The Interaction of Fungicide and Nitrogen for Aboveground Biomass from Flag Leaf Emergence and Grain Yield Generation under Tan Spot Infection in Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Environmental Conditions
2.2. Treatments
2.3. Inoculum Preparation and Fungicide Treatments
2.4. Measurements
2.5. Statistical Analysis and Experimental Design
3. Results
3.1. Meteorological Data
3.2. Disease Severity, Green Leaf Area Dynamics, and Aboveground Biomass Production
3.3. Yield and Main Components
3.4. HAD Acts as an Indicator of the Positive Effects Generated by Fungicide Mixtures and N Fertilization under Tan Spot Epidemics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization. 2014. Available online: http://www.fao.org/resources/infographics/infographics-details/es/c/240943/ (accessed on 29 January 2022).
- Hawkesford, M.J. Reducing the reliance on nitrogen fertilizer for wheat production. J. Cereal Sci. 2014, 59, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Sheteiwy, M.S.; Zhu, S.; Zhu, L.; Batool, A.; Jia, T.; Xiong, Y. Differentiate responses of tetraploid and hexaploid wheat (Triticum aestivum L.) to moderate and severe drought stress: A cue of wheat domestication. Plant Signal. Behav. 2021, 16, 1839710. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.W.; Sheteiwy, M.S.; Zhu, S.G.; Batool, A.; Xiong, Y.C. Differentiate effects of non-hydraulic and hydraulic root signaling on yield and water use efficiency in diploid and tetraploid wheat under drought stress. Environ. Exp. Bot. 2021, 181, 104287. [Google Scholar] [CrossRef]
- Liu, Z.; Li, G.; Zhang, H.; Zhang, Y.; Zhang, Y.; Duan, S.; Sheteiwy, M.S.A.; Zhang, H.; Shao, H.; Guo, X. TaHsfA2-1, a new gene for thermotolerance in wheat seedlings: Characterization and functional roles. J. Plant Physiol. 2020, 246, 153135. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.; Ulhassan, Z.; Qi, W.; Lu, H.; AbdElgawad, H.; Minkina, T.; Sushkova, S.; Rajput, V.D.; El-Keblawy, A.; Josko, I.; et al. Association of jasmonic acid priming with multiple defense mechanisms in wheat plants under high salt stress. Front. Plant Sci. 2022, 13, 2614. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fang, C.; Li, Y.; Wu, Y.; Fransson, P.; Rillig, M.C.; Zhai, S.; Xie, J.; Tong, Z.; Zhang, Q.; et al. Temporal complementarity between roots and mycorrhizal fungi drives wheat nitrogen use efficiency. New Phytol. 2022, 236, 1168–1181. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Ahmed, M.; Mohammed, A.E.; Alotaibi, M.O.; Yehia, R.S.; Selim, S.; Saleh, A.M.; Beemster, G.T.; Sheteiwy, M.S. Increasing atmospheric CO2 differentially supports arsenite stress mitigating impact of arbuscular mycorrhizal fungi in wheat and soybean plants. Chemosphere 2022, 296, 134044. [Google Scholar] [CrossRef]
- Moreno, M.V.; Stenglein, S.A.; Perelló, A.E. Pyrenophora tritici-repentis, causal agent of tan spot: A review of intraspecific genetic diversity. In The Molecular Basis of Plant Genetic Diversity; Caliskan, M., Ed.; Intech Open: London, UK, 2012; pp. 297–330. [Google Scholar] [CrossRef]
- Dimmock, J.P.R.E.; Gooding, M.J. The effects of fungicide on rate and duration of grain filling in winter wheat in relation to maintenance of flag leaf green area. J. Agric. Sci. 2002, 138, 1–16. [Google Scholar] [CrossRef]
- Schierenbeck, M.; Fleitas, M.C.; Miralles, D.J.; Simón, M.R. Does radiation interception or radiation use efficiency limit the growth of wheat inoculated with tan spot or leaf rust? Field Crops Res. 2016, 199, 65–76. [Google Scholar] [CrossRef]
- Fischer, R.A. Number of kernels in wheat crops and the influence of solar radiation and temperature. J. Agric. Sci. 1985, 105, 447–461. [Google Scholar] [CrossRef]
- Gonzalez, F.G.; Slafer, G.A.; Miralles, D.J. Photoperiod during stem elongation in wheat: Is its impact on fertile floret and grain number determination Reducing sink limitations to wheat yield 147 similar to that of radiation? Funct. Plant Biol. 2005, 32, 181–188. [Google Scholar] [CrossRef]
- Slafer, G.A.; Andrade, F.H. Physiological attributes related to the generation of grain yield in bread wheat cultivars released at different eras. Field Crops Res. 1993, 31, 351–367. [Google Scholar] [CrossRef]
- Simón, M.R.; Perelló, A.E.; Cordo, C.A.; Struik, P.C. Influence of Septoria tritici on yield, yield components, and test weight of wheat under two nitrogen fertilization conditions. Crop Sci. 2002, 42, 1974–1981. [Google Scholar] [CrossRef]
- Leitch, M.H.; Jenkins, P.D. Influence of nitrogen on the development of septoria epidemics in winter wheat. J. Agric. Sci. 1995, 124, 361–368. [Google Scholar] [CrossRef]
- Simón, M.R.; Ayala, F.; Terrile, I.; Golik, S.; Perelló, A.; Cordo, C.A.; Chidichimo, H. Integrated foliar disease management to prevent yield loss in Argentinian wheat production. Agron. J. 2011, 103, 1441–1451. [Google Scholar] [CrossRef]
- Simón, M.R.; Perelló, A.E.; Cordo, C.A.; Arriaga, H.O. Influencia de la infección tardía de Septoria tritici Rob. ex Desm. sobre el peso de mil granos y algunos parámetros de calidad en Triticum aestivum. Investig. Agrar. 1996, 11, 161–171. [Google Scholar]
- Ishikawa, S.; Hare, M.C.; Kettlewell, P.S. Effects of strobilurin fungicide programmes and fertilizer nitrogen rates on winter wheat: Severity of Septoria tritici, leaf senescence and yield. J. Agric. Sci. 2012, 150, 411–426. [Google Scholar] [CrossRef]
- Castro, A.C.; Simón, M.R. The effect of tolerance to Septoria tritici blotch in grain yield, yield components and quality among Argentinean wheat cultivars. Crop Prot. 2016, 90, 66–76. [Google Scholar] [CrossRef]
- Fleitas, M.C.; Schierenbeck, M.; Gerard, G.S.; Dietz, J.I.; Golik, S.I.; Simón, M.R. Breadmaking quality and yield response to the green leaf area duration caused by fluxapyroxad under three nitrogen rates in wheat affected with tan spot. Crop Prot. 2018, 106, 201–209. [Google Scholar] [CrossRef]
- Serrago, R.A.; Carretero, R.; Bancal, M.O.; Miralles, D.J. Grain weight response to foliar diseases control in wheat (Triticum aestivum L.). Field Crops Res. 2011, 120, 352–359. [Google Scholar] [CrossRef]
- Carretero, R.; Bancal, M.O.; Miralles, D.J. Effect of leaf rust (Puccinia triticina) on photosynthesis and related processes of leaves in wheat crops grown at two contrasting sites and with different nitrogen levels. Eur. J. Agron. 2011, 35, 237–246. [Google Scholar] [CrossRef]
- Bhathal, J.S.; Loughman, R.; Speijers, J. Yield reduction in wheat in relation to leaf disease from yellow (tan) spot and Septoria nodorum blotch. Eur. J. Plant Pathol. 2003, 109, 435–443. [Google Scholar] [CrossRef]
- Wegulo, S.; Stevens, J.; Zwingman, M.; Baenziger, P.S. Yield response to foliar fungicide application in winter wheat. In Fungicides for Plant and Animal Diseases; Dharumadurai, D., Ed.; Intech Open: London, UK, 2012; pp. 227–244. [Google Scholar] [CrossRef]
- Kremer, M.; Hoffmann, G.M. Effect to Drechslera tritici-repentis as the cause of wheat yellow leaf spot disease on kernel yield and dry matter production. J. Phytopathol. 1992, 99, 509–605. [Google Scholar]
- Gooding, M.J.; Gregory, P.J.; Ford, K.E.; Pepler, S. Fungicide and cultivar affect post-anthesis patterns of nitrogen uptake, remobilization and utilization efficiency in wheat. J. Agric. Sci. 2005, 143, 503–518. [Google Scholar] [CrossRef]
- Jørgensen, L.N.; Olsen, L.V. Control of tan spot (Drechslera tritici-repentis) using cultivar resistance, tillage methods and fungicides. Crop Prot. 2007, 26, 1606–1616. [Google Scholar] [CrossRef]
- Bhatta, M.; Regassa, T.; Wegulo, S.N.; Baenziger, P.S. Foliar fungicide effects on disease severity, yield, and agronomic characteristics of modern winter wheat genotypes. Agron. J. 2018, 110, 602–610. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhao, J.S. Canopy photosynthesis capacity and the contribution from different organs in high-yielding winter wheat. Acta Agron. Sin. 1995, 21, 204–209. [Google Scholar]
- Berdugo, C.A.; Mahlein, A.K.; Steiner, U.; Dehne, H.W.; Oerke, E.C. Sensors and imaging techniques for the assessment of the delay of wheat senescence induced by fungicides. Funct. Plant Biol. 2012, 40, 677–689. [Google Scholar] [CrossRef]
- Simón, M.R.; Fleitas, M.C.; Castro, A.C.; Schierenbeck, M. How foliar fungal diseases affect nitrogen dynamics, milling and end-use quality of wheat. Front. Plant Sci. 2020, 11, 1568. [Google Scholar] [CrossRef]
- Sautua, F.J.; Carmona, M.A. Detection and characterization of QoI resistance in Pyrenophora tritici-repentis populations causing tan spot of wheat in Argentina. Plant Pathol. 2021, 70, 2125–2136. [Google Scholar] [CrossRef]
- Tonin, R.B.; Reis, E.M.; Avozani, A. Reduction in the in vitro sensitivity of Drechslera tritici-repentis, isolated from wheat, to strobilurin and triazole fungicides. Summa Phytopathol. 2017, 43, 20–25. [Google Scholar] [CrossRef][Green Version]
- Carmona, M.; Sautua, F.; Pérez-Hérnandez, O.; Reis, E.M. Role of Fungicide Applications on the Integrated Management of Wheat Stripe Rust. Front. Plant Sci. 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, S.; Semar, M. The contribution of BASF SDHI chemistry to cereal yield performance. In Proceedings of the Crop Protection in Northern Britain, Dundee, UK, 28–29 February 2012; pp. 151–156. [Google Scholar]
- Ajigboye, O.O.; Murchie, E.; Ray, R.V. Foliar application of isopyrazam and epoxiconazole improves photosystem II efficiency, biomass and yield in winter wheat. Pestic. Biochem. Physiol. 2014, 114, 52–60. [Google Scholar] [CrossRef]
- Snoeijers, S.S.; Pérez-García, A.; Joosten, M.H.A.J.; De Wit, P.J.G.M. The effect of nitrogen on disease development and gene expression in bacterial and fungal plant pathogens. Eur. J. Plant Pathol. 2000, 106, 493–506. [Google Scholar] [CrossRef]
- Carignano, M.; Staggenborg, S.A.; Shroyer, J.P. Management practices to minimize tan spot in a continuous wheat rotation. Agron. J. 2008, 100, 145–153. [Google Scholar] [CrossRef]
- Bockus, W.W.; Davis, M.A. Effect of nitrogen fertilizers on severity of tan spot of winter wheat. Plant Dis. 1993, 77, 508–510. [Google Scholar] [CrossRef]
- Savary, S.; Teng, P.S.; Willocquet, L.; Nutter, F.W. Quantification and modeling crop losses: A review of purposes. Annu. Rev. Phytopathol. 2006, 44, 89–112. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Raymond, P.J.; Bockus, W.W. An in vitro technique for profuse sporulation of Drechslera tritici-repentis. Phytopathology 1982, 72, 934. [Google Scholar]
- Tsialtas, J.T.; Theologidou, G.S.; Karaoglanidis, G.S. Effects of pyraclostrobin on leaf diseases, leaf physiology, yield and quality of durum wheat under Mediterranean conditions. Crop Prot. 2018, 113, 48–55. [Google Scholar] [CrossRef]
- Waggoner, P.E.; Berger, R.D. Defoliation, disease and growth. Phytopathology 1987, 77, 393–398. [Google Scholar]
- VSNI. GenStat for Windows, 14th ed.; VSN International: Hemel Hempstead, UK, 2015. [Google Scholar]
- Levene, H. Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Olkin, I., Ghurye, S.G., Hoeffding, W., Madow, W.G., Mann, H.B., Eds.; Stanford University Press: Redwood City, CA, USA, 1960. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Carretero, R.; Serrago, R.A.; Bancal, M.O.; Perelló, A.E.; Miralles, D.J. Absorbed radiation and radiation use efficiency as affected by foliar diseases in relation to their vertical position into the canopy in wheat. Field Crops Res. 2010, 116, 184–195. [Google Scholar] [CrossRef]
- Pastore, M. Efecto de la Fertilización Nitrogenada y Aplicación de Fungicidas Sobre la Severidad de la Roya de la Hoja, Mancha de la Hoja y Mancha Amarilla en Trigo. Master’s Thesis, Facultad de Ciencias Agrarias y Forestales, UNLP, La Plata, Argentina, 2008; 83p. [Google Scholar]
- Verret, E. Principles of integrated pest management. IPM Wheat Model. Pflanzenschutz Nachr. 1995, 48, 303. [Google Scholar]
- Walters, D.R.; Bingham, I.J. Influence of nutrition on disease development caused by fungal pathogens: Implications for plant disease control. Ann. Appl. Biol. 2007, 151, 307–324. [Google Scholar] [CrossRef]
- Hobbelen, P.H.F.; Paveley, N.D.; Oliver, R.P.; van den Bosch, F. The usefulness of fungicide mixtures and alternation for delaying the selection for resistance in populations of Mycosphaerella graminicola on winter wheat: A modeling analysis. Phytopathology 2013, 103, 690–707. [Google Scholar] [CrossRef]
- Fleitas, M.C.; Castro, A.C.; Simón, M.R. Quality and yield response to the control of Mycosphaerella graminicola in wheat as affected by nitrogen rate and cultivar bread-making characteristics. Crop Pasture Sci. 2017, 68, 317–327. [Google Scholar] [CrossRef]
- Fleitas, M.C.; Gerard, G.S.; Simón, M.R. Eficacia residual de fungicidas sobre la roya de la hoja del trigo y su efecto sobre componentes del rendimiento y porcentaje de proteínas en grano. FAVE Secc. Cienc. Agrar. 2015, 14, 69–84. [Google Scholar] [CrossRef]
- Fleitas, M.C.; Schierenbeck, M.; Gerard, G.S.; Dietz, J.I.; Golik, S.I.; Campos, P.E.; Simón, M.R. How leaf rust disease and its control with fungicides affect dough properties, gluten quality and loaf volume under different N rates in wheat. J. Cereal Sci. 2018, 80, 119–127. [Google Scholar] [CrossRef]
- Bancal, M.O.; Roche, R.; Bancal, P. Late foliar diseases in wheat crops decrease nitrogen yield through N uptake rather than through variations in N remobilization. Ann. Bot. 2008, 102, 579–590. [Google Scholar] [CrossRef]
- Serrago, R.A.; Carretero, R.; Bancal, M.O.; Miralles, D.J. Foliar diseases affect the ecophysiological attributes linked with yield and biomass in wheat (Triticum aestivum L). Eur. J. Agron. 2009, 31, 95–203. [Google Scholar] [CrossRef]
- Smith, J.; Grimmer, M.; Waterhouse, S.; Paveley, N. Quantifying the non-fungicidal effects of foliar applications of fluxapyroxad (Xemium) on stomatal conductance, water use efficiency and yield in winter wheat. Commun. Agric. Appl. Biol. Sci. 2013, 78, 523–535. [Google Scholar] [PubMed]
- Murdock, L.; Jones, S.; Bowley, C.; Needham, P.; James, J.; Howe, P. Using a Chlorophyll Meter to Make Nitrogen Recommendations on Wheat; Kentucky Cooperative Extension Service: Whitesburg, KY, USA, 1997; p. 4. [Google Scholar]
- Rosyara, U.R.; Sharma, R.C.; Duveiller, E. Variation of canopy temperature depression and chlorophyll content in spring wheat genotypes and association with foliar blight resistance. J. Plant Breed. 2006, 1, 45–52. [Google Scholar]
- Zuckerman, E.; Eshel, A.; Eyal, Z. Physiological aspects related to tolerance of spring wheat cultivars to Septoria tritici blotch. Phytopathology 1997, 87, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Barbottin, A.; Lecompte, C.; Bouchard, C.; Jeuffroy, M.H. Nitrogen remobilization during grain filling in wheat: Genotypic and environmental effects. Crop Sci. 2005, 45, 1141–1150. [Google Scholar] [CrossRef]
- Gooding, M.J. Influence of foliar diseases and their control by fungicides on grain yield and quality in wheat. In Wheat Production in Stressed Environments, Proceedings of the 7th International Wheat Conference, Mar del Plata, Argentina, 27 November–2 December 2005; Buck, H.T., Nisi, J.E., Salomón, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 567–581. [Google Scholar]
- De Oliveira Silva, A.; Slafer, G.A.; Fritz, A.K.; Lollato, R.P. Physiological basis of genotypic response to management in dryland wheat. Front. Plant Sci. 2020, 10, 1644. [Google Scholar] [CrossRef]
- Barraclough, P.B.; Lopez-Bellido, R.; Hawkesford, M.J. Genotypic variation in the uptake, partitioning and remobilisation of nitrogen during grain-filling in wheat. Field Crops Res. 2014, 156, 242–248. [Google Scholar] [CrossRef]
- Schierenbeck, M.; Fleitas, M.C.; Gerard, G.S.; Dietz, J.I.; Simón, M.R. Combinations of fungicide molecules and nitrogen fertilization revert nitrogen yield reductions generated by Pyrenophora tritici-repentis infections in bread wheat. Crop Prot. 2019, 121, 173–181. [Google Scholar] [CrossRef]
- Schierenbeck, M.; Fleitas, M.C.; Cortese, F.; Golik, S.I.; Simón, M.R. Nitrogen accumulation in grains, remobilization and post-anthesis uptake under tan spot and leaf rust infections on wheat. Field Crops Res. 2019, 235, 27–37. [Google Scholar] [CrossRef]
- Brinkman, J.M.P.; Deen, W.; Lauzon, J.D.; Hooker, D.C. Synergism of nitrogen rate and foliar fungicides in soft red winter wheat. Agron. J. 2014, 106, 491–510. [Google Scholar] [CrossRef]
- Ruske, R.E.; Gooding, M.J.; Jones, S.A. The effects of adding picoxystrobin, azoxystrobin and nitrogen to a triazole programme on disease control, flag leaf senescence, yield and grain quality of winter wheat. Crop Prot. 2003, 22, 975–987. [Google Scholar] [CrossRef]
- Dietz, J.I.; Schierenbeck, M.; Simón, M.R. Impact of foliar diseases and its interaction with nitrogen fertilization and fungicides mixtures on green leaf area dynamics and yield in oat genotypes with different resistance. Crop Prot. 2019, 121, 80–88. [Google Scholar] [CrossRef]
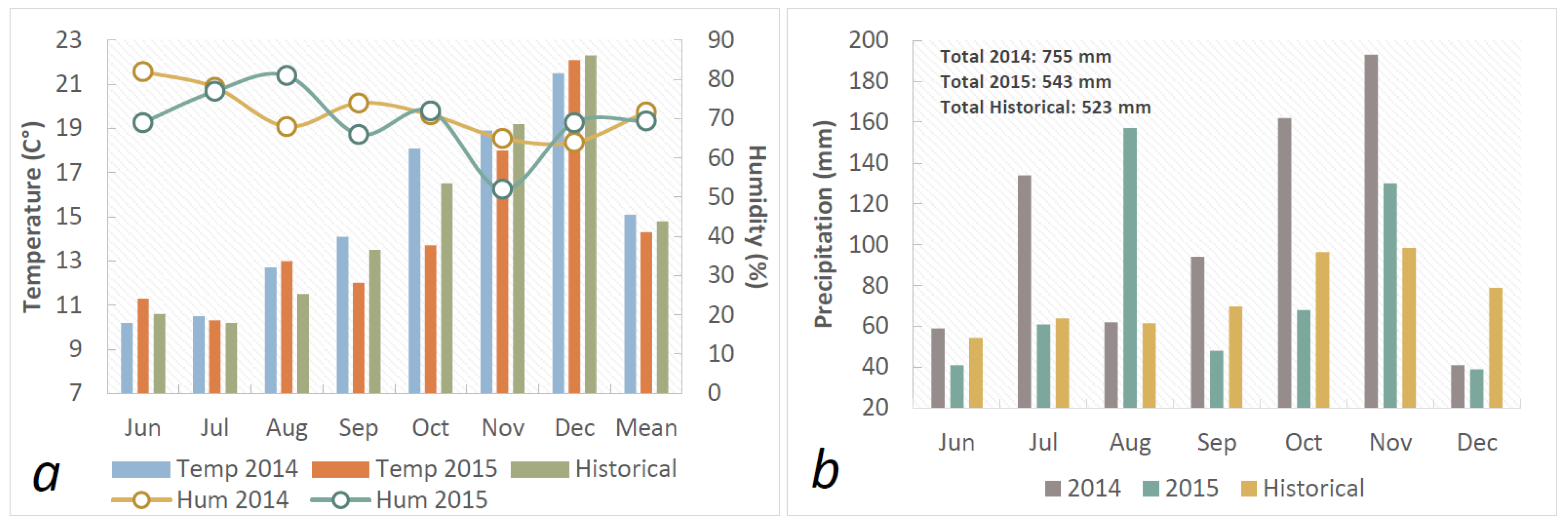

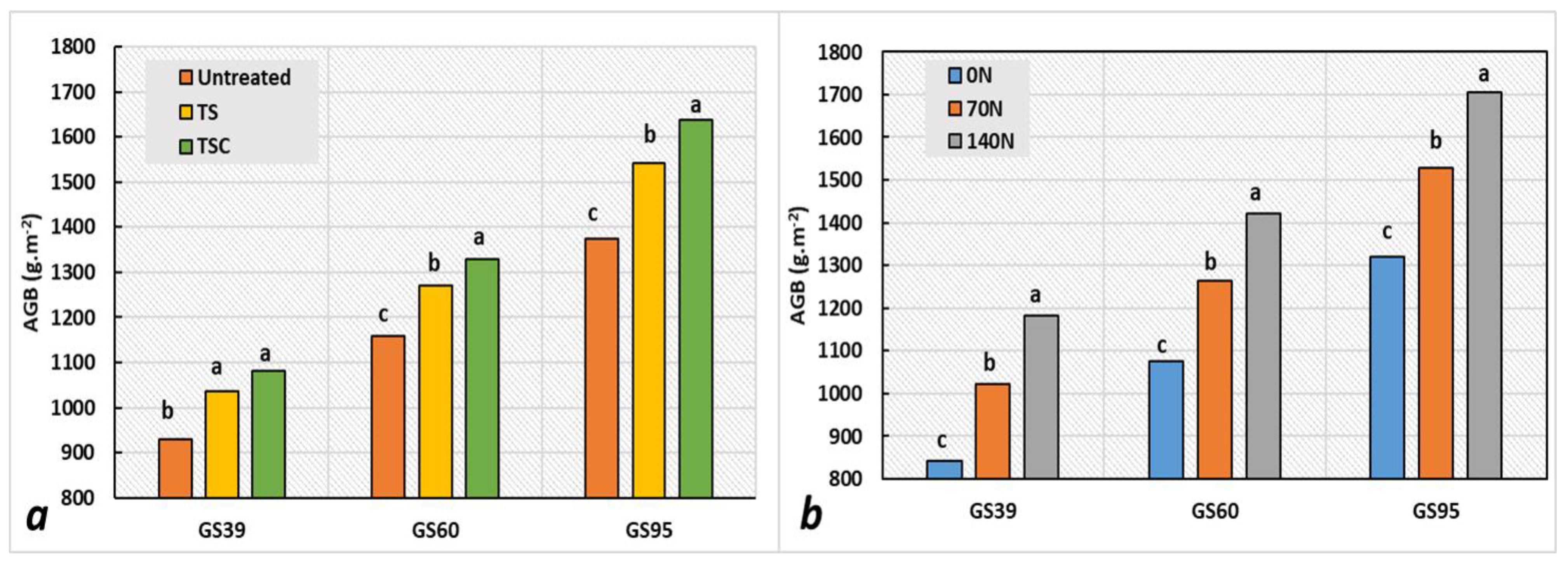
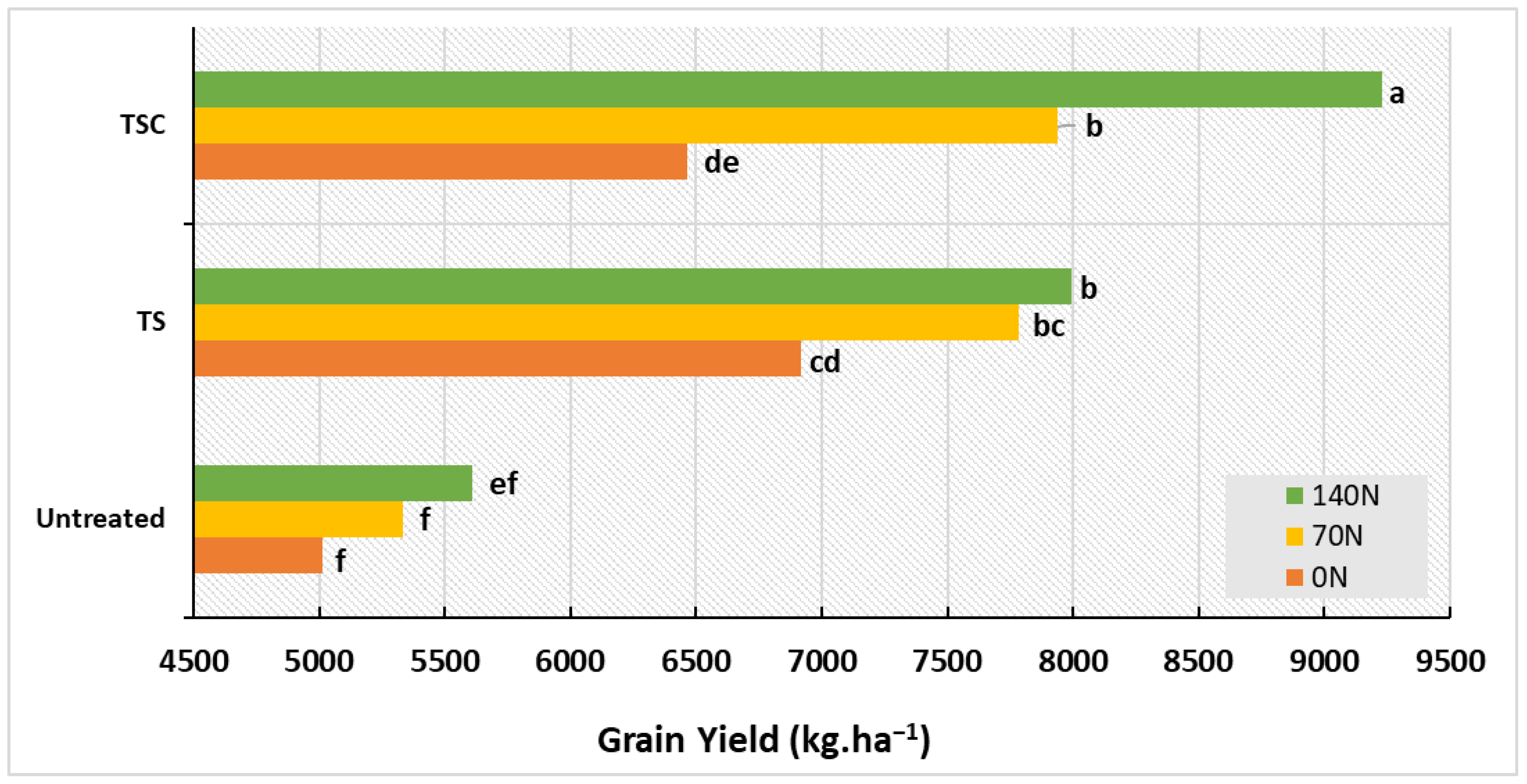

| Source of variation | D.F. | %SEV39 | % SEV60 | %SEV82 | GLAI39 | GLAI60 | GLAI82 | HAD |
|---|---|---|---|---|---|---|---|---|
| Years (Yr) | 1 | 111 (0.009) | 2861 (<0.001) | 858 (0.005) | 10.8 (0.002) | 1.01 (<0.001) | 0.11 (0.003) | 261.(0.004) |
| Error A | 2 | 0.97 | 0.19 | 4.41 | 0.02 | 0.0003 | 0.0003 | 1.00 |
| Fungicide (Fun) | 2 | 88.4 (<0.001) | 4061 (<0.001) | 1181 (0.001) | 35.3 (<0.001) | 27.9 (<0.001) | 1.88 (<0.001) | 12,455 (<0.001) |
| Yr × Fun | 2 | 7.92(0.004) | 142 (0.339) | 16.0 (0.796) | 0.14 (0.892) | 0.03 (0.687) | 0.01 (0.748) | 1.41 (0.988) |
| Error B | 8 | 0.69 | 114 | 68.2 | 1.22 | 0.078 | 0.039 | 118 |
| Nitrogen (Ni) | 2 | 14.5 (<0.001) | 472(<0.001) | 536(<0.001) | 49.9 (<0.001) | 26.4 (<0.001) | 1.52 (<0.001) | 14,330 (<0.001) |
| Yr × Ni | 2 | 1.30 (0.072) | 16.5 (0.438) | 9.86 (0.343) | 0.19 (0.573) | 0.03 (0.642) | 0.009 (0.227) | 3.20 (0.94) |
| Fun × Ni | 4 | 2.48 (0.002) | 93.8 (0.005) | 14.9 (0.018) | 1.86 (0.003) | 0.72 (<0.001) | 0.087 (<0.001) | 445 (<0.001) |
| Y × Fun × Ni | 4 | 0.22 (0.734) | 3.28 (0.952) | 10.6 (0.334) | 0.007 (0.999) | 0.0008 (1.000) | 0.0005 (0.985) | 0.20 (0.522) |
| Error C | 24 | 0.44 | 19.3 | 8.81 | 0.35 | 0.67 | 0.006 | 51.9 |
| Total | 53 |
| Source of Variation | D.F. | SPAD39 | SPAD60 | SPAD82 | AGB39 | AGB60 | AGB95 |
|---|---|---|---|---|---|---|---|
| Years (Yr) | 1 | 51.8 (0.462) | 302 (0.206) | 414 (0.102) | 876,369 (<0.001) | 1,877,710 (0.014) | 2,834,048 (0.009) |
| Error A | 2 | 63.6 | 88.8 | 49.4 | 8157 | 27,799 | 26,219 |
| Fungicide (Fun) | 2 | 322 (0.002) | 543 (<0.001) | 961 (<0.001) | 108,715 (<0.001) | 134,419 (<0.001) | 319,933 (<0.001) |
| Yr × Fun | 2 | 6.36 (0.742) | 8.48 (0.602) | 7.31(0.729) | 8157 (0.191) | 4007 (0.110) | 19,475 (0.014) |
| Error B | 8 | 20.5 | 15.6 | 22.2 | 3981 | 1358 | 2575 |
| Nitrogen (Ni) | 2 | 111 (<0.001) | 71.3 (<0.001) | 346 (<0.001) | 514,680 (<0.001) | 543,652 (<0.001) | 673,632 (<0.001) |
| Yr × Ni | 2 | 7.22 (0.353) | 15.5 (0.081) | 2.37 (0.657) | 7882 (0.289) | 3497 (0.466) | 6718 (0.206) |
| Fun × Ni | 4 | 35.1 (0.003) | 37.9 (<0.001) | 38.1 (<0.001) | 1637 (0.893) | 2122 (0.752) | 6799 (0.181) |
| Yr × Fun × Ni | 4 | 8.05 (0.331) | 17.4 (0.033) | 0.34 (0.993) | 2024 (0.851) | 686 (0.959) | 7470 (0.147) |
| Error C | 24 | 6.63 | 5.53 | 5.55 | 6031 | 4442 | 3978 |
| Total | 53 |
| %SEV39 | %SEV60 | %SEV82 | SPAD39 | SPAD60 | SPAD82 | GLAI39 | GLAI60 | GLAI82 | HAD | |
|---|---|---|---|---|---|---|---|---|---|---|
| Years | ||||||||||
| 2014 | 6.21 a | 46.2 a | 90.1 a | 30.4 | 28.7 | 25.1 | 7.52 a | 3.98 b | 0.51 b | 110 a |
| 2015 | 3.35 b | 31.6 b | 82.2 b | 32.4 | 33.4 | 30.6 | 6.62 b | 4.26 a | 0.60 a | 106 b |
| LSD | 1.15 | 0.51 | 2.46 | 9.34 | 11.0 | 8.23 | 0.15 | 0.02 | 0.02 | 1.17 |
| Fungicide | ||||||||||
| Untreated | 7.34 a | 56.0 a | 95.0 a | 26.5 b | 24.8 b | 19.5 b | 5.57 c | 2.72 c | 0.21 c | 78.8 c |
| TS | 3.47 b | 32.9 b | 84.5 b | 33.4 a | 33.6 a | 30.9 a | 7.28 b | 4.54 b | 0.62 b | 114.2 b |
| TSC | 3.53 b | 27.9 b | 79.0 b | 34.3 a | 34.8 a | 33.2 a | 8.35 a | 5.10 a | 0.84 a | 130.1 a |
| LSD | 0.64 | 8.22 | 6.35 | 3.48 | 3.04 | 3.62 | 0.85 | 0.22 | 0.15 | 8.39 |
| Nitrogen | ||||||||||
| 0 N | 5.79 a | 43.1 a | 91.2 a | 28.9 c | 29.5 b | 23.5 c | 5.39 c | 2.86 c | 0.26 c | 78.9 c |
| 70 N | 4.47 b | 40.5 a | 86.9 b | 31.4 b | 30.4 b | 27.8 b | 7.09 b | 4.22 b | 0.57 b | 109 b |
| 140 N | 4.08 b | 33.2 b | 80.4 c | 33.9 a | 33.3 a | 32.3 a | 8.72 a | 5.28 a | 0.84 a | 135 a |
| LSD | 0.46 | 3.03 | 2.04 | 1.77 | 1.62 | 1.62 | 0.41 | 0.18 | 0.05 | 4.95 |
| Source of variation | D.F. | GPS | TKW | SPKN | GN | Grain Yield |
|---|---|---|---|---|---|---|
| Years (Y) | 1 | 122 (0.010) | 0.019 (0.987) | 92,955 (<0.001) | 2.1067 (<0.001) | 114,030 (0.875) |
| Error A | 2 | 1.19 | 56.2 | 44.1 | 1.8798 | 3,600,132 |
| Fungicide (Fu) | 2 | 138 (0.065) | 285 (<0.001) | 26,459 (0.014) | 1.5368 (<0.001) | 35,112,519 (<0.001) |
| Y × Fu | 2 | 0.13 (0.996) | 11.3 (0.433) | 989 (0.756) | 7.5955 (0.973) | 380,523 (0.748) |
| Error B | 8 | 35.2 | 12.1 | 3422 | 0.25 | 1,262,760 |
| Nitrogen (N) | 2 | 25.1 (0.004) | 3.36 (0.352) | 66,771 (<0.001) | 1.4548 (<0.001) | 9,987,390 (<0.001) |
| Y × N | 2 | 11.3 (0.064) | 11.3 (0.041) | 20,797 (<0.001) | 1.6977 (0.002) | 854,157 (0.103) |
| Fu × N | 4 | 1.36 (0.827) | 3.20 (0.407) | 644 (0.510) | 8.8977 (0.773) | 1,998,794 (0.002) |
| Y × Fu × N | 4 | 1.36 (0.817) | 3.20 (0.406) | 646 (0.505) | 1.1106 (0.695) | 171,130 (0.734) |
| Error C | 24 | 3.66 | 3.07 | 762 | 1.9896 | 340,526 |
| Total | 53 |
| Source of Variation | AGB39 | AGB60 | AGB95 | TKW | GPS | SPKN | GN | Grain Yield |
|---|---|---|---|---|---|---|---|---|
| Year | ||||||||
| 2014 | 888 a | 1067 b | 1289 b | 38.0 | 37.6 a | 616 a | 23,282 a | 6966 a |
| 2015 | 1143 a | 1440 a | 1747 a | 38.0 | 34.6 b | 533 b | 18,573 b | 6874 a |
| LSD | 264 | 195 | 190 | 8.78 | 1.27 | 7.80 | 161 | 2221 |
| Fungicide | ||||||||
| Untreated | 929 a | 1159 c | 1375 c | 33.4 b | 32.9 b | 531 b | 17,555 b | 5317 b |
| TS | 1037 b | 1271 b | 1542 b | 40.0 a | 37.6 a | 601 a | 22,693 a | 7563 a |
| TSC | 1080 b | 1329 a | 1638 a | 40.6 a | 37.8 a | 593 a | 22,533 a | 7878 a |
| LSD | 48.50 | 28.3 | 39 | 2.68 | 4.56 | 45.0 | 4031 | 864 |
| Nitrogen | ||||||||
| 0N | 843 c | 1075 c | 1319 c | 38.2 | 35.1 b | 510 c | 18,029 c | 6131 c |
| 70N | 1023 b | 1263 b | 1530 b | 38.4 | 35.7 b | 582 b | 21,042 b | 7018 b |
| 140N | 1081 a | 1422 a | 1706 a | 37.5 | 37.4 a | 631 a | 23,710 a | 7611 a |
| LSD | 53.4 | 45.9 | 43.4 | 1.21 | 1.32 | 19.0 | 0.41 | 401 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schierenbeck, M.; Fleitas, M.C.; Simón, M.R. The Interaction of Fungicide and Nitrogen for Aboveground Biomass from Flag Leaf Emergence and Grain Yield Generation under Tan Spot Infection in Wheat. Plants 2023, 12, 212. https://doi.org/10.3390/plants12010212
Schierenbeck M, Fleitas MC, Simón MR. The Interaction of Fungicide and Nitrogen for Aboveground Biomass from Flag Leaf Emergence and Grain Yield Generation under Tan Spot Infection in Wheat. Plants. 2023; 12(1):212. https://doi.org/10.3390/plants12010212
Chicago/Turabian StyleSchierenbeck, Matías, María Constanza Fleitas, and María Rosa Simón. 2023. "The Interaction of Fungicide and Nitrogen for Aboveground Biomass from Flag Leaf Emergence and Grain Yield Generation under Tan Spot Infection in Wheat" Plants 12, no. 1: 212. https://doi.org/10.3390/plants12010212
APA StyleSchierenbeck, M., Fleitas, M. C., & Simón, M. R. (2023). The Interaction of Fungicide and Nitrogen for Aboveground Biomass from Flag Leaf Emergence and Grain Yield Generation under Tan Spot Infection in Wheat. Plants, 12(1), 212. https://doi.org/10.3390/plants12010212






