Overlapping Root Architecture and Gene Expression of Nitrogen Transporters for Nitrogen Acquisition of Tomato Plants Colonized with Isolates of Funneliformis mosseae in Hydroponic Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.1.1. Experimental Protocol and Growing Seasons
2.1.2. AMF Material and Inoculation
2.1.3. Growth Conditions of Tomato Plants
2.2. Analysis of Root Biomass, Architecture, Nitrogen Concentration, and Colonization Rates
2.3. Root Sampling and Relative Expression of Transporters
- 5′-CCGCCGCTTCATACATCTGCAA (forward),
- 5′-GCGAAACCAAGCTGCATGGAGA (reverse) for LeAMT1.1,
- 5′-TTCCCTCATCTCGGCAGCTCAG (forward),
- 5′-CCGCGTAGGTGGTGTTTGTGAG (reverse) for LeAMT1.2
- 5′-GGGCTACTACACTTCCTCTGG (forward),
- 5′- CCTCCAGCTCCTGTCATACC (reverse) for LeNRT2.3.
- 5′-TCGTAAGGAGTGCCCTAATGCTGA (forward),
- 5′- CAATCGCCTCCAGCCTTGTTGTAA (reverse) for LeUBI [32]
- 5′-CTCCATTGGGTCGTTTTGCT (forward),
- 5′-GGTCACCTTGGCACCAGTTG (reverse) for LeEF [33]
2.4. Statistical Analysis
3. Results
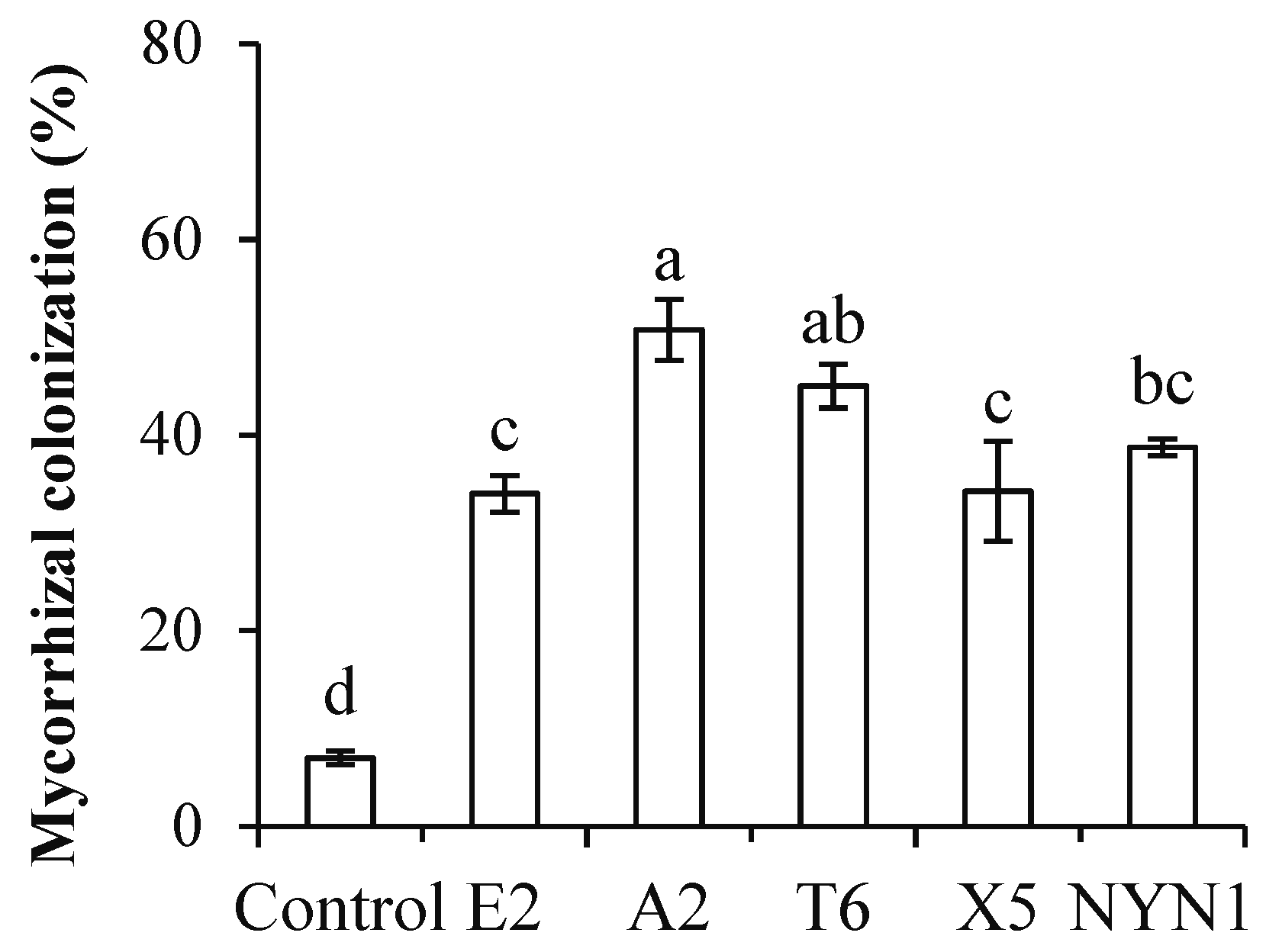
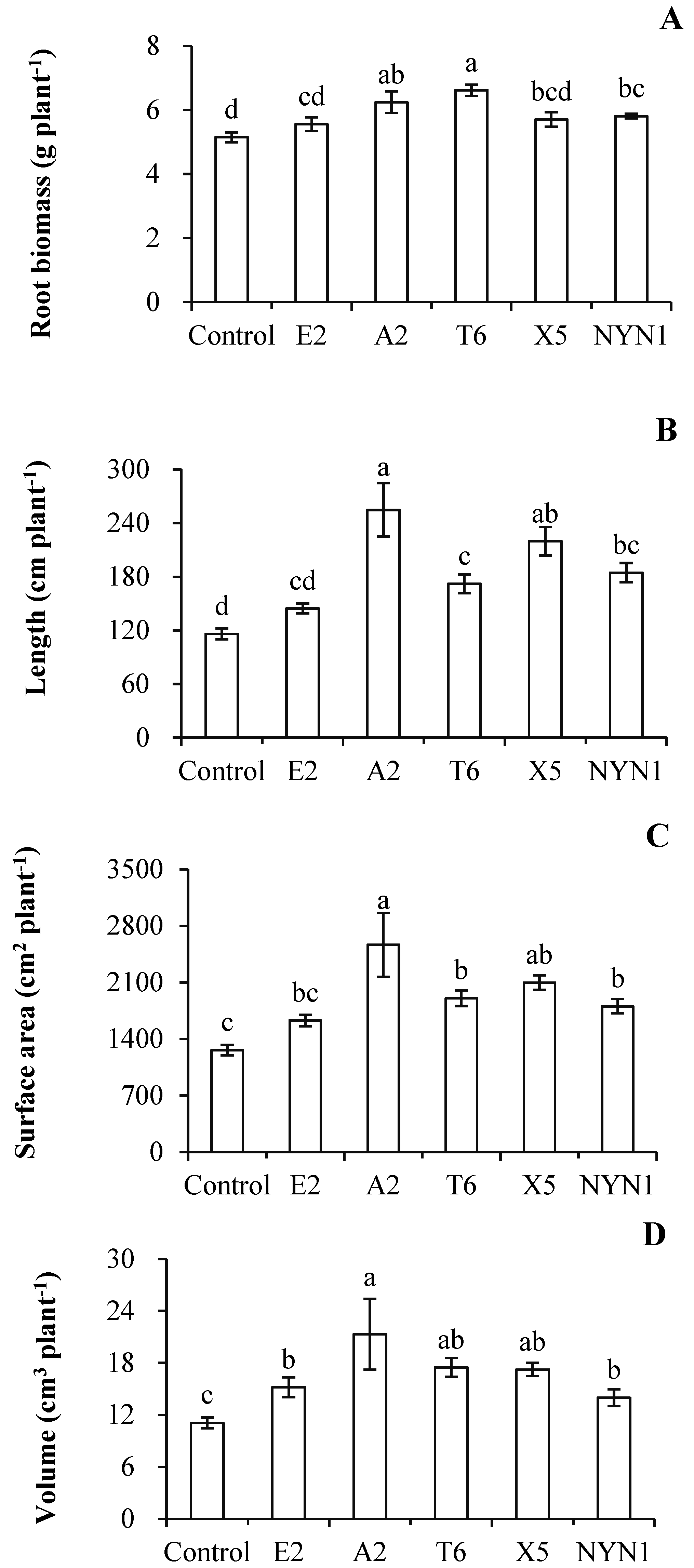
3.1. AMF Colonization and Its Effects on the Root Growth of Tomato Plants
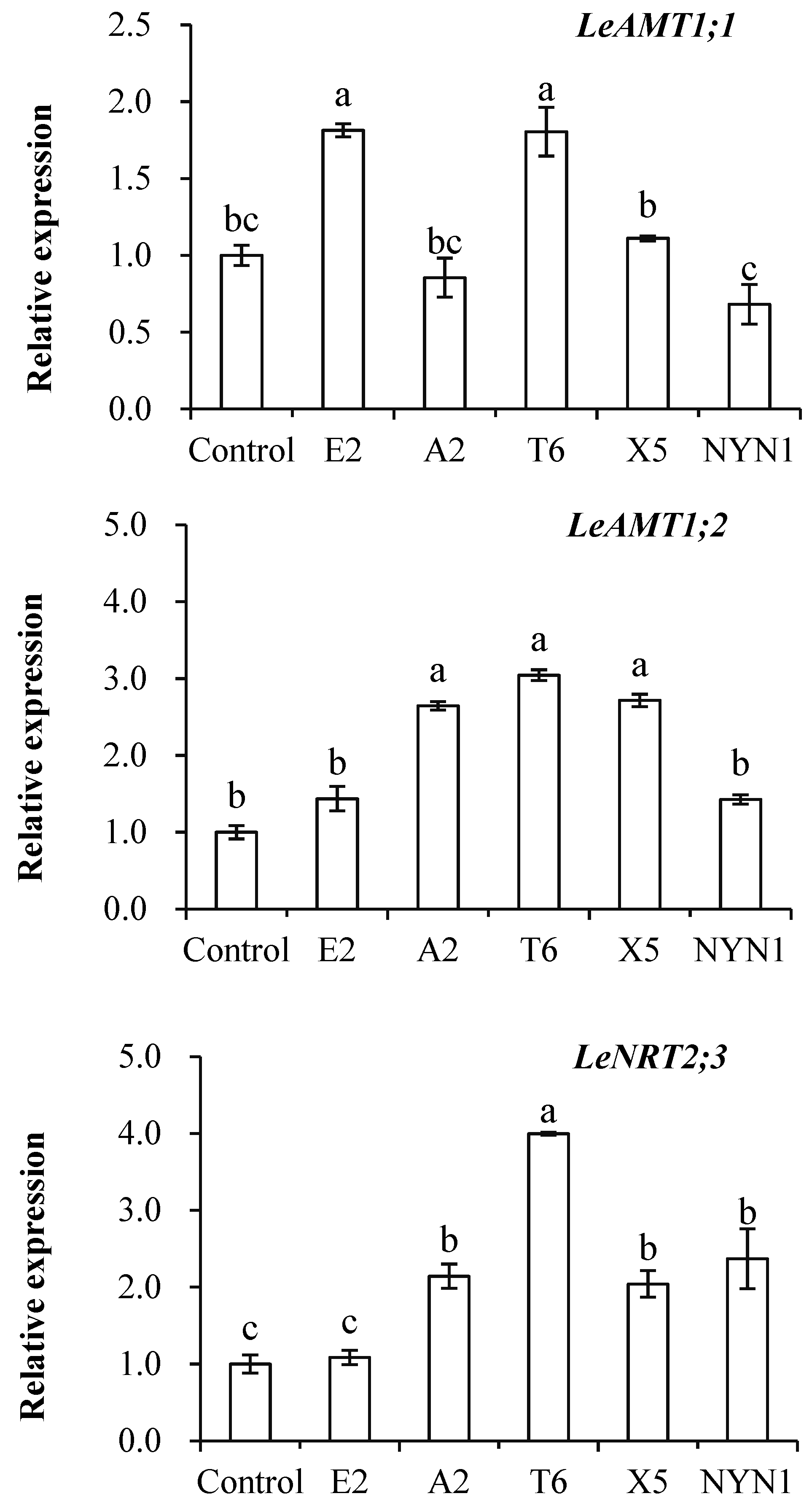
3.2. Effects of AMF on Nitrogen Transporters Expression
3.3. Influence of AMF Inoculation on Nitrogen Acquisition of Plants
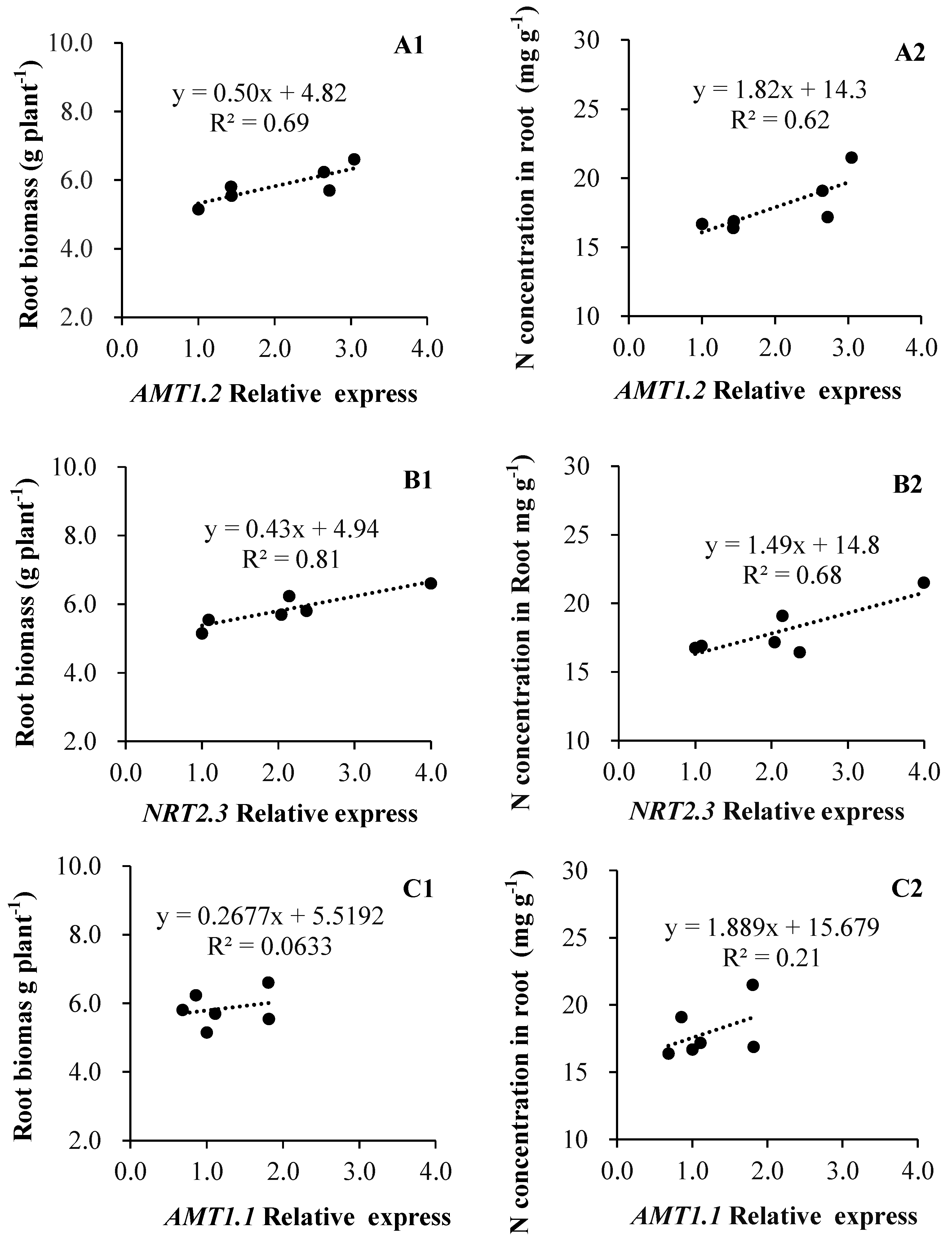
3.4. Nitrogen Transporters Expression Is Co-Related to Root Biomass and N Concentration in Root Tissue
4. Discussion
4.1. Root Growth and N Acquisition of Mycorrhizal Plants
4.2. Transporter Genes LeAMT1.1, LeAMT1.2, and LeNRT2.3 Were Up-Regulated by Inoculation with AMF Isolates
4.3. Overlapping Effects of AMF Upon Root Growth and N Transporters Expression
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maboko, M.M.; Plooy, C.P.D.; Bertling, I. Comparative performance of tomato cultivars cultivated in two hydroponic production systems. S. Afr. J. Plant Soil 2011, 28, 97–102. [Google Scholar] [CrossRef]
- Kowalska, I.; Konieczny, A.; Gastol, M.; Sady, W.; Hanus-Fajerska, E. Effect of mycorrhiza and phosphorus content in nutrient solution on the yield and nutritional status of tomato plants grown on rockwool or coconut coir. Agr. Food Sci. 2015, 24, 39–51. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Ferrol, N.; Pérez-Tienda, J. Coordinated nutrient exchange in arbuscular mycorrhiza. In Mycorrhizas-Functional Processes and Ecological Impact; Springer: Berlin/Heidelberg, Germany, 2009; pp. 73–87. [Google Scholar]
- Hariprasad, P.; Venkateswaran, G.; Niranjana, S.R. Diversity of cultivable rhizobacteria across tomato growing regions of Karnataka. Biol. Control 2014, 72, 9–16. [Google Scholar] [CrossRef]
- Lynch, J. Root architecture and plant productivity. Plant Physiol. 1995, 109, 7–13. [Google Scholar] [CrossRef]
- Cruz, C.; Green, J.J.; Watson, C.A.; Wilson, F.; Martins-Loução, M.A. Functional aspects of root architecture and mycorrhizal inoculation with respect to nutrient uptake capacity. Mycorrhiza 2004, 14, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Berta, G.; Fusconi, A.; Trotta, A.; Scannerini, S. Morphogenetic modifications induced by the mycorrhizal fungus Glomus isolate E3 in the root system of Allium porrum L. New Phytol. 1990, 114, 207–215. [Google Scholar] [CrossRef]
- Schellenbaum, L.; Berta, G.; Ravolanirina, F.; Tisserant, B.; Gianinazzi, S.; Fitter, A.H. Influence of endomycorrhizal infection on root morphology in a micropropagated woody plant species (Vitis vinifera L.). Ann. Bot. 1991, 68, 135–141. [Google Scholar] [CrossRef]
- Ning, Y.; Xiao, Z.; Weinmann, M.; Li, Z. Phosphate uptake is correlated with the root length of celery plants following the association between arbuscular mycorrhizal fungi, Pseudomonas sp. and biochar with different phosphate fertilization levels. Agronomy 2019, 9, 824. [Google Scholar] [CrossRef] [Green Version]
- Amtmann, A.; Armengaud, P. Effects of N, P, K and S on metabolism: New knowledge gained from multi-level analysis. Curr. Opin. Plant Biol. 2009, 12, 275–283. [Google Scholar] [CrossRef]
- Crawford, N.M.; Glass, A.D. Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 1998, 3, 389–395. [Google Scholar] [CrossRef]
- Ludewig, U.; Neuhäuser, B.; Dynowski, M. Molecular mechanisms of ammonium transport and accumulation in plants. FEBS Lett. 2007, 581, 2301–2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraiser, T.; Gras, D.E.; Gutiérrez, A.G.; González, B.; Gutiérrez, R.A. A holistic view of nitrogen acquisition in plants. J. Exp. Bot. 2011, 62, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.K.; Javot, H.; Deewatthanawong, P.; Torres-Jerez, I.; Tang, Y.; Blancaflor, E.B.; Udvardi, M.K.; Harrison, M.J. Medicago truncatula and Glomus intraradices gene expression in cortical cells harboring arbuscules in the arbuscular mycorrhizal symbiosis. BMC Plant Biol. 2009, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobae, Y.; Tamura, Y.; Takai, S.; Banba, M.; Hata, S. Localized expression of arbuscular mycorrhiza-inducible ammonium transporters in soybean. Plant Cell Physiol. 2010, 51, 1411–1415. [Google Scholar] [CrossRef]
- Koegel, S.; Lahmidi, N.A.; Arnould, C.; Chatagnier, O.; Walder, F.; Ineichen, K.; Boller, T.; Wipf, D.; Wiemken, A.; Courty, P.E. The family of ammonium transporters (AMT) in Sorghum bicolor: Two AMT members are induced locally, but not systemically in roots colonized by arbuscular mycorrhizal fungi. New Phytol. 2013, 198, 853–865. [Google Scholar] [CrossRef]
- von Wittgenstein, N.J.; Le, C.H.; Hawkins, B.J.; Ehlting, J. Evolutionary classification of ammonium, nitrate, and peptide transporters in land plants. BMC Evol. Biol. 2014, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Lauter, F.R.; Ninnemann, O.; Bucher, M.; Riesmeier, J.W.; Frommer, W.B. Preferential expression of an ammonium transporter and of two putative nitrate transporters in root hairs of tomato. Proc. Natl. Acad. Sci. USA 1996, 93, 8139–8144. [Google Scholar] [CrossRef] [Green Version]
- Von Wirén, N.; Gazzarrini, S.; Gojon, A.; Frommer, W.B. The molecular physiology of ammonium uptake and retrieval. Curr. Opin. Plant Biol. 2000, 3, 254–261. [Google Scholar] [CrossRef]
- Becker, D.; Stanke, R.; Fendrik, I.; Frommer, W.B.; Hedrich, R. Expression of the nh4+-transporter gene leAMT1.2 is induced in tomato roots upon association with N2-fixing bacteria. Planta 2002, 215, 424–429. [Google Scholar] [CrossRef]
- Guether, M.; Neuhäuser, B.; Balestrini, R.; Dynowski, M.; Ludewig, U.; Bonfante, P. A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol. 2009, 150, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Tienda, J.; Corrêa, A.; Azcón-Aguilar, C.; Ferrol, N. Transcriptional regulation of host nh transporters and gs/gogat pathway in arbuscular mycorrhizal rice roots. Plant Physiol. Biochem. 2014, 75, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hohnjec, N.; Vieweg, M.F.; Pühler, A.; Becker, A.; Küster, H. Overlaps in the transcriptional profiles of Medicago truncatula roots inoculated with two different Glomus fungi provide insights into the genetic program activated during arbuscular mycorrhiza. Plant Physiol. 2005, 137, 1283–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Chen, A.; Xie, K.; Yang, X.; Luo, Z.; Chen, J.; Zeng, D.; Ren, Y.; Yang, C.; Wang, L. Functional analysis of the OsNPF4. 5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 16649–16659. [Google Scholar] [CrossRef]
- Fu, Y.; Yi, H.; Bao, J.; Gong, J. LeNRT2.3 functions in nitrate acquisition and long-distance transport in tomato. FEBS Lett. 2015, 589, 1072–1079. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, U.; Schmelzer, E.; Bothe, H. Expression of nitrate transporter genes in tomato colonized by an arbuscular mycorrhizal fungus. Physiol. Plant. 2002, 115, 125–136. [Google Scholar] [CrossRef]
- Ngwene, B.; Gabriel, E.; George, E. Influence of different mineral nitrogen sources (NO3− -N vs. NH4+− -N) on arbuscular mycorrhiza development and N transfer in a Glomus intraradices cowpea symbiosis. Mycorrhiza 2013, 23, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Huang, Z.; Zhang, Y.; Rui, W.; Lei, X.; Li, Z. Beneficial effects of the five isolates of Funneliformis mosseae on the tomato plants were not related to their evolutionary distances of SSU rDNA or PT1 sequences in the nutrition solution production. Plants 2021, 10, 1948. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn 1950, 347, 357–359. [Google Scholar] [CrossRef]
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- Mascia, T.; Santovito, E.; Gallitelli, D.; Cillo, F. Evaluation of reference genes for quantitative reverse-transcription polymerase chain reaction normalization in infected tomato plants. Mol. Plant Pathol. 2010, 11, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Ca Scone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Yamauchi, A.; Kono, Y. Localized alteration in lateral root development in roots colonized by an arbuscular mycorrhizal fungus. Mycorrhiza 1996, 6, 409–415. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, L.R.; Zhu, H.H.; Chen, J.Z. Effect of arbuscular mycorrhizal fungal inoculation on root system architecture of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings. Sci. Hortic. 2009, 121, 458–461. [Google Scholar] [CrossRef]
- Padilla, I.M.G.; Encina, C.L. Changes in root morphology accompanying mycorrhizal alleviation of phosphorus deficiency in micropropagated Annona cherimola Mill. plants. Sci. Hortic. 2005, 106, 360–369. [Google Scholar] [CrossRef]
- Kaldorf, M.; Ludwig-Müller, J. AMF might affect the root morphology of maize by increasing indole-3-butyric acid biosynthesis. Physiol. Plant. 2000, 109, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Wiersum, L.K. Density of root branching as affected by substrate and separate ions. Acta Bot. Neerl. 1958, 7, 174–190. [Google Scholar] [CrossRef]
- Fan, X.; Naz, M.; Fan, X.; Xuan, W.; Miller, A.J.; Xu, G. Plant nitrate transporters: From gene function to application. J. Exp. Bot. 2017, 68, 2463–2475. [Google Scholar] [CrossRef]
- Saia, S.; Rappa, V.; Ruisi, P.; Abenavoli, M.R.; Sunseri, F.; Giambalvo, D.; Frenda, A.S.; Martinelli, F. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant Sci. 2015, 6, 815. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Garvin, D.H.; Kochian, L.V. Nitrate-induced genes in tomato roots: Array analysis reveals novel genes that may play a role in nitrogen nutrition. Plant Physiol. 2001, 127, 345–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filiz, E.; Akbuddak, M.A. Ammonium transporter 1 (AMT1) gene family in tomato (Solanum lycopersicum L.): Bioinformatics, physiological and expression analyses under drought and salt stresses. Genomics 2020, 112, 3773–3782. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, F.; Gebauer, M.; Ponce, C.; Cabeza, R.A. LeNRT1.1 improves nitrate uptake in grafted tomato plants under high nitrogen demand. Int. J. Mol. Sci. 2018, 19, 3921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansen, A.; Jakobsen, I.; Jensen, E.S. External hyphae of vesicular–arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 3. Hyphal transport of 32P and 15N. New Phytol. 1993, 124, 61–68. [Google Scholar] [CrossRef]
- Ramírez-Flores, M.; Bello-Bello, E.; Rellán-Álvarez, R.; Sawers, R.J.H.; Olalde-Portugal, V. Inoculation with the mycorrhizal fungus Rhizophagus irregulars increases nutrient uptake in maize (Zea mays) through hyphal foraging and promotion of root growth. Plant Direct 2019, 3, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Lv, W.; Xu, J.; Huang, Z.; Rui, W.; Lei, X.; Ju, X.; Li, Z. Overlapping Root Architecture and Gene Expression of Nitrogen Transporters for Nitrogen Acquisition of Tomato Plants Colonized with Isolates of Funneliformis mosseae in Hydroponic Production. Plants 2022, 11, 1176. https://doi.org/10.3390/plants11091176
Feng J, Lv W, Xu J, Huang Z, Rui W, Lei X, Ju X, Li Z. Overlapping Root Architecture and Gene Expression of Nitrogen Transporters for Nitrogen Acquisition of Tomato Plants Colonized with Isolates of Funneliformis mosseae in Hydroponic Production. Plants. 2022; 11(9):1176. https://doi.org/10.3390/plants11091176
Chicago/Turabian StyleFeng, Jingyu, Weixing Lv, Jing Xu, Zhe Huang, Wenjing Rui, Xihong Lei, Xuehai Ju, and Zhifang Li. 2022. "Overlapping Root Architecture and Gene Expression of Nitrogen Transporters for Nitrogen Acquisition of Tomato Plants Colonized with Isolates of Funneliformis mosseae in Hydroponic Production" Plants 11, no. 9: 1176. https://doi.org/10.3390/plants11091176
APA StyleFeng, J., Lv, W., Xu, J., Huang, Z., Rui, W., Lei, X., Ju, X., & Li, Z. (2022). Overlapping Root Architecture and Gene Expression of Nitrogen Transporters for Nitrogen Acquisition of Tomato Plants Colonized with Isolates of Funneliformis mosseae in Hydroponic Production. Plants, 11(9), 1176. https://doi.org/10.3390/plants11091176





