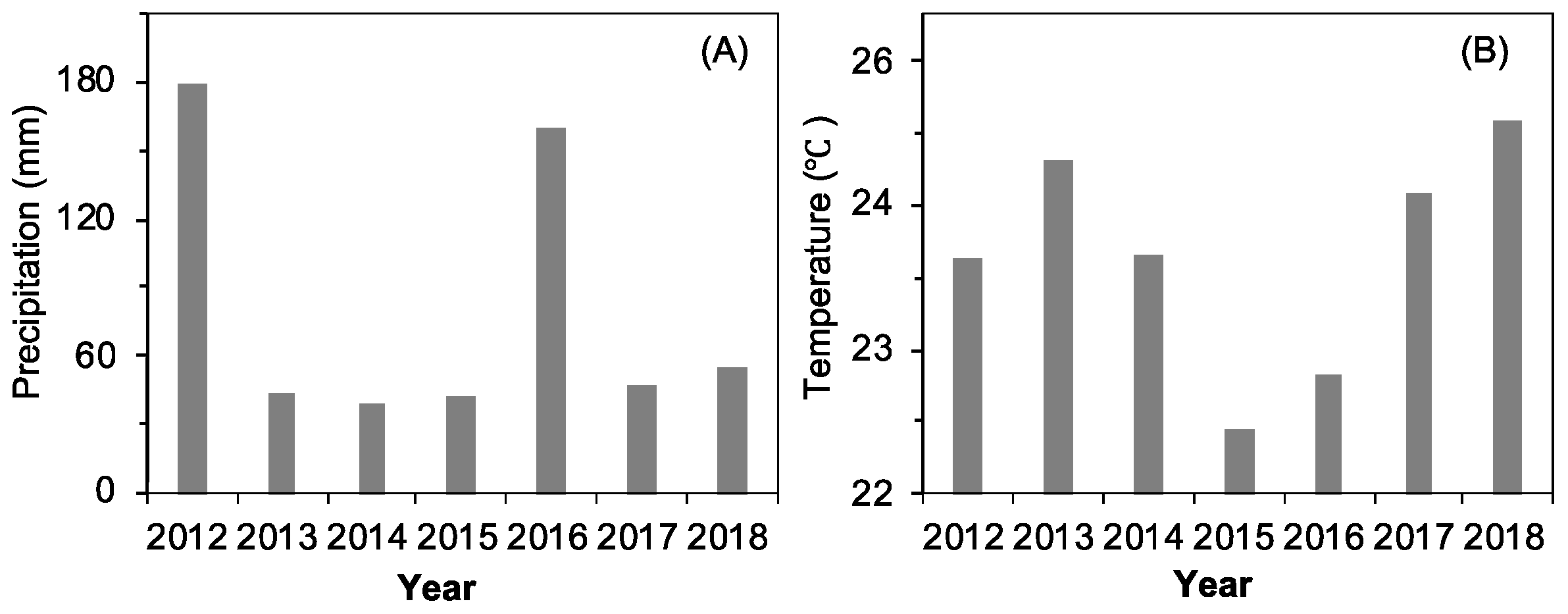
2.1. Interannual Dynamics of Meteorological Factors
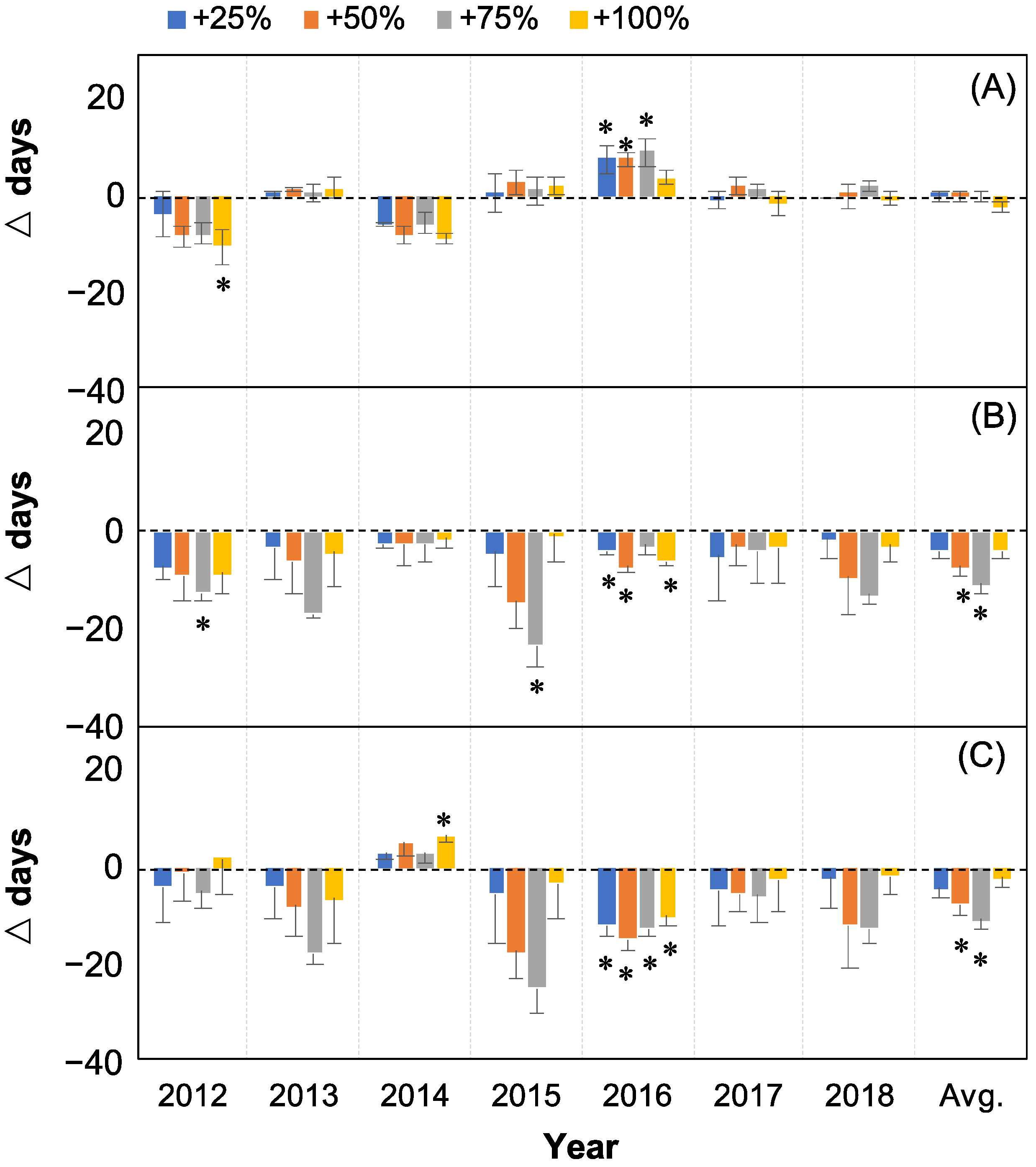
2.2. Changes in Seed Release
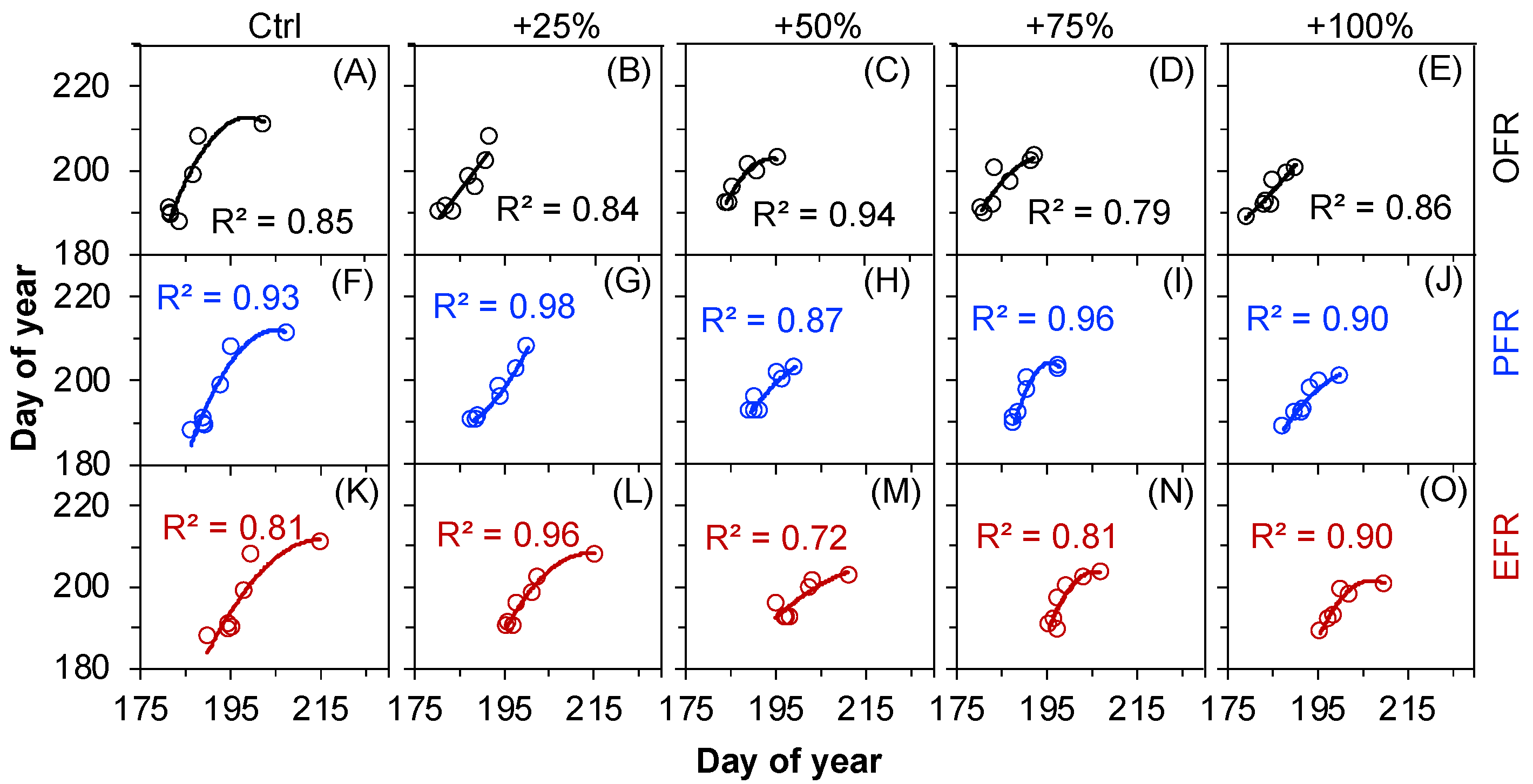
2.3. The Correlations between Seed Release Events and Water Addition Amounts
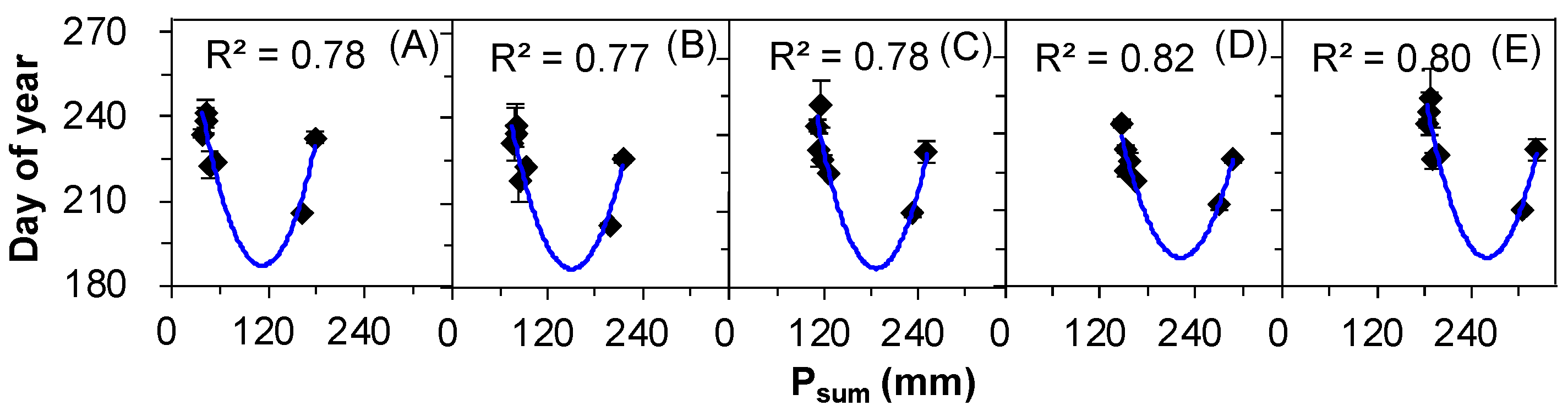
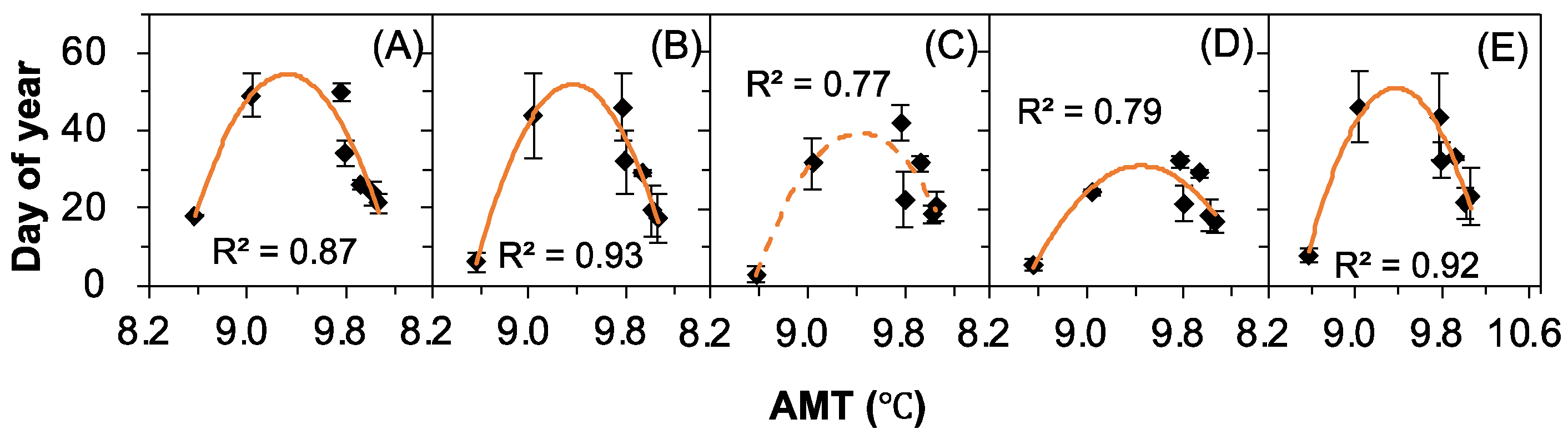
2.4. The Correlations between Seed Release Events and Meteorological Factors
2.5. The Correlations between Seed Release Events and the Other Phenological Events
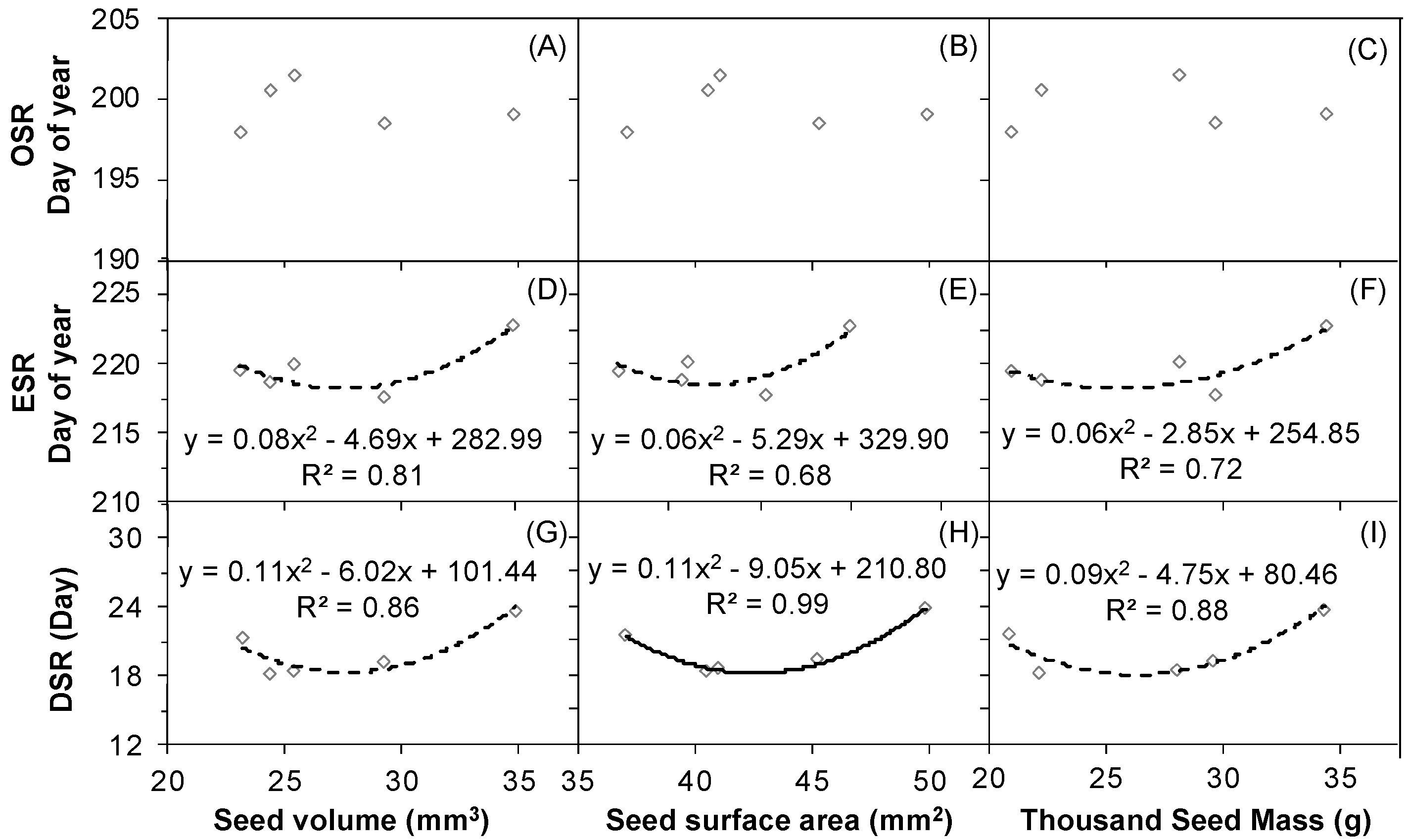
2.6. The Correlations between Seed Release Events and Seed Size and Weight
3. Discussion
3.1. Effects of Water Addition Treatments on Seed Release Events
3.2. Drivers of Inter-Annual Variations of Seed Release Phenology
4. Materials and Methods
4.1. Study Area
4.2. Simulated Precipitation Enhancement
4.3. Phenological Observations
4.4. Data Processing
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, Y.; Ma, K. Advancements and prospects in forest seed rain studies. Biodivers. Sci. 2012, 20, 94–107. [Google Scholar]
- Hao, P.; Li, J.; Zhou, W.; Liu, Q.; Zhang, Y. Spatial pattern and utility of Populus euphratica seed rain: A simulation study combined with field investigations. For. Stud. China 2012, 14, 169–179. [Google Scholar] [CrossRef]
- Eriksson, O. Seed dispersal and colonization ability of plants assessment and implications for conservation. Folia Geobot. 2000, 35, 115–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wang, M.; Liu, J.; Luo, F.; Lee, Y. Seed dispersal in Neuwiedia singapureana: Novel evidence for avian endozoochory in the earliest diverging clade in Orchidaceae. Bot. Stud. 2021, 62, 3. [Google Scholar] [CrossRef] [PubMed]
- Klips, R.A.; Peñalosa, J. The timing of seed fall, innate dormancy, and ambient temperature in Lythrum salicaria. Aquat. Bot. 2003, 75, 1–7. [Google Scholar] [CrossRef]
- Du, Y.; Mi, X.; Liu, X.; Chen, L.; Ma, K. Seed dispersal phenology and dispersal syndromes in a subtropical broad–leaved forest of China. For. Ecol. Manag. 2009, 258, 1147–1152. [Google Scholar] [CrossRef]
- Price, M.V.; Kelly, P.A. An age-structured demographic model for the endangered Stephens’ kangaroo rat. Conserv. Biol. 1994, 8, 810–821. [Google Scholar] [CrossRef]
- Price, M.V.; Joyner, J.W. What resources are available to desert granivores: Seed rain or soil seed bank? Ecology 1997, 78, 764–773. [Google Scholar] [CrossRef]
- M’Closkey, R.T. Desert rodent activity: Response to seed production by two perennial plant species. Oikos 1983, 41, 233–238. [Google Scholar] [CrossRef]
- Maity, A.; Lamichaney, A.; Joshi, D.C.; Bajwa, A.; Subramanian, N.; Walsh, M.; Bagavathiannan, M. Seed shattering: A trait of evolutionary importance in plants. Front. Plant Sci. 2021, 12, 657773. [Google Scholar] [CrossRef]
- Schwartz-Lazaro, L.M.; Shergill, L.S.; Evans, J.A.; Bagavathiannan, M.V.; Beam, S.C.; Bish, M.D.; Bond, J.A.; Bradley, K.W.; Curran, W.S.; Davis, A.S.; et al. Seed-shattering phenology at soybean harvest of economically important weeds in multiple regions of the United States. Part 2: Grass species. Weed Sci. 2021, 69, 104–110. [Google Scholar] [CrossRef]
- Schwartz-Lazaro, L.M.; Shergill, L.S.; Evans, J.A.; Bagavathiannan, M.V.; Beam, S.C.; Bish, M.D.; Bond, J.A.; Bradley, K.W.; Curran, W.S.; Davis, A.S.; et al. Seed-shattering phenology at soybean harvest of economically important weeds in multiple regions of the United States. Part 3: Drivers of seed shatter. Weed Sci. 2022, 70, 79–86. [Google Scholar] [CrossRef]
- Garaventa, J.M.; Parker, V.T. Extended seed rain period of Adenostoma fasciculatum impacts diverse seed predators. PLoS ONE 2021, 16, e0250290. [Google Scholar] [CrossRef] [PubMed]
- Chanyenga, T.T.; Geldenhuys, C.J.; Harvey, J. Variation in seed rain from Widdringtonia whytei growing in different conditions on Mulanje Mountain in Malawei. South. Forests 2011, 73, 123–129. [Google Scholar] [CrossRef]
- Hardesty, B.D.; Parker, V. Community seed rain patterns and comparison to adult community structure in a West African tropical forest. Plant Ecol. 2002, 164, 49–64. [Google Scholar] [CrossRef]
- Bradford, K.J. Water stress and the water relations of seed development: A critical review. Crop Sci. 1994, 34, 1–11. [Google Scholar] [CrossRef]
- Narita, K. Effects of seed release timing on plant life–history and seed production in a population of a desert annual, Blepharis sindica (Acanthaceae). Plant Ecol. 1998, 136, 195–203. [Google Scholar] [CrossRef]
- Herbison, B.; Polzin, M.L.; Rood, S.B. Hydration as a possible colonization cue: Rain may promote seed release from black cottonwood trees. For. Ecol. Manag. 2015, 350, 22–29. [Google Scholar] [CrossRef]
- Dunham, A.E.; Razafindratsima, O.H.; Rakotonirina, P.; Wright, P.C. Fruiting phenology is linked to rainfall variability in a tropical rain forest. Biotropica 2018, 50, 396–404. [Google Scholar] [CrossRef]
- Howe, H.F.; Smallwood, J. Ecology of seed dispersal. Ann. Rev. Ecol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Woutersen, A.; Jardine, P.E.; Bogotá-Angel, R.G.; Zhang, H.; Silvestro, D.; Antonelli, A.; Gogna, E.; Erkens, R.H.J.; Gosling, W.D.; Dupont-Nivet, G.; et al. A novel approach to study the morphology and chemistry of pollen in a phylogenetic context, applied to the halophytic taxon Nitraria L. (Nitrariaceae). PeerJ 2018, 6, e5055. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Hu, N.; Suo, Y.; Chi, X.; Wang, H. The complete chloroplast genome sequences of two species from Nitraria. Mitochondrial DNA Part B 2019, 4, 1229–1230. [Google Scholar] [CrossRef]
- The Plant List. 2013. Version 1.1. Available online: http://www.theplantlist.org/browse/A/Nitrariaceae/Nitraria/ (accessed on 28 October 2022).
- Pan, X.; Shen, G.; Chen, P. A preliminary research on taxonomy and systematics of genus Nitraria. Acta Bot. Yunnanica 1999, 21, 287–295. [Google Scholar]
- Noble, J.C.; Whalley, R.D.B. The biology and autecology of Nitraria L. in Australia. II. Seed germination, seedling establishment and response to salinity. Austral Ecol. 1978, 2, 165–177. [Google Scholar] [CrossRef]
- Xue, H.; Li, Q.; Xu, J.; Zhang, J. Effects of sand burial on growth and biomass allocation of Nitraria tangutorum. Pratacultural Sci. 2016, 33, 2062–2070. [Google Scholar]
- Zhao, Y.; Liu, W.; Wang, H.; Wu, H.; Xiao, Y.; Yan, Y. Effects of exogenous CaCl2 on reactive oxygen species metabolism in Nitraria sibirica under NaCl stress. Plant Physiol. J. 2021, 57, 1105–1112. [Google Scholar]
- El-Sheikh, M.A.; Abbadi, G.A.; Bianco, P.M. Vegetation ecology of phytogenic hillocks (Nabkhas) in coastal habitats of Jal Az-Zor National Park, Kuwait: Role of patches and edaphic factors. Flora 2010, 205, 832–840. [Google Scholar] [CrossRef]
- Bao, F.; Xin, Z.; Li, J.; Liu, M.; Cao, Y.; Lu, Q.; Gao, Y.; Wu, B. Preceding phenological events rather than climate drive the variations in fruiting phenology in the desert shrub Nitraria tangutorum. Plants 2022, 11, 1578. [Google Scholar] [CrossRef]
- Turghun, C.; Bakri, M.; Zou, G.A.; Bobakulov, K.M.; Aisa, H.A. Phenolic Compounds from Leaves of Nitraria sibirica. Chem. Nat. Compd. 2018, 54, 987–989. [Google Scholar] [CrossRef]
- Turghun, C.; Bobakulov, K.M.; Bakri, M.; Aisa, H.A. Flavonoids from Leaves of Nitraria sibirica. Chem. Nat. Compd. 2019, 55, 1156–1158. [Google Scholar] [CrossRef]
- Liu, Z.; Mei, L.; Wang, Q.; Shao, Y.; Tao, Y. Optimization of subcritical fluid extraction of seed oil from Nitraria tangutorum using response surface methodology. Food Sci. Technol. 2014, 56, 168–174. [Google Scholar] [CrossRef]
- Wu, D.; Gao, T.; Yang, H.; Du, Y.; Li, C.; Wei, L.; Zhou, T.; Lu, J.; Bi, H. Simultaneous microwave/ultrasonic-assisted enzymatic extraction of antioxidant ingredients from Nitraria tangutorun Bobr. juice by-products. Ind. Crop. Prod. 2015, 66, 229–238. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Yang, Y.; Zeng, Y.; Wang, Q.; Shao, Y.; Mei, L.; Shi, Y.; Tao, Y. Isolation and identification of antioxidant and a-glucosidase inhibitory compounds from fruit juice of Nitraria tangutorum. Food Chem. 2017, 227, 93–101. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Zhao, X.; Shao, Y.; Wei, W.; Tao, Y.; Yue, H. A new flavonol acylglycoside from the fruits of Nitraria tangutorum Bobr. Nat. Prod. Res. 2020, 35, 3652–3657. [Google Scholar] [CrossRef]
- Chaabane, M.; Koubaa, M.; Soudani, N.; Elwej, A.; Grati, M.; Jamoussi, K.; Boudawara, T.; Chaabouni, S.E.; Zeghal, N. Nitraria retusa fruit prevents penconazole-induced kidney injury in adult rats through modulation of oxidative stress and histopathological changes. Pharm. Biol. 2017, 55, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, N.; Bai, B.; Zhang, L.; Song, L. Physical Properties and Antioxidant Activity of Polysaccharide from Nitraria roborowskii Kom Fruit. Sci. Technol. Food Ind. 2021, 42, 87–94. [Google Scholar]
- Zhi, D.; Zhang, N.; Li, J. Microwave–ultrasonic extraction of proanthocyanidins from Nitraria and its antioxidant analysis. Sci. Technol. Food Ind. 2022, 43, 171–179. [Google Scholar]
- Suo, Y.; Gao, H.; Wang, H. Chinese Nitraria tangutorum Bobr.: Chemical constituents of seed oil extracted by SFE–CO2. Nat. Prod. Res. Dev. 2004, 16, 16–18. [Google Scholar]
- Suo, Y.; Wang, H.; Chen, G. Research on security and decreasing blood lipid effect of Nitraria seed oil from Qaidam Basin. Nat. Prod. Res. Dev. 2005, 26, 217–219. [Google Scholar]
- Suo, Y.; Gao, H.; Wang, H. The protective effect of Nitraria tangutorum Bohr. seed oil from Qaidam basin on the liver injury of mice. Nat. Prod. Res. Dev. 2005, 17, 573–576. [Google Scholar]
- Suo, Y.; Wang, H.; Lin, Y.; Chen, G. The anti–fatigue effect of Nitraria tangutorum Bohr. seed oil from Qaidam basin on the mice. Natural Product Research and Development. Nat. Prod. Res. Dev. 2006, 18, 88–91. [Google Scholar]
- Bao, F.; Liu, M.; Cao, Y.L.; Li, J.; Yao, B.; Xin, Z.; Lu, Q.; Wu, B. Water addition prolonged the length of the growing season of the desert shrub Nitraria tangutorum in a temperate desert. Front. Plant Sci. 2020, 11, 1099. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Xin, Z.; Li, J.; Liu, M.; Cao, Y.; Lu, Q.; Gao, Y.; Wu, B. Effects of the simulated enhancement of precipitation on the phenology of Nitraria tangutorum under extremely dry and wet years. Plants 2021, 10, 1474. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.J.; Shi, Y.; Zhang, D.F.; Giorgi, F. Climate change in China in the 21st century as simulated by a high-resolution regional climate model. Chin. Sci. Bull. 2012, 57, 1188–1195. [Google Scholar] [CrossRef]
- Chen, H. Projected change in extreme rainfall events in China by the end of the 21st century using CMIP5 models. Chin. Sci. Bull. 2013, 58, 1462–1472. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, B.; Qin, D.; Wu, J.; Gao, R.; Song, L. Changes in mean and extreme temperature and precipitation over the arid region of northwestern China: Observation and projection. Adv. Atmos. Sci. 2017, 343, 289–305. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, X.; Xin, Z.; Liu, M.; Zhang, R.; Huang, Y.; Sun, F. Effects of artificial simulated precipitation on seed characters and germination of Nitraria tangutorum. Southwest China J. Agric. Sci. 2019, 32, 1181–1186. [Google Scholar]
- Ren, H.; Xu, Z.; Isbell, F.; Huang, J.; Han, X.; Wan, S.; Chen, S.; Wang, R.; Zeng, D.; Jiang, Y.; et al. Exacerbated nitrogen limitation ends transient stimulation of grassland productivity by increased precipitation. Ecol. Monogr. 2017, 87, 457–469. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Richard, J.H. Nitrogen addition increase fecundity in the desert shrub Sarcobatus vermiculatus. Oecologica 2005, 143, 349–356. [Google Scholar] [CrossRef]
- Stanton, M.L. Seed variation in wild radish: Effect of seed size on components of seedling and adult fitness. Ecology 1984, 65, 1105–1112. [Google Scholar] [CrossRef]
- Thompson, K.; Band, S.R.; Hodgson, J.G. Seed size and shape predict persistence in soil. Funct. Ecol. 1993, 7, 236–241. [Google Scholar] [CrossRef]
- Breen, A.N.; Richards, J.H. Irrigation and fertilization effects on seed number, size, germination and seedling growth: Implications for desert shrub establishment. Oecologia 2008, 157, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.E. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 2000, 5, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Volis, S. Correlated patterns of variation in phenology and seed production in populations of two annual grasses along an aridity gradient. Evol. Ecol. 2007, 21, 381–393. [Google Scholar] [CrossRef]
- Collins, C.G.; Elmendorf, S.C.; Hollister, R.D.; Henry, G.H.R.; Clark, K.; Bjorkman, A.D.; Myers-Smith, I.H.; Prevéy, J.S.; Ashton, I.W.; Assmann, J.J.; et al. Experimental warming differentially affects vegetative and reproductive phenology of tundra plants. Nat. Commun. 2021, 12, 3442. [Google Scholar] [CrossRef]
- Donnelly, A.; Yu, R. Temperate deciduous shrub phenology: The overlooked forest layer. Int. J. Biometerol. 2021, 65, 343–355. [Google Scholar] [CrossRef]
- Wan, M.W.; Liu, X. Phenology Observation Methodology in China; Science Press: Beijing, China, 1979. [Google Scholar]


| Source | OSR | ESR | DSR | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Water | 2.85 | 0.04 | 2.85 | 0.04 | 7.43 | 0.00 |
| Year | 2.4 | 0.05 | 2.49 | 0.05 | 21.90 | 0.00 |
| PFR | 8.3 | 0.00 | 8.35 | 0.00 | 2.42 | 0.01 |
| Water × Year | 1.54 | 0.17 | 1.54 | 0.17 | 1.09 | 0.40 |
| PFR × Year | 2.72 | 0.02 | 2.72 | 0.02 | 1.23 | 0.31 |
| Water × PFR | 1.11 | 0.38 | 1.1 | 0.38 | 2.68 | 0.00 |
| Water × Year × PFR | 0.38 | 0.77 | 0.38 | 0.77 | 1.20 | 0.32 |
| Meteorological Factors | OSR | |||||||||
| Ctrl | +25% | +50% | +75% | +100% | ||||||
| R2 | p | R2 | p | R2 | p | R2 | p | R2 | p | |
| AMP | 0.15 | 0.46 | 0.36 | 0.41 | 0.18 | 0.67 | 0.21 | 0.63 | 0.19 | 0.69 |
| PWin | 0.08 | 0.92 | 0.13 | 0.68 | 0.32 | 0.24 | 0.37 | 0.37 | 0.20 | 0.42 |
| PSpr | 0.78 | 0.11 | 0.56 | 0.20 | 0.43 | 0.32 | 0.45 | 0.30 | 0.70 | 0.07 |
| Psum | 0.63 | 0.12 | 0.66 | 0.12 | 0.39 | 0.30 | 0.45 | 0.30 | 0.63 | 0.12 |
| PAut | 0.13 | 0.71 | 0.27 | 0.53 | 0.32 | 0.47 | 0.32 | 0.46 | 0.13 | 0.81 |
| AMT | 0.65 | 0.09 | 0.71 | 0.08 | 0.80 | 0.04 | 0.74 | 0.07 | 0.60 | 0.15 |
| Twin | 0.46 | 0.06 | 0.56 | 0.19 | 0.57 | 0.19 | 0.38 | 0.38 | 0.51 | 0.23 |
| Tspr | 0.54 | 0.29 | 0.54 | 0.21 | 0.55 | 0.20 | 0.67 | 0.11 | 0.77 | 0.06 |
| Tsum | 0.70 | 0.09 | 0.84 | 0.03 | 0.78 | 0.05 | 0.93 | 0.00 | 0.82 | 0.03 |
| Taut | 0.28 | 0.83 | 0.10 | 0.82 | 0.07 | 0.85 | 0.14 | 0.73 | 0.20 | 0.63 |
| Meteorological Factors | ESR | |||||||||
| Ctrl | +25% | +50% | +75% | +100% | ||||||
| R2 | p | R2 | p | R2 | R2 | p | R2 | p | R2 | |
| AMP | 0.49 | 0.37 | 0.44 | 0.58 | 0.73 | 0.23 | 0.73 | 0.39 | 0.41 | 0.47 |
| PWin | 0.19 | 0.62 | 0.18 | 0.50 | 0.03 | 0.83 | 0.06 | 0.77 | 0.13 | 0.59 |
| PSpr | 0.68 | 0.29 | 0.57 | 0.19 | 0.57 | 0.19 | 0.73 | 0.06 | 0.52 | 0.23 |
| Psum | 0.78 | 0.05 | 0.77 | 0.05 | 0.78 | 0.05 | 0.82 | 0.03 | 0.80 | 0.04 |
| PAut | 0.50 | 0.14 | 0.48 | 0.27 | 0.38 | 0.39 | 0.04 | 0.75 | 0.53 | 0.22 |
| AMT | 0.69 | 0.15 | 0.77 | 0.06 | 0.62 | 0.15 | 0.54 | 0.18 | 0.74 | 0.07 |
| Twin | 0.04 | 1.00 | 0.02 | 0.95 | 0.02 | 0.96 | 0.07 | 0.93 | 0.05 | 0.90 |
| Tspr | 0.11 | 0.68 | 0.11 | 0.78 | 0.39 | 0.38 | 0.55 | 0.10 | 0.11 | 0.80 |
| Tsum | 0.03 | 0.87 | 0.06 | 0.89 | 0.14 | 0.74 | 0.33 | 0.28 | 0.05 | 0.90 |
| Taut | 0.08 | 0.87 | 0.10 | 0.80 | 0.25 | 0.56 | 0.62 | 0.13 | 0.14 | 0.74 |
| Meteorological Factors | DSR | |||||||||
| Ctrl | +25% | +50% | +75% | +100% | ||||||
| R2 | p | R2 | p | R2 | R2 | p | R2 | p | R2 | |
| AMP | 0.09 | 0.83 | 0.13 | 0.76 | 0.45 | 0.30 | 0.45 | 0.30 | 0.14 | 0.74 |
| PWin | 0.36 | 0.41 | 0.26 | 0.45 | 0.12 | 0.58 | 0.04 | 0.74 | 0.25 | 0.44 |
| PSpr | 0.31 | 0.48 | 0.28 | 0.52 | 0.36 | 0.39 | 0.31 | 0.47 | 0.41 | 0.35 |
| Psum | 0.41 | 0.35 | 0.62 | 0.15 | 0.75 | 0.07 | 0.82 | 0.03 | 0.65 | 0.12 |
| PAut | 0.79 | 0.05 | 0.63 | 0.14 | 0.47 | 0.28 | 0.27 | 0.53 | 0.64 | 0.13 |
| AMT | 0.87 | 0.02 | 0.93 | 0.01 | 0.77 | 0.06 | 0.79 | 0.05 | 0.92 | 0.03 |
| Twin | 0.17 | 0.69 | 0.16 | 0.70 | 0.06 | 0.88 | 0.12 | 0.77 | 0.11 | 0.79 |
| Tspr | 0.12 | 0.78 | 0.12 | 0.77 | 0.17 | 0.68 | 0.26 | 0.55 | 0.08 | 0.85 |
| Tsum | 0.44 | 0.31 | 0.32 | 0.46 | 0.11 | 0.80 | 0.16 | 0.70 | 0.23 | 0.59 |
| Taut | 0.06 | 0.89 | 0.03 | 0.94 | 0.15 | 0.71 | 0.30 | 0.49 | 0.04 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, F.; Xin, Z.; Liu, M.; Li, J.; Gao, Y.; Lu, Q.; Wu, B. Contrasting Regulators of the Onset and End of the Seed Release Phenology of a Temperate Desert Shrub Nitraria tangutorum. Plants 2023, 12, 88. https://doi.org/10.3390/plants12010088
Bao F, Xin Z, Liu M, Li J, Gao Y, Lu Q, Wu B. Contrasting Regulators of the Onset and End of the Seed Release Phenology of a Temperate Desert Shrub Nitraria tangutorum. Plants. 2023; 12(1):88. https://doi.org/10.3390/plants12010088
Chicago/Turabian StyleBao, Fang, Zhiming Xin, Minghu Liu, Jiazhu Li, Ying Gao, Qi Lu, and Bo Wu. 2023. "Contrasting Regulators of the Onset and End of the Seed Release Phenology of a Temperate Desert Shrub Nitraria tangutorum" Plants 12, no. 1: 88. https://doi.org/10.3390/plants12010088
APA StyleBao, F., Xin, Z., Liu, M., Li, J., Gao, Y., Lu, Q., & Wu, B. (2023). Contrasting Regulators of the Onset and End of the Seed Release Phenology of a Temperate Desert Shrub Nitraria tangutorum. Plants, 12(1), 88. https://doi.org/10.3390/plants12010088


.png)




