Biochemical Characterization of Orange-Colored Rice Calli Induced by Target Mutagenesis of OsOr Gene
Abstract
1. Introduction
2. Results
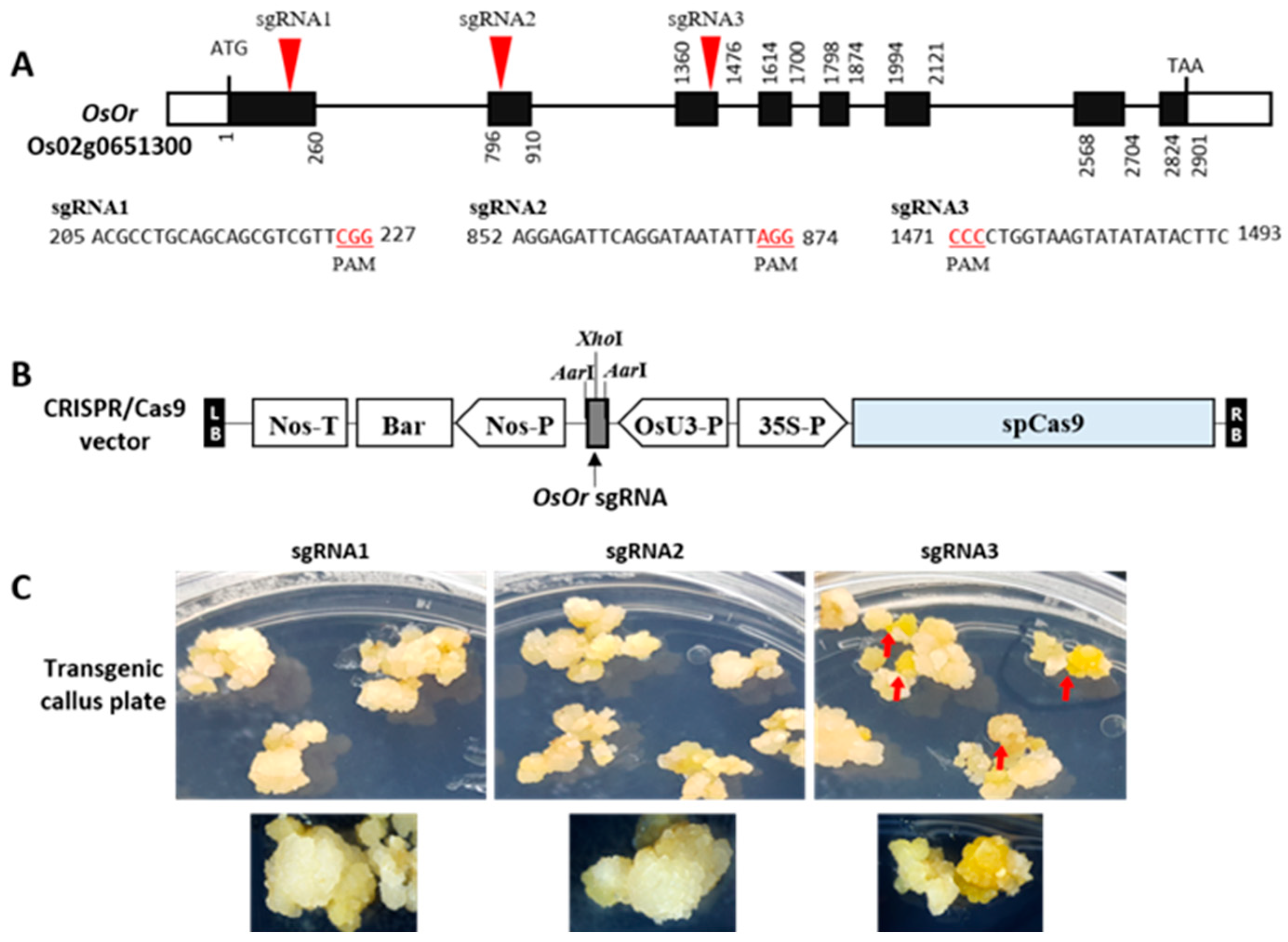
2.1. CRISPR/Cas9 Targeted Mutagenesis of OsOr
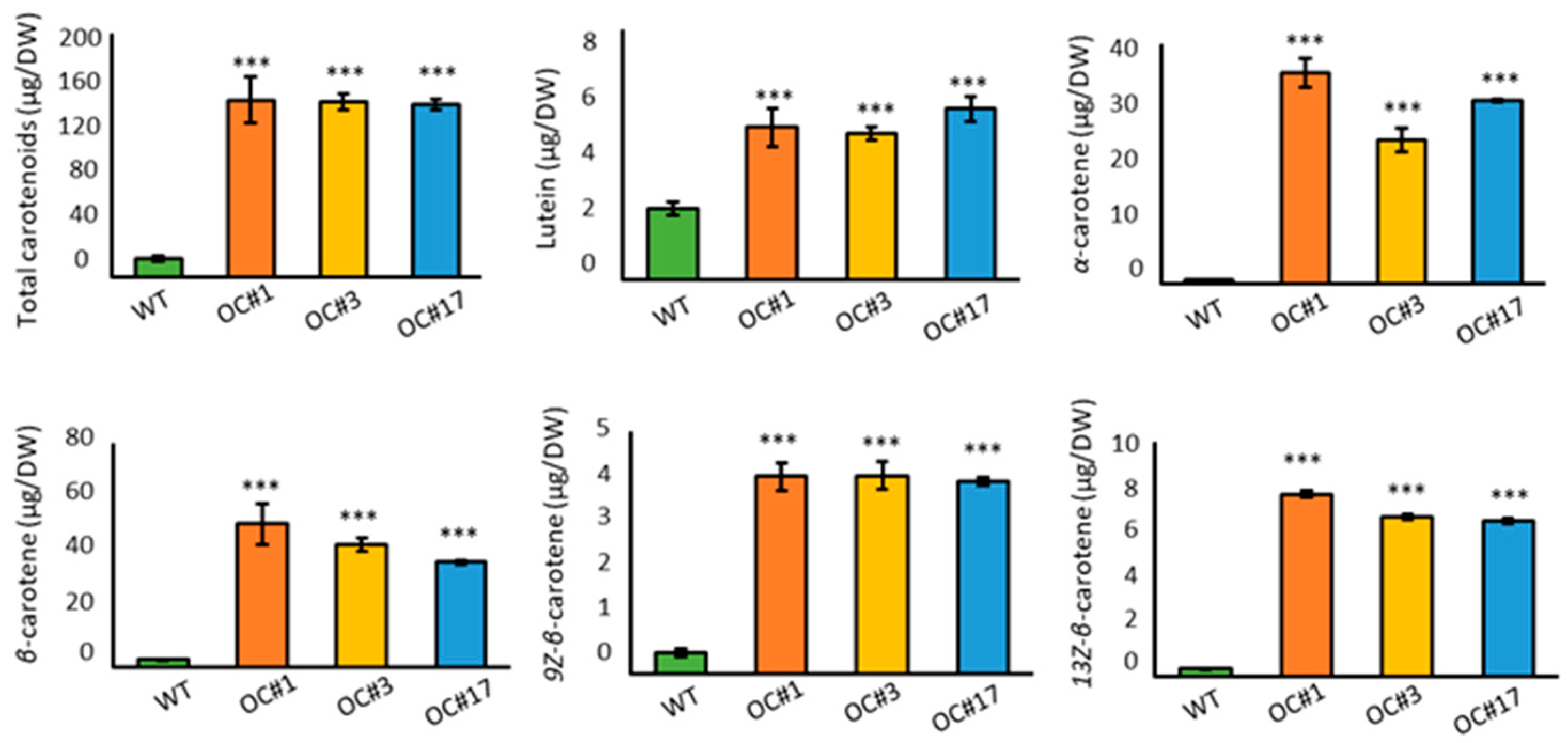
2.2. Overaccumulation of Carotenoids in OC #1, OC#3, and OC#17 Line
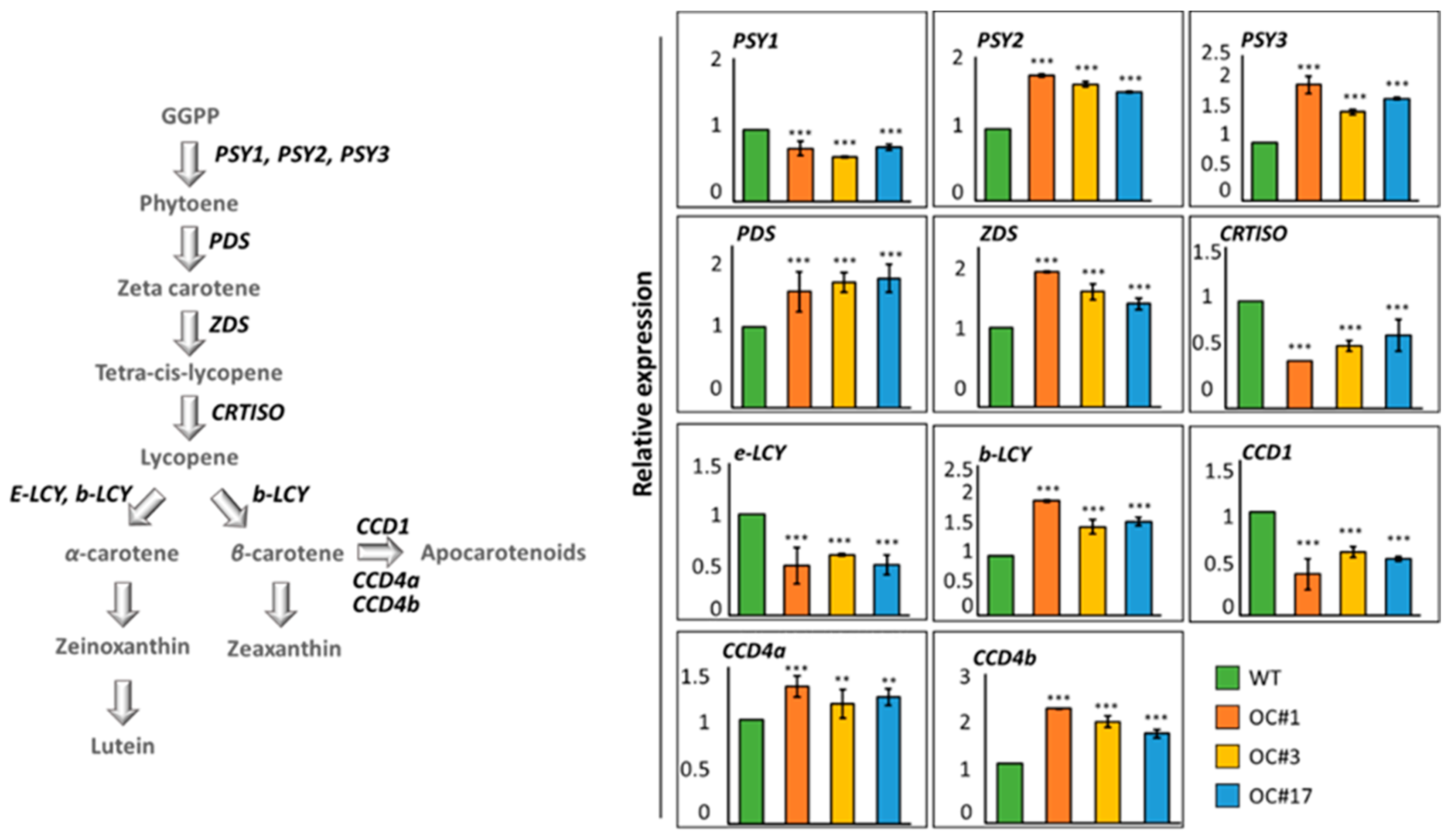
2.3. Analysis of Expression of Carotenoid Pathway Genes
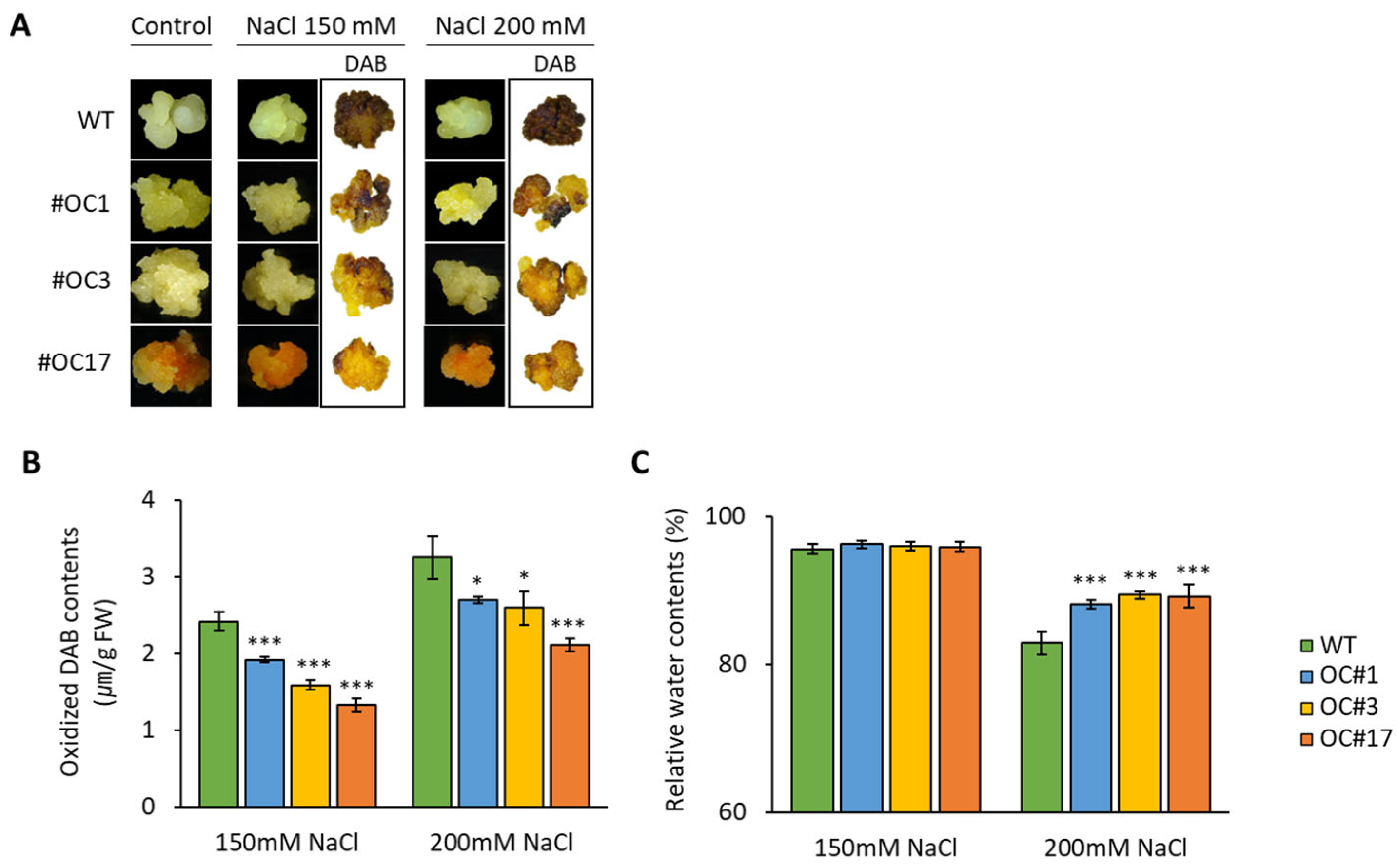
2.4. Salt-Induces H2O2 Accumulation in OC#1, OC#3, and OC#17 Lines
3. Discussion
4. Materials and Methods
4.1. Plasmid Construction and Genetic Transformation of Rice
4.2. qRT-PCR Analysis
4.3. Extraction of Pigments and Spectrophotometric Analysis
4.4. Determination of Carotenoid Content
4.5. Salt Stress
4.6. Qualitative and Quantitative Analysis of H2O2
4.7. Analysis of RWC
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yahia, E.M.; de Jesús Ornelas-Paz, J.; Emanuelli, T.; Jacob-Lopes, E.; Zepka, L.Q.; Cervantes-Paz, B. Chemistry, stability, and biological actions of carotenoids. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health; Wiley: Hoboken, NJ, USA, 2017; Volume 2, pp. 285–346. [Google Scholar]
- Young, A.J.; Pallett, K.E. Carotenoids. In Antioxidants in HIGHER Plants; CRC Press: Boca Raton, FL, USA, 2017; pp. 59–89. [Google Scholar]
- Hughes, D.A. Effects of carotenoids on human immune function. Proc. Nutr. Soc. 1999, 58, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Cazzonelli, C.I.; Pogson, B.J. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010, 15, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, Y.H.; Ahn, Y.O.; Ahn, M.J.; Jeong, J.C.; Lee, H.S.; Kwak, S.S. Downregulation of the lycopene ε-cyclase gene increases carotenoid synthesis via the β-branch-specific pathway and enhances salt-stress tolerance in sweetpotato transgenic calli. Physiol. Plant. 2013, 147, 432–442. [Google Scholar] [PubMed]
- Fraser, P.D.; Romer, S.; Shipton, C.A.; Mills, P.B.; Kiano, J.W.; Misawa, N.; Bramley, P.M. Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc. Natl. Acad. Sci. USA 2002, 99, 1092–1097. [Google Scholar] [CrossRef]
- Ducreux, L.J.; Morris, W.L.; Hedley, P.E.; Shepherd, T.; Davies, H.V.; Millam, S.; Taylor, M.A. Metabolic engineering of high carotenoid potato tubers containing enhanced levels of β-carotene and lutein. J. Exp. Bot. 2005, 56, 81–89. [Google Scholar] [CrossRef]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.M.; Kennedy, M.J.; Vernon, G.; Drake, R. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef]
- Diretto, G.; Al-Babili, S.; Tavazza, R.; Papacchioli, V.; Beyer, P.; Giuliano, G. Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway. PLoS ONE 2007, 2, e350. [Google Scholar] [CrossRef]
- Welsch, R.; Arango, J.; Bär, C.; Salazar, B.; Al-Babili, S.; Beltrán, J.; Beyer, P. Provitamin A accumulation in cassava (Manihot esculenta) roots driven by a single nucleotide polymorphism in a phytoene synthase gene. Plant Cell 2010, 22, 3348–3356. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid metabolism in plants: The role of plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef]
- Tang, G.; Qin, J.; Dolnikowski, G.G.; Russell, R.M.; Grusak, M.A. Golden Rice is an effective source of vitamin A. Am. J. Clin. 2009, 89, 1776–1783. [Google Scholar] [CrossRef]
- Bai, C.; Rivera, S.M.; Medina, V.; Alves, R.; Vilaprinyo, E.; Sorribas, A.; Zhu, C. An in vitro system for the rapid functional characterization of genes involved in carotenoid biosynthesis and accumulation. Plant J. 2014, 77, 464–475. [Google Scholar] [CrossRef]
- Stein, A.J.; Sachdev, H.P.S.; Qaim, M. Genetic engineering for the poor: Golden Rice and public health in India. World Dev. 2008, 36, 144–158. [Google Scholar] [CrossRef]
- Kim, H.S.; Ji, C.Y.; Lee, C.J.; Kim, S.E.; Park, S.C.; Kwak, S.S. Orange: A target gene for regulating carotenoid homeostasis and increasing plant tolerance to environmental stress in marginal lands. J. Exp. Bot. 2018, 69, 3393–3400. [Google Scholar] [CrossRef]
- Cordero, B.F.; Couso, I.; León, R.; Rodríguez, H.; Vargas, M.Á. Enhancement of carotenoids biosynthesis in Chlamydomonas reinhardtii by nuclear transformation using a phytoene synthase gene isolated from Chlorella zofingiensis. Appl. Microbiol. Biotechnol. 2011, 91, 341–351. [Google Scholar] [CrossRef]
- Endo, A.; Saika, H.; Takemura, M.; Misawa, N.; Tok, S. A novel approach to carotenoid accumulation in rice callus by mimicking the cauliflower orange mutation via genome editing. Rice 2019, 12, 81–85. [Google Scholar] [CrossRef]
- Lu, S.; Van Eck, J.; Zhou, X.; Lopez, A.B.; O’Halloran, D.M.; Cosman, K.M.; Li, L. The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of β-carotene accumulation. Plant Cell 2006, 18, 3594–3605. [Google Scholar] [CrossRef]
- Berman, J.; Zorrilla-López, U.; Medina, V.; Farré, G.; Sandmann, G.; Capell, T.; Zhu, C. The Arabidopsis Orange (AtOR) gene promotes carotenoid accumulation in transgenic corn hybrids derived from parental lines with limited carotenoid pools. Plant Cell Rep. 2017, 36, 933–945. [Google Scholar] [CrossRef]
- Tzuri, G.; Zhou, X.; Chayut, N.; Yuan, H.; Portnoy, V.; Meir, A.; Tadmor, Y. A ‘golden’ SNP in CmOr governs the fruit flesh color of melon (Cucumis melo). Plant J. 2015, 82, 267–279. [Google Scholar] [CrossRef]
- Yuan, H.; Owsiany, K.; Sheeja, T.; Zhou, X.; Rodriguez, C.; Li, Y.; Welsch, R.; Chayut, N.; Yang, Y.; Thannhauser, T.W.; et al. A single amino acid substitution in an ORANGE protein promotes carotenoid overaccumulation in Arabidopsis. Plant Physiol. 2015, 169, 421–431. [Google Scholar] [CrossRef]
- Yazdani, M.; Sun, Z.; Yuan, H.; Zeng, S.; Thannhauser, T.W.; Vrebalov, J.; Ma, Q.; Xu, Y.; Fei, Z.; Van Eck, J.; et al. Ectopic expression of ORANGE promotes carotenoid accumulation and fruit development in tomato. Plant Biotechnol. J. 2018, 17, 33–49. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.S.; Jung, Y.J.; Kim, S.H.; Ji, C.Y.; Wang, Z.; Kwak, S.S. Orange protein has a role in phytoene synthase stabilization in sweetpotato. Sci. Rep. 2016, 6, 33563. [Google Scholar] [CrossRef]
- Kang, L.; Kim, H.S.; Kwon, Y.S.; Ke, Q.; Ji, C.Y.; Park, S.C.; Kwak, S.S. IbOr regulates photosynthesis under heat stress by stabilizing IbPsbP in sweetpotato. Front. Plant Sci. 2017, 8, 989. [Google Scholar] [CrossRef]
- Wang, Z.; Ke, Q.; Kim, M.D.; Kim, S.H.; Ji, C.Y.; Jeong, J.C.; Kwak, S.S. Transgenic alfalfa plants expressing the sweetpotato Orange gene exhibit enhanced abiotic stress tolerance. PLoS ONE 2015, 10, e0126050. [Google Scholar] [CrossRef]
- Cho, K.S.; Han, E.H.; Kwak, S.S.; Cho, J.H.; Im, J.S.; Hong, S.Y.; Lee, S.W. Expressing the sweet potato orange gene in transgenic potato improves drought tolerance and marketable tuber production. Comptes Rendus. Biol. 2016, 339, 207–213. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, J.; Wang, Q.; Wang, J.; Zhao, G.; Wu, H.; Fang, J. Overexpression of the rice ORANGE gene OsOR negatively regulates carotenoid accumulation, leads to higher tiller numbers and decreases stress tolerance in Nipponbare rice. Plant Sci. 2021, 310, 110962. [Google Scholar] [CrossRef]
- Jung, Y.J.; Kim, J.H.; Lee, H.J.; Kim, D.H.; Yu, J.; Bae, S.; Kang, K.K. Generation and Transcriptome Profiling of Slr1-d7 and Slr1-d8 Mutant Lines with a New Semi-Dominant Dwarf Allele of SLR1 Using the CRISPR/Cas9 System in Rice. Int. J. Mol. Sci. 2020, 21, 5492. [Google Scholar] [CrossRef]
- Ruiz-Sola, M.Á.; Rodríguez-Concepción, M. Carotenoid biosynthesis in Arabidopsis: A colorful pathway. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2012; Volume 10. [Google Scholar]
- Zhou, X.; Welsch, R.; Yang, Y.; Álvarez, D.; Riediger, M.; Yuan, H.; Li, L. Arabidopsis OR proteins are the major posttranscriptional regulators of phytoene synthase in controlling carotenoid biosynthesis. Proc. Natl. Acad. Sci. USA 2015, 112, 3558–3563. [Google Scholar] [CrossRef]
- Osorio, C.E. The role of orange gene in carotenoid accumulation: Manipulating chromoplasts toward a colored future. Front. Plant Sci. 2019, 10, 1235. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, H.S.; Wang, Z.; Ke, Q.; Lee, C.J.; Park, S.U.; Lim, Y.H.; Park, W.S.; Ahn, M.J.; Kwak, S.S. A single amino acid change at position 96 (Arg to His) of the sweetpotato Orange protein leads to carotenoid overaccumulation. Plant Cell Rep. 2019, 38, 1393–1402. [Google Scholar] [CrossRef]
- Jung, Y.J.; Go, J.Y.; Lee, H.J.; Park, J.S.; Kim, J.Y.; Lee, Y.J.; Ahn, M.J.; Kim, M.S.; Cho, Y.G.; Kwak, S.S.; et al. Overexpression of Orange Gene (OsOr-R115H) Enhances Heat Tolerance and Defense-Related Gene Expression in Rice (Oryza sativa L.). Genes 2021, 12, 1891. [Google Scholar] [CrossRef]
- Ali, F.; Wang, Q.; Fazal, A.; Wang, L.J.; Song, S.; Kong, M.J.; Lu, S. The DnaJ-like Zinc Finger Protein ORANGE Promotes Proline Biosynthesis in Drought-Stressed Arabidopsis Seedlings. Int. J. Mol. Sci. 2022, 23, 3907. [Google Scholar] [CrossRef] [PubMed]
- Arango, J.; Jourdan, M.; Geoffriau, E.; Beyer, P.; Welsch, R. Carotene hydroxylase activity determines the levels of both α-carotene and total carotenoids in orange carrots. Plant Cell 2014, 26, 2223–2233. [Google Scholar] [CrossRef]
- Ronen, G.; Cohen, M.; Zamir, D.; Hirschberg, J. Regulation of carotenoid biosynthesis during tomato fruit development: Expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant Delta. Plant J. 1999, 17, 341–351. [Google Scholar] [CrossRef]
- Galpaz, N.; Wang, Q.; Menda, N.; Zamir, D.; Hirschberg, J. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J. 2008, 53, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Kolotilin, I.; Koltai, H.; Tadmor, Y.; Bar-Or, C.; Reuveni, M.; Meir, A.; Levin, I. Transcriptional profiling of high pigment-2dg tomato mutant links early fruit plastid biogenesis with its overproduction of phytonutrients. Plant Physiol. 2007, 145, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, A.; Kishimoto, S.; Aida, R.; Yoshioka, S.; Sumitomo, K. Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white color formation in chrysanthemum petals. Plant Physiol. 2006, 142, 1193–1201. [Google Scholar] [CrossRef]
- Kim, S.H.; Ahn, Y.O.; Ahn, M.J.; Lee, H.S.; Kwak, S.S. Down-regulation of β-carotene hydroxylase increases β-carotene and total carotenoids enhancing salt stress tolerance in transgenic cultured cells of sweetpotato. Phytochemistry 2012, 74, 69–78. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.B.; Dong, Y.X.; Gao, X.Q.; Zhang, X.S. Expression of a putative alfalfa helicase increases tolerance to abiotic stress in Arabidopsis by enhancing the capacities for ROS scavenging and osmotic adjustment. J. Plant Physiol. 2009, 166, 385–394. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, H.J.; Kim, J.H.; Kim, D.H.; Kim, H.K.; Cho, Y.G.; Kang, K.K. CRISPR/Cas9-targeted mutagenesis of F3′ H, DFR and LDOX, genes related to anthocyanin biosynthesis in black rice (Oryza sativa L.). Plant Biotechnol. Rep. 2019, 13, 521–531. [Google Scholar] [CrossRef]
- Park, J.; Lim, K.; Kim, J.S.; Bae, S. Cas-analyzer: An online tool for assessing genome editing results using NGS data. Bioinformatics 2017, 33, 286–288. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Zhang, R.; Huang, R. The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J. 2012, 71, 273–287. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ishihara, A.; Ohishi, K.; Yamada, T.; Shibata-Hatta, M.; Arai-Kichise, Y.; Watanabe, S.; Wakasa, K. Biochemical and molecular characterization of orange-and tangerine-colored rice calli. Plant Biotechnol. J. 2015, 32, 193–203. [Google Scholar] [CrossRef]
- Lim, O.; Suntornsuk, W.; Suntornsuk, L. Capillary zone electrophoresis for enumeration of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus in yogurt. J. Chromatogr. B 2009, 877, 710–718. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Guo, J.; Yang, Y.; Li, B.; Zhang, L. Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol. 2004, 134, 849–857. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.K.; Kim, J.Y.; Kim, J.H.; Go, J.Y.; Jung, Y.-S.; Lee, H.J.; Ahn, M.-J.; Yu, J.; Bae, S.; Kim, H.S.; et al. Biochemical Characterization of Orange-Colored Rice Calli Induced by Target Mutagenesis of OsOr Gene. Plants 2023, 12, 56. https://doi.org/10.3390/plants12010056
Kim HK, Kim JY, Kim JH, Go JY, Jung Y-S, Lee HJ, Ahn M-J, Yu J, Bae S, Kim HS, et al. Biochemical Characterization of Orange-Colored Rice Calli Induced by Target Mutagenesis of OsOr Gene. Plants. 2023; 12(1):56. https://doi.org/10.3390/plants12010056
Chicago/Turabian StyleKim, Hee Kyoung, Jin Young Kim, Jong Hee Kim, Ji Yun Go, Yoo-Seob Jung, Hyo Ju Lee, Mi-Jeong Ahn, Jihyeon Yu, Sangsu Bae, Ho Soo Kim, and et al. 2023. "Biochemical Characterization of Orange-Colored Rice Calli Induced by Target Mutagenesis of OsOr Gene" Plants 12, no. 1: 56. https://doi.org/10.3390/plants12010056
APA StyleKim, H. K., Kim, J. Y., Kim, J. H., Go, J. Y., Jung, Y.-S., Lee, H. J., Ahn, M.-J., Yu, J., Bae, S., Kim, H. S., Kwak, S.-S., Kim, M.-S., Cho, Y.-G., Jung, Y. J., & Kang, K. K. (2023). Biochemical Characterization of Orange-Colored Rice Calli Induced by Target Mutagenesis of OsOr Gene. Plants, 12(1), 56. https://doi.org/10.3390/plants12010056











