Time of Day Analysis over a Field Grown Developmental Time Course in Rice
Abstract
1. Introduction
2. Results
2.1. Identification of Time of Day (TOD) Expression in the Rice Developmental Time Course
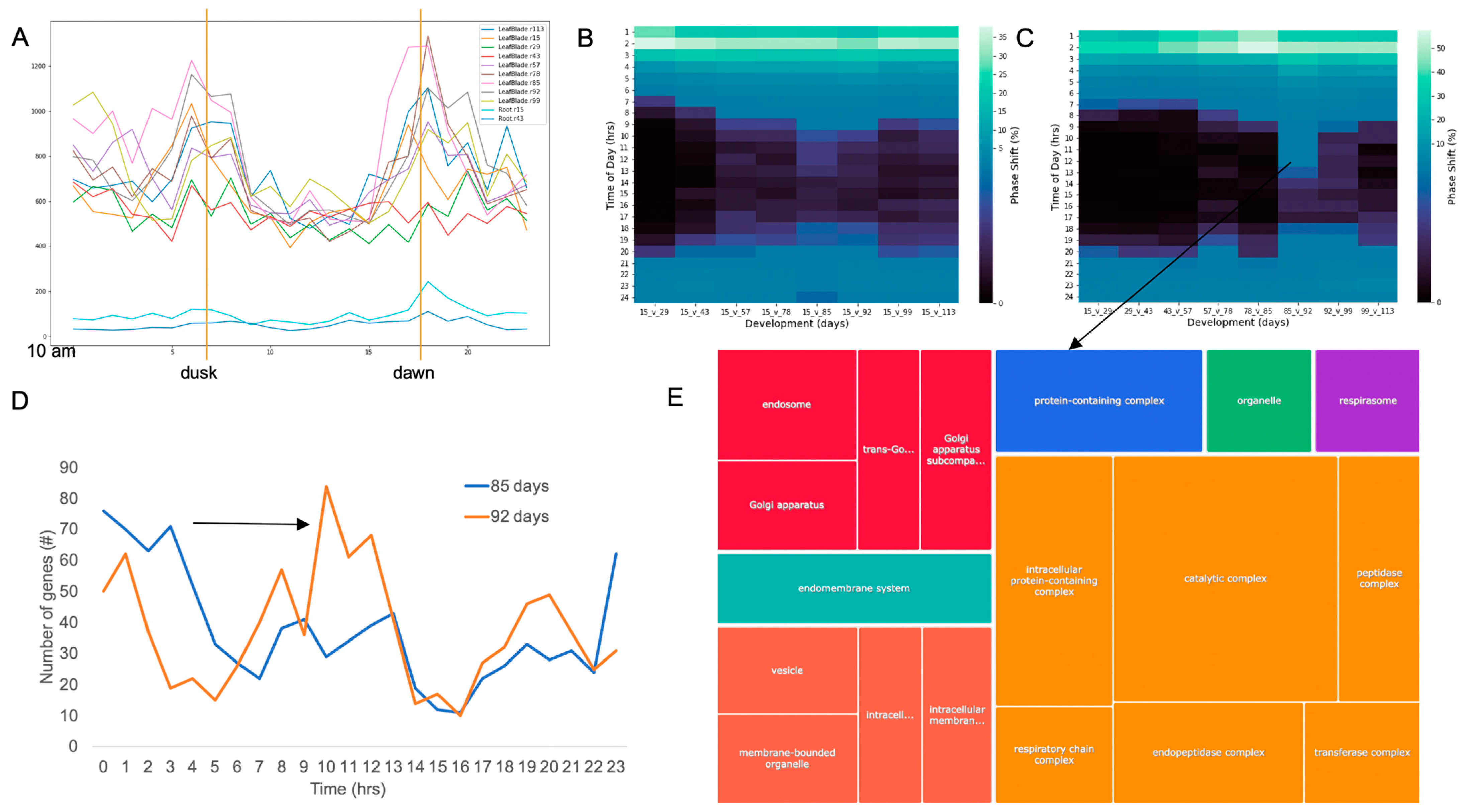
2.2. The TOD of Expression Changes over Developmental Time
2.3. The Core Circadian Clock and Flowering Time Genes over Developmental Time
2.4. ELF3 Is a Photo and Thermocycle Integrator over Developmental Time
2.5. Element Analysis over the Developmental Time Course
2.6. Gene Ontology (GO) Analysis Reveals Rewiring of the Plant over Development
3. Discussion
4. Materials and Methods
4.1. Developmental Rice Time Course
4.2. Time Course Analysis
4.3. Circadian Clock Ortholog Analysis
4.4. Analysis of Other Time Courses
4.5. ELEMENT Time of Day (TOD) Cis-Element Identification
4.6. Gene Ontology (GO) Analysis
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michael, T.P.; Salomé, P.A.; Yu, H.J.; Spencer, T.R.; Sharp, E.L.; McPeek, M.A.; Alonso, J.M.; Ecker, J.R.; McClung, C.R. Enhanced Fitness Conferred by Naturally Occurring Variation in the Circadian Clock. Science 2003, 302, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Michael, T.P. Core Circadian Clock and Light Signaling Genes Brought into Genetic Linkage across the Green Lineage. Plant Physiol. 2022, 190, 1037–1056. [Google Scholar] [CrossRef]
- Michael, T.P.; Mockler, T.C.; Breton, G.; McEntee, C.; Byer, A.; Trout, J.D.; Hazen, S.P.; Shen, R.; Priest, H.D.; Sullivan, C.M.; et al. Network Discovery Pipeline Elucidates Conserved Time-of-Day–Specific Cis-Regulatory Modules. PLoS Genet. 2008, 4, e14. [Google Scholar] [CrossRef]
- Michael, T.P.; Breton, G.; Hazen, S.P.; Priest, H.; Mockler, T.C.; Kay, S.A.; Chory, J. A Morning-Specific Phytohormone Gene Expression Program Underlying Rhythmic Plant Growth. PLoS Biol. 2008, 6, e225. [Google Scholar] [CrossRef] [PubMed]
- Izawa, T. Physiological Significance of the Plant Circadian Clock in Natural Field Conditions. Plant Cell Environ. 2012, 35, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Panter, P.E.; Muranaka, T.; Cuitun-Coronado, D.; Graham, C.A.; Yochikawa, A.; Kudoh, H.; Dodd, A.N. Circadian Regulation of the Plant Transcriptome Under Natural Conditions. Front. Genet. 2019, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Oravec, M.W.; Greenham, K. The Adaptive Nature of the Plant Circadian Clock in Natural Environments. Plant Physiol. 2022, 190, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Filichkin, S.A.; Breton, G.; Priest, H.D.; Dharmawardhana, P.; Jaiswal, P.; Fox, S.E.; Michael, T.P.; Chory, J.; Kay, S.A.; Mockler, T.C. Global Profiling of Rice and Poplar Transcriptomes Highlights Key Conserved Circadian-Controlled Pathways and Cis-Regulatory Modules. PLoS ONE 2011, 6, e16907. [Google Scholar] [CrossRef]
- Cronn, R.; Dolan, P.C.; Jogdeo, S.; Wegrzyn, J.L.; Neale, D.B.; St Clair, J.B.; Denver, D.R. Transcription through the Eye of a Needle: Daily and Annual Cyclic Gene Expression Variation in Douglas-Fir Needles. BMC Genom. 2017, 18, 558. [Google Scholar] [CrossRef]
- MacKinnon, K.J.-M.; Cole, B.J.; Yu, C.; Coomey, J.H.; Hartwick, N.T.; Remigereau, M.-S.; Duffy, T.; Michael, T.P.; Kay, S.A.; Hazen, S.P. Changes in Ambient Temperature Are the Prevailing Cue in Determining Brachypodium Distachyon Diurnal Gene Regulation. New Phytol. 2020, 227, 1709–1724. [Google Scholar] [CrossRef]
- Ferrari, C.; Proost, S.; Janowski, M.; Becker, J.; Nikoloski, Z.; Bhattacharya, D.; Price, D.; Tohge, T.; Bar-Even, A.; Fernie, A.; et al. Kingdom-Wide Comparison Reveals the Evolution of Diurnal Gene Expression in Archaeplastida. Nat. Commun. 2019, 10, 737. [Google Scholar] [CrossRef] [PubMed]
- Nose, M.; Watanabe, A. Clock Genes and Diurnal Transcriptome Dynamics in Summer and Winter in the Gymnosperm Japanese Cedar (Cryptomeria Japonica (L.f.) D.Don). BMC Plant Biol. 2014, 14, 308. [Google Scholar]
- Sato, Y.; Takehisa, H.; Kamatsuki, K.; Minami, H.; Namiki, N.; Ikawa, H.; Ohyanagi, H.; Sugimoto, K.; Antonio, B.A.; Nagamura, Y. RiceXPro Version 3.0: Expanding the Informatics Resource for Rice Transcriptome. Nucleic Acids Res. 2013, 41, D1206–D1213. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Rowe, S.C.; Harmon, F.G. Coordination of the Maize Transcriptome by a Conserved Circadian Clock. BMC Plant Biol. 2010, 10, 126. [Google Scholar] [CrossRef]
- Michael, T.P.; Ernst, E.; Hartwick, N.; Chu, P.; Bryant, D.; Gilbert, S.; Ortleb, S.; Baggs, E.L.; Sree, K.S.; Appenroth, K.J.; et al. Genome and Time-of-Day Transcriptome of Wolffia Australiana Link Morphological Minimization with Gene Loss and Less Growth Control. Genome Res. 2020, 31, 225–238. [Google Scholar] [CrossRef]
- Wai, C.M.; Weise, S.E.; Ozersky, P.; Mockler, T.C.; Michael, T.P.; VanBuren, R. Time of Day and Network Reprogramming during Drought Induced CAM Photosynthesis in Sedum Album. PLoS Genet. 2019, 15, e1008209. [Google Scholar] [CrossRef]
- Wickell, D.; Kuo, L.-Y.; Yang, H.-P.; Dhabalia Ashok, A.; Irisarri, I.; Dadras, A.; de Vries, S.; de Vries, J.; Huang, Y.-M.; Li, Z.; et al. Underwater CAM Photosynthesis Elucidated by Isoetes Genome. Nat. Commun. 2021, 12, 6348. [Google Scholar] [CrossRef]
- Lai, X.; Bendix, C.; Yan, L.; Zhang, Y.; Schnable, J.C.; Harmon, F.G. Interspecific Analysis of Diurnal Gene Regulation in Panicoid Grasses Identifies Known and Novel Regulatory Motifs. BMC Genom. 2020, 21, 428. [Google Scholar] [CrossRef]
- Greenham, K.; Sartor, R.C.; Zorich, S.; Lou, P.; Mockler, T.C.; McClung, C.R. Expansion of the Circadian Transcriptome in Brassica Rapa and Genome-Wide Diversification of Paralog Expression Patterns. Elife 2020, 9, e58993. [Google Scholar] [CrossRef]
- Greenham, K.; Lou, P.; Puzey, J.R.; Kumar, G.; Arnevik, C.; Farid, H.; Willis, J.H.; McClung, C.R. Geographic Variation of Plant Circadian Clock Function in Natural and Agricultural Settings. J. Biol. Rhythm. 2017, 32, 26–34. [Google Scholar] [CrossRef]
- Edwards, K.D.; Lynn, J.R.; Gyula, P.; Nagy, F.; Millar, A.J. Natural Allelic Variation in the Temperature-Compensation Mechanisms of the Arabidopsis Thaliana Circadian Clock. Genetics 2005, 170, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Green, R.M.; Tingay, S.; Wang, Z.-Y.; Tobin, E.M. Circadian Rhythms Confer a Higher Level of Fitness to Arabidopsis Plants. Plant Physiol. 2002, 129, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.N.; Salathia, N.; Hall, A.; Kévei, E.; Tóth, R.; Nagy, F.; Hibberd, J.M.; Millar, A.J.; Webb, A.A.R. Plant Circadian Clocks Increase Photosynthesis, Growth, Survival, and Competitive Advantage. Science 2005, 309, 630–633. [Google Scholar] [CrossRef] [PubMed]
- McClung, C.R. The Plant Circadian Oscillator. Biology 2019, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Matsushika, A.; Makino, S.; Kojima, M.; Mizuno, T. Circadian Waves of Expression of the APRR1/TOC1 Family of Pseudo-Response Regulators in Arabidopsis Thaliana: Insight into the Plant Circadian Clock. Plant Cell Physiol. 2000, 41, 1002–1012. [Google Scholar] [CrossRef]
- Jung, J.-H.; Barbosa, A.D.; Hutin, S.; Kumita, J.R.; Gao, M.; Derwort, D.; Silva, C.S.; Lai, X.; Pierre, E.; Geng, F.; et al. A Prion-like Domain in ELF3 Functions as a Thermosensor in Arabidopsis. Nature 2020, 585, 256–260. [Google Scholar] [CrossRef]
- Huang, H.; McLoughlin, K.E.; Sorkin, M.L.; Burgie, E.S.; Bindbeutel, R.K.; Vierstra, R.D.; Nusinow, D.A. PCH1 Regulates Light, Temperature, and Circadian Signaling as a Structural Component of Phytochrome B-Photobodies in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 8603–8608. [Google Scholar] [CrossRef]
- Michael, T.P.; Salome, P.A.; McClung, C.R. Two Arabidopsis Circadian Oscillators Can Be Distinguished by Differential Temperature Sensitivity. Proc. Natl. Acad. Sci. USA 2003, 100, 6878–6883. [Google Scholar] [CrossRef]
- Creux, N.; Harmer, S. Circadian Rhythms in Plants. Cold Spring Harb. Perspect. Biol. 2019, 11, a034611. [Google Scholar] [CrossRef]
- Bendix, C.; Marshall, C.M.; Harmon, F.G. Circadian Clock Genes Universally Control Key Agricultural Traits. Mol. Plant 2015, 8, 1135–1152. [Google Scholar] [CrossRef]
- Steed, G.; Ramirez, D.C.; Hannah, M.A.; Webb, A.A.R. Chronoculture, Harnessing the Circadian Clock to Improve Crop Yield and Sustainability. Science 2021, 372, eabc9141. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-W.; Liu, W.; Lam, H.-M.; Gendron, J.M. Characterization of Two Growth Period QTLs Reveals Modification of PRR3 Genes During Soybean Domestication. Plant Cell Physiol. 2019, 60, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Dong, L.; Fang, C.; Liu, S.; Kong, L.; Cheng, Q.; Chen, L.; Su, T.; Nan, H.; Zhang, D.; et al. Stepwise Selection on Homeologous PRR Genes Controlling Flowering and Maturity during Soybean Domestication. Nat. Genet. 2020, 52, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-W.; Lam, H.-M. The Modification of Circadian Clock Components in Soybean During Domestication and Improvement. Front. Genet. 2020, 11, 571188. [Google Scholar] [CrossRef]
- Murphy, R.L.; Klein, R.R.; Morishige, D.T.; Brady, J.A.; Rooney, W.L.; Miller, F.R.; Dugas, D.V.; Klein, P.E.; Mullet, J.E. Coincident Light and Clock Regulation of Pseudoresponse Regulator Protein 37 (PRR37) Controls Photoperiodic Flowering in Sorghum. Proc. Natl. Acad. Sci. USA 2011, 108, 16469–16474. [Google Scholar] [CrossRef]
- Koo, B.-H.; Yoo, S.-C.; Park, J.-W.; Kwon, C.-T.; Lee, B.-D.; An, G.; Zhang, Z.; Li, J.; Li, Z.; Paek, N.-C. Natural Variation in OsPRR37 Regulates Heading Date and Contributes to Rice Cultivation at a Wide Range of Latitudes. Mol. Plant 2013, 6, 1877–1888. [Google Scholar] [CrossRef]
- Nagano, A.J.; Sato, Y.; Mihara, M.; Antonio, B.A.; Motoyama, R.; Itoh, H.; Nagamura, Y.; Izawa, T. Deciphering and Prediction of Transcriptome Dynamics under Fluctuating Field Conditions. Cell 2012, 151, 1358–1369. [Google Scholar] [CrossRef]
- Sato, Y.; Antonio, B.A.; Namiki, N.; Takehisa, H.; Minami, H.; Kamatsuki, K.; Sugimoto, K.; Shimizu, Y.; Hirochika, H.; Nagamura, Y. RiceXPro: A Platform for Monitoring Gene Expression in Japonica Rice Grown under Natural Field Conditions. Nucleic Acids Res. 2011, 39, D1141–D1148. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Kawahara, Y.; Izawa, T. Punctual Transcriptional Regulation by the Rice Circadian Clock under Fluctuating Field Conditions. Plant Cell 2015, 27, 633–648. [Google Scholar] [CrossRef]
- Sato, Y.; Antonio, B.; Namiki, N.; Motoyama, R.; Sugimoto, K.; Takehisa, H.; Minami, H.; Kamatsuki, K.; Kusaba, M.; Hirochika, H.; et al. Field Transcriptome Revealed Critical Developmental and Physiological Transitions Involved in the Expression of Growth Potential in Japonicarice. BMC Plant Biol. 2011, 11, 10. [Google Scholar] [CrossRef]
- Mockler, T.C.; Michael, T.P.; Priest, H.D.; Shen, R.; Sullivan, C.M.; Givan, S.A.; McEntee, C.; Kay, S.A.; Chory, J. The DIURNAL Project: DIURNAL and Circadian Expression Profiling, Model-Based Pattern Matching, and Promoter Analysis. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Strayer, C.; Oyama, T.; Schultz, T.F.; Raman, R.; Somers, D.E.; Más, P.; Panda, S.; Kreps, J.A.; Kay, S.A. Cloning of the Arabidopsis Clock Gene TOC1, an Autoregulatory Response Regulator Homolog. Science 2000, 289, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Tobin, E.M. Constitutive Expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) Gene Disrupts Circadian Rhythms and Suppresses Its Own Expression. Cell 1998, 93, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, R.; Ramsay, N.; Samach, A.; Corden, S.; Putterill, J.; Carré, I.A.; Coupland, G. The Late Elongated Hypocotyl Mutation of Arabidopsis Disrupts Circadian Rhythms and the Photoperiodic Control of Flowering. Cell 1998, 93, 1219–1229. [Google Scholar] [CrossRef]
- Chaudhury, A.; Okada, K.; Raikhel, N.V.; Shinozaki, K.; Sundaresan, V.V. A Weed Reaches New Heights down under. Plant Cell 1999, 11, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.J.; Stelzl, L.S.; Pietrek, L.; Hummer, G.; Wigge, P.A.; Hanson, S.M. Helical Region near Poly-Q Tract in Prion-like Domain of Arabidopsis ELF3 Plays Role in Temperature-Sensing Mechanism. Biophys. J. 2022, 121, 355a–356a. [Google Scholar] [CrossRef]
- Press, M.O.; Queitsch, C. The Variable ELF3 Polyglutamine Tract Mediates Complex Epistatic Interactions in Arabidopsis Thaliana. Genetics 2017, 205, 455–464. [Google Scholar] [CrossRef]
- Zhang, L.L.; Shao, Y.J.; Ding, L.; Wang, M.J.; Davis, S.J.; Liu, J.X. XBAT31 Regulates Thermoresponsive Hypocotyl Growth through Mediating Degradation of the Thermosensor ELF3 in Arabidopsis. Sci. Adv. 2021, 7, eabf4427. [Google Scholar] [CrossRef]
- Box, M.S.; Huang, B.E.; Domijan, M.; Jaeger, K.E.; Khattak, A.K.; Yoo, S.J.; Sedivy, E.L.; Jones, D.M.; Hearn, T.J.; Webb, A.A.R.; et al. ELF3 Controls Thermoresponsive Growth in Arabidopsis. Curr. Biol. 2015, 25, 194–199. [Google Scholar] [CrossRef]
- Matsubara, K.; Ogiso-Tanaka, E.; Hori, K.; Ebana, K.; Ando, T.; Yano, M. Natural Variation in Hd17, a Homolog of Arabidopsis ELF3 That Is Involved in Rice Photoperiodic Flowering. Plant Cell Physiol. 2012, 53, 709–716. [Google Scholar] [CrossRef]
- Saito, H.; Ogiso-Tanaka, E.; Okumoto, Y.; Yoshitake, Y.; Izumi, H.; Yokoo, T.; Matsubara, K.; Hori, K.; Yano, M.; Inoue, H.; et al. Ef7 Encodes an ELF3-like Protein and Promotes Rice Flowering by Negatively Regulating the Floral Repressor Gene Ghd7 under Both Short- and Long-Day Conditions. Plant Cell Physiol. 2012, 53, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, Y.; Tang, W.; Chen, X.; Lin, C.; Liu, Y.; Ye, Y.; Wu, W.; Duan, Y. LUX ARRHYTHMO Interacts With ELF3a and ELF4a to Coordinate Vegetative Growth and Photoperiodic Flowering in Rice. Front. Plant Sci. 2022, 13, 853042. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.; Lu, Y.; Cordeiro, A.; Costa, J.M.F.; Wigge, P.A.; Saibo, N.J.M.; Jaeger, K.E. The Evening Complex Integrates Photoperiod Signals to Control Flowering in Rice. Proc. Natl. Acad. Sci. USA 2022, 119, e2122582119. [Google Scholar] [CrossRef] [PubMed]
- Zdepski, A.; Wang, W.; Priest, H.D.; Ali, F.; Alam, M.; Mockler, T.C.; Michael, T.P. Conserved Daily Transcriptional Programs in Carica Papaya. Trop. Plant Biol. 2008, 1, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, R.; Li, M.; Xing, Z.; Yang, W.; Chen, G.; Guo, H.; Gong, X.; Du, Z.; Zhang, Z.; et al. Transcriptome Phase Distribution Analysis Reveals Diurnal Regulated Biological Processes and Key Pathways in Rice Flag Leaves and Seedling Leaves. PLoS ONE 2011, 6, e17613. [Google Scholar] [CrossRef]
- Liu, C.; Qu, X.; Zhou, Y.; Song, G.; Abiri, N.; Xiao, Y.; Liang, F.; Jiang, D.; Hu, Z.; Yang, D. OsPRR37 Confers an Expanded Regulation of the Diurnal Rhythms of the Transcriptome and Photoperiodic Flowering Pathways in Rice. Plant Cell Environ. 2018, 41, 630–645. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Zhou, Y.; Wei, X.; Peng, Y.; Dai, Y.; Zhang, L.; Zhu, Z. Diurnal Transcriptomics Analysis Reveals the Regulatory Role of the Circadian Rhythm in Super-Hybrid Rice LY2186. Genomics 2021, 113, 1281–1290. [Google Scholar] [CrossRef]
- Deng, L.; Gao, B.; Zhao, L.; Zhang, Y.; Zhang, Q.; Guo, M.; Yang, Y.; Wang, S.; Xie, L.; Lou, H.; et al. Diurnal RNAPII-Tethered Chromatin Interactions Are Associated with Rhythmic Gene Expression in Rice. Genome Biol. 2022, 23, 7. [Google Scholar] [CrossRef]
- Matos, D.A.; Cole, B.J.; Whitney, I.P.; MacKinnon, K.J.-M.; Kay, S.A.; Hazen, S.P. Daily Changes in Temperature, Not the Circadian Clock, Regulate Growth Rate in Brachypodium Distachyon. PLoS ONE 2014, 9, e100072. [Google Scholar] [CrossRef]
- Swift, J.; Greenham, K.; Ecker, J.R.; Coruzzi, G.M.; Robertson McClung, C. The Biology of Time: Dynamic Responses of Cell Types to Developmental, Circadian and Environmental Cues. Plant J. 2022, 109, 764–778. [Google Scholar] [CrossRef]
- Hashida, Y.; Tezuka, A.; Nomura, Y.; Kamitani, M.; Kashima, M.; Kurita, Y.; Nagano, A.J. Fillable and Unfillable Gaps in Plant Transcriptome under Field and Controlled Environments. Plant Cell Environ. 2022, 45, 2410–2427. [Google Scholar] [CrossRef] [PubMed]
- Dantas, L.L.B.; Dourado, M.M.; de Lima, N.O.; Cavaçana, N.; Nishiyama, M.Y., Jr.; Souza, G.M.; Carneiro, M.S.; Caldana, C.; Hotta, C.T. Field Microenvironments Regulate Crop Diel Transcript and Metabolite Rhythms. New Phytol. 2021, 232, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T. Around the Circadian Clock: Review and Preview. In Circadian Rhythms in Bacteria and Microbiomes; Springer: Berlin/Heidelberg, Germany, 2021; pp. 21–52. [Google Scholar]
- Emms, D.M.; Kelly, S. OrthoFinder: Solving Fundamental Biases in Whole Genome Comparisons Dramatically Improves Orthogroup Inference Accuracy. Genome Biol. 2015, 16, 157. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A Comparative Platform for Green Plant Genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Cepas, J.; Forslund, K.; Coelho, L.P.; Szklarczyk, D.; Jensen, L.J.; von Mering, C.; Bork, P. Fast Genome-Wide Functional Annotation through Orthology Assignment by eggNOG-Mapper. Mol. Biol. Evol. 2017, 34, 2115–2122. [Google Scholar] [CrossRef]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramírez, F.; Warwick Vesztrocy, A.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A Python Library for Gene Ontology Analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef] [PubMed]




| Leaf 15 d | Leaf 29 d | Leaf 43 d | Leaf 57 d | Leaf 78 d | Leaf 85 d | Leaf 92 d | Leaf 99 d | Leaf 113 d | Root 15 d | Root 43 d | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| total | 27,648 | 27,648 | 27,648 | 27,648 | 27,648 | 27,648 | 27,648 | 27,648 | 27,648 | 27,648 | 27,648 |
| Not expressed (#) | 4735 | 4893 | 4713 | 4945 | 5180 | 5213 | 4552 | 5676 | 4701 | 1637 | 2265 |
| Not Expressed (%) | 17 | 18 | 17 | 18 | 19 | 19 | 16 | 21 | 17 | 6 | 8 |
| Not rhythmic (#) | 7298 | 9687 | 9618 | 5794 | 5473 | 2587 | 4862 | 4322 | 5354 | 23,652 | 24,167 |
| Not rhythmic (%) | 26 | 35 | 35 | 21 | 20 | 9 | 18 | 16 | 19 | 86 | 87 |
| Rhythmic genes (#) | 15,615 | 13,068 | 13,317 | 16,909 | 16,995 | 19,848 | 18,234 | 17,650 | 17,593 | 2359 | 1216 |
| Rhythmic genes (%) | 68 | 57 | 58 | 74 | 76 | 88 | 79 | 80 | 77 | 9 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michael, T.P. Time of Day Analysis over a Field Grown Developmental Time Course in Rice. Plants 2023, 12, 166. https://doi.org/10.3390/plants12010166
Michael TP. Time of Day Analysis over a Field Grown Developmental Time Course in Rice. Plants. 2023; 12(1):166. https://doi.org/10.3390/plants12010166
Chicago/Turabian StyleMichael, Todd P. 2023. "Time of Day Analysis over a Field Grown Developmental Time Course in Rice" Plants 12, no. 1: 166. https://doi.org/10.3390/plants12010166
APA StyleMichael, T. P. (2023). Time of Day Analysis over a Field Grown Developmental Time Course in Rice. Plants, 12(1), 166. https://doi.org/10.3390/plants12010166





