In Dormant Red Rice Seeds, the Inhibition of Early Seedling Growth, but Not of Germination, Requires Extracellular ABA
Abstract
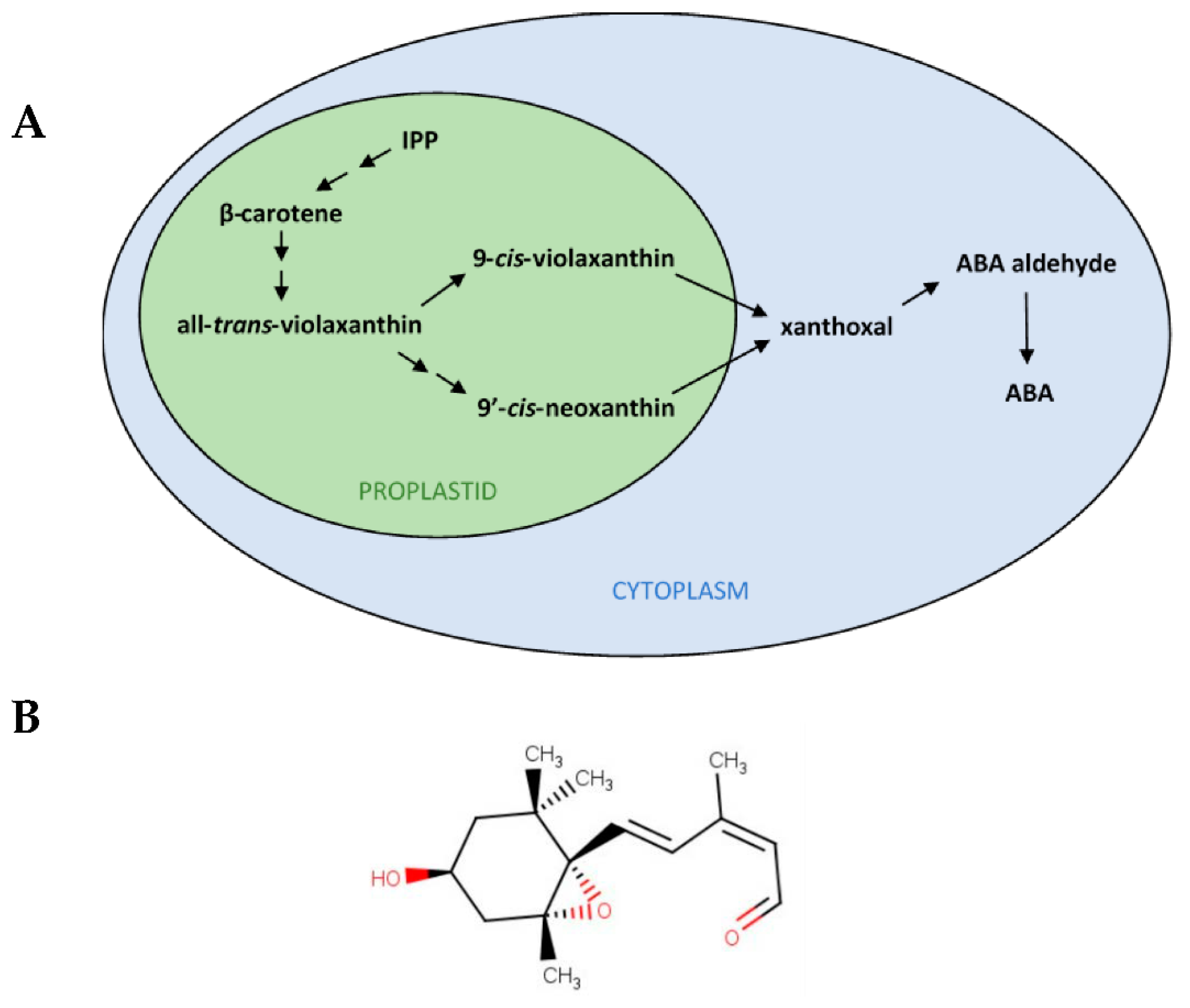
1. Introduction
2. Results and Discussion
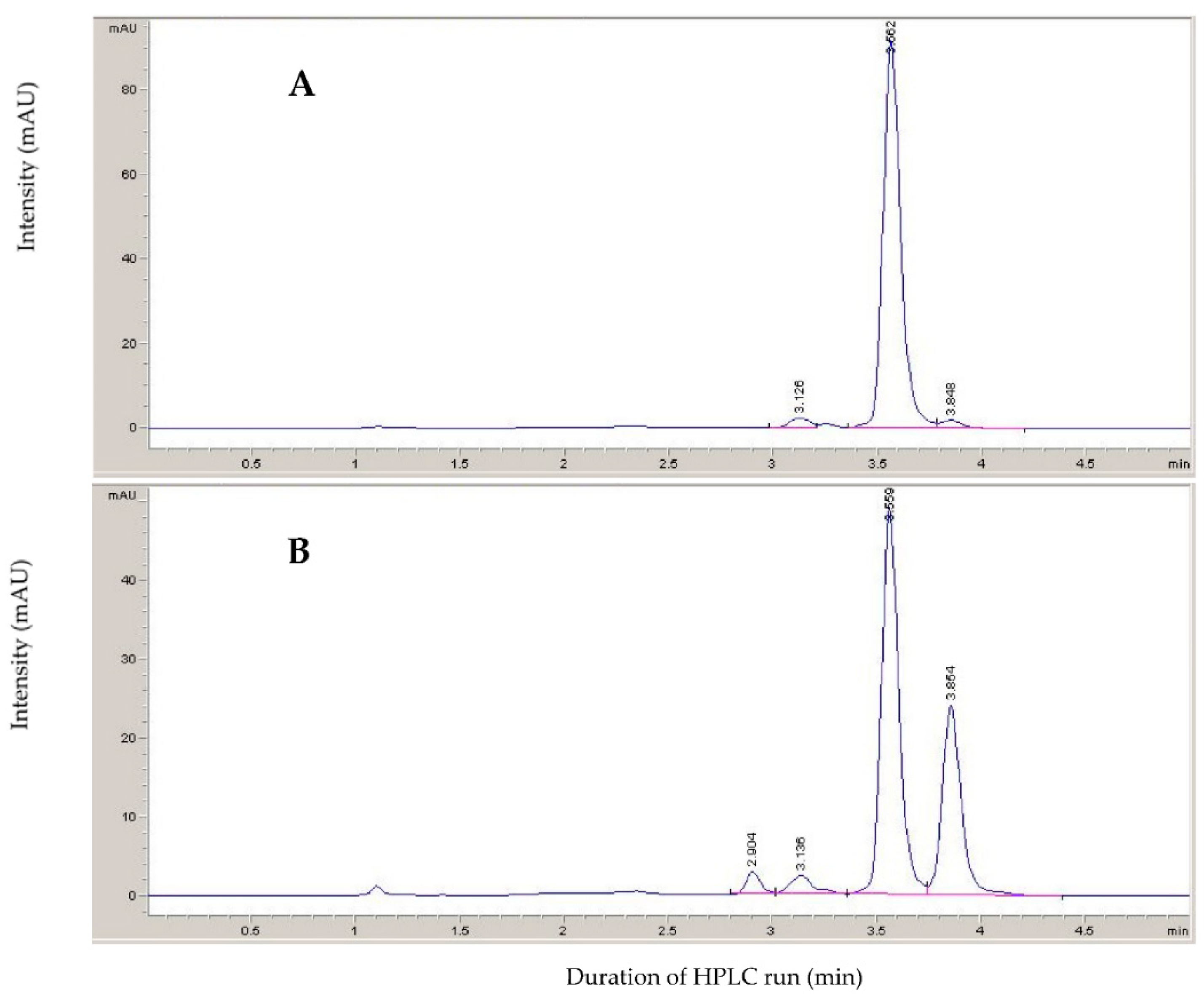
2.1. Xanthoxal Characterization
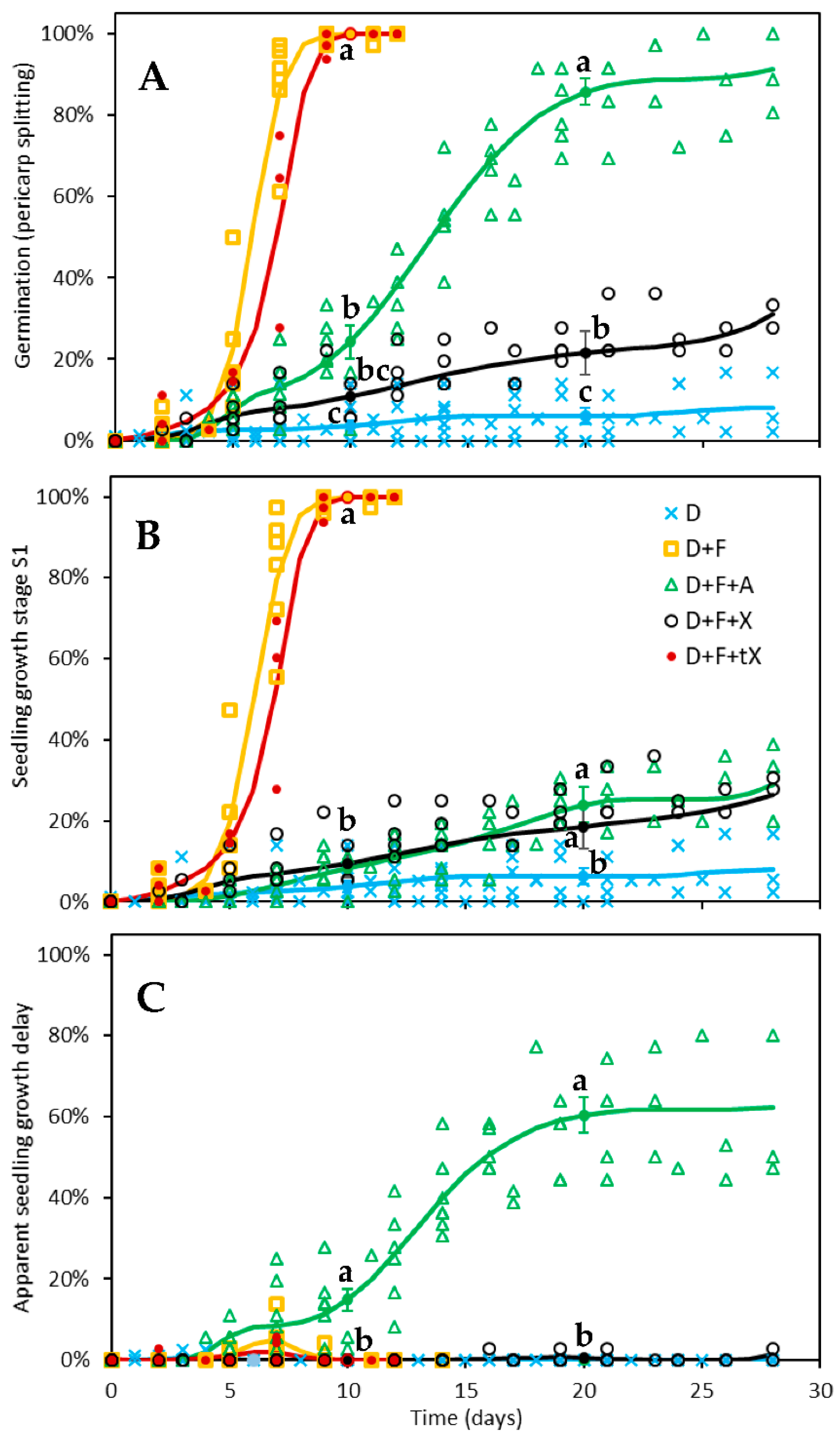
2.2. Germination Test
3. Materials and Methods
3.1. Chemicals
3.2. Xanthoxal Production
3.2.1. Extraction of Xanthophylls
3.2.2. Liquid-Liquid Partition of Xanthophylls
3.2.3. Purification of Violaxanthin and Neoxanthin with Solid-Phase Extraction
3.2.4. Production of Xanthoxal by Oxidation of Epoxydic Xanthophylls
3.2.5. Purification of Xanthoxal with Reversed-Phase SPE
3.2.6. Photoisomerization
3.2.7. Final Purification of Xanthoxal with Normal-Phase SPE
3.3. Chemical Analyses
3.3.1. HPLC Analyses
3.3.2. Mass Spectrometry
3.4. Chemical Editor
3.5. Seed Materials and Experimental Setup
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Statistical Details and Notes
| /* conditional model with continuous time */ proc GLIMMIX method = Laplace; effect spltime = spline(day); class rep exp trt; model VAR/n = trt trt * spltime/link = probit; random trt * exp; random rep(trt * exp)/type = AR(1); lsmeans trt/at day = 10 adjust = sidak stepdown ilink; lsmeans trt/at day = 20 adjust = sidak stepdown ilink; lsmestimate trt ‘D vs. D + F + A’ 1 0 −1 0 0, ‘D vs. D + F + X’ 1 0 0 −1 0, ‘D + F + A vs. D + F + X’ 0 0 1 −1 0/ at day = 20 adjust = sidak stepdown elsm; run; |
| Type III Tests of Fixed Effects | ||||
|---|---|---|---|---|
| Effect | Num DF | Den DF | F Value | Pr > F |
| trt | 4 | 726 | 0.72 | 0.5802 |
| spltime*trt | 26 | 726 | 49.47 | <0.0001 |
| Type III Tests of Fixed Effects | ||||
|---|---|---|---|---|
| Effect | Num DF | Den DF | F Value | Pr > F |
| trt | 4 | 726 | 0.37 | 0.8305 |
| spltime*trt | 26 | 726 | 27.63 | <0.0001 |
| Type III Tests of Fixed Effects | ||||
|---|---|---|---|---|
| Effect | Num DF | Den DF | F Value | Pr > F |
| trt | 4 | 726 | 0.11 | 0.9796 |
| spltime*trt | 23 | 726 | 18.61 | <0.0001 |
References
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-4692-7. [Google Scholar]
- Ziska, L.H.; Gealy, D.R.; Burgos, N.; Caicedo, A.L.; Gressel, J.; Lawton-Rauh, A.L.; Avila, L.A.; Theisen, G.; Norsworthy, J.; Ferrero, A.; et al. Weedy (Red) Rice: An Emerging Constraint to Global Rice Production. Adv. Agron. 2015, 129, 181–228. [Google Scholar] [CrossRef]
- Cohn, M.A. Chemical Mechanisms of Breaking Seed Dormancy. Seed Sci. Res. 1996, 6, 95–99. [Google Scholar] [CrossRef]
- Chao, W.S.; Horvath, D.P.; Anderson, J.V.; Foley, M.E. Potential Model Weeds to Study Genomics, Ecology, and Physiology in the 21st Century. Weed Sci. 2005, 53, 929–937. [Google Scholar] [CrossRef]
- Liu, X.; Hou, X. Antagonistic Regulation of ABA and GA in Metabolism and Signaling Pathways. Front. Plant Sci. 2018, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.; Bressan, R.A.; Song, C.; Zhu, J.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, P.; Bajracharya, D.; Plachy, C. Control of Seed Germination by Abscisic Acid: I. Time Course of Action in Sinapis alba L. Plant Physiol. 1979, 64, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Molina, L.; Mongrand, S.; Chua, N.-H. A Postgermination Developmental Arrest Checkpoint Is Mediated by Abscisic Acid and Requires the ABI5 Transcription Factor in Arabidopsis. Proc. Natl. Acad. Sci. USA 2001, 98, 4782–4787. [Google Scholar] [CrossRef]
- Koornneef, M.; Jorna, M.L.; Brinkhorst-van der Swan, D.L.C.; Karssen, C.M. The Isolation of Abscisic Acid (ABA) Deficient Mutants by Selection of Induced Revertants in Non-Germinating Gibberellin Sensitive Lines of Arabidopsis thaliana (L.) Heynh. Theor. Appl. Genet. 1982, 61, 385–393. [Google Scholar] [CrossRef]
- Frey, A.; Effroy, D.; Lefebvre, V.; Seo, M.; Perreau, F.; Berger, A.; Sechet, J.; To, A.; North, H.M.; Marion-Poll, A. Epoxycarotenoid Cleavage by NCED5 Fine-Tunes ABA Accumulation and Affects Seed Dormancy and Drought Tolerance with Other NCED Family Members. Plant J. 2012, 70, 501–512. [Google Scholar] [CrossRef]
- Groot, S.P.C.; Karssen, C.M. Dormancy and Germination of Abscisic Acid-Deficient Tomato Seeds: Studies with the Sitiens Mutant. Plant Physiol. 1992, 99, 952–958. [Google Scholar] [CrossRef]
- Marin, E.; Nussaume, L.; Quesada, A.; Gonneau, M.; Sotta, B.; Hugueney, P.; Frey, A.; Marion-Poll, A. Molecular Identification of Zeaxanthin Epoxidase of Nicotiana plumbaginifolia, a Gene Involved in Abscisic Acid Biosynthesis and Corresponding to the ABA Locus of Arabidopsis thaliana. EMBO J. 1996, 15, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Heimovaara-Dijkstra, S.; Van Duijn, B. Modulation of Germination of Embryos Isolated from Dormant and Nondormant Barley Grains by Manipulation of Endogenous Abscisic Acid. Planta 1995, 195, 586–592. [Google Scholar] [CrossRef]
- Gianinetti, A.; Vernieri, P. On the Role of Abscisic Acid in Seed Dormancy of Red Rice. J. Exp. Bot. 2007, 58, 3449–3462. [Google Scholar] [CrossRef] [PubMed]
- Goggin, D.E.; Steadman, K.J.; Emery, R.J.N.; Farrow, S.C.; Benech-Arnold, R.L.; Powles, S.B. ABA Inhibits Germination but Not Dormancy Release in Mature Imbibed Seeds of Lolium rigidum Gaud. J. Exp. Bot. 2009, 60, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Gianinetti, A.; Finocchiaro, F.; Bagnaresi, P.; Zechini, A.; Faccioli, P.; Cattivelli, L.; Valè, G.; Biselli, C. Seed Dormancy Involves a Transcriptional Program That Supports Early Plastid Functionality during Imbibition. Plants 2018, 7, 35. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the Mark: Early Seed Germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef]
- Millar, A.A.; Jacobsen, J.V.; Ross, J.J.; Helliwell, C.A.; Poole, A.T.; Scofield, G.; Reid, J.B.; Gubler, F. Seed Dormancy and ABA Metabolism in Arabidopsis and Barley: The Role of ABA 8′-Hydroxylase. Plant J. 2006, 45, 942–954. [Google Scholar] [CrossRef]
- Liu, A.; Gao, F.; Kanno, Y.; Jordan, M.C.; Kamiya, Y.; Seo, M.; Ayele, B.T. Regulation of Wheat Seed Dormancy by After-Ripening Is Mediated by Specific Transcriptional Switches That Induce Changes in Seed Hormone Metabolism and Signaling. PLoS ONE 2013, 8, e56570. [Google Scholar] [CrossRef]
- Carrera, E.; Holman, T.; Medhurst, A.; Dietrich, D.; Footitt, S.; Theodoulou, F.L.; Holdsworth, M.J. Seed After-Ripening Is a Discrete Developmental Pathway Associated with Specific Gene Networks in Arabidopsis. Plant J. 2008, 53, 214–224. [Google Scholar] [CrossRef]
- Chibani, K.; Ali-Rachedi, S.; Job, C.; Job, D.; Jullien, M.; Grappin, P. Proteomic Analysis of Seed Dormancy in Arabidopsis. Plant Physiol. 2006, 142, 1493–1510. [Google Scholar] [CrossRef]
- Matusova, R.; Rani, K.; Verstappen, F.W.A.; Franssen, M.C.R.; Beale, M.H.; Bouwmeester, H.J. The Strigolactone Germination Stimulants of the Plant-Parasitic Striga and Orobanche spp. Are Derived from the Carotenoid Pathway. Plant Physiol. 2005, 139, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Ali-Rachedi, S.; Bouinot, D.; Wagner, M.-H.; Bonnet, M.; Sotta, B.; Grappin, P.; Jullien, M. Changes in Endogenous Abscisic Acid Levels during Dormancy Release and Maintenance of Mature Seeds: Studies with the Cape Verde Islands Ecotype, the Dormant Model of Arabidopsis thaliana. Planta 2004, 219, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Leymarie, J.; Benech-Arnold, R.L.; Farrant, J.M.; Corbineau, F. Thermodormancy and ABA Metabolism in Barley Grains. Plant Signal. Behav. 2009, 4, 205–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alvarado, V.; Bradford, K.J. Hydrothermal Time Analysis of Seed Dormancy in True (Botanical) Potato Seeds. Seed Sci. Res. 2005, 15, 77–88. [Google Scholar] [CrossRef]
- Grappin, P.; Bouinot, D.; Sotta, B.; Miginiac, E.; Jullien, M. Control of Seed Dormancy in Nicotiana plumbaginifolia: Post-Imbibition Abscisic Acid Synthesis Imposes Dormancy Maintenance. Planta 2000, 210, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.H.; Yoneyama, K.; Takeuchi, Y.; Joel, D.M. Fluridone and Norflurazon, Carotenoid-Biosynthesis Inhibitors, Promote Seed Conditioning and Germination of the Holoparasite Orobanche minor. Physiol. Plant 2004, 120, 328–337. [Google Scholar] [CrossRef]
- Awan, S.Z.; Chandler, J.O.; Harrison, P.J.; Sergeant, M.J.; Bugg, T.D.H.; Thompson, A.J. Promotion of Germination Using Hydroxamic Acid Inhibitors of 9-Cis-Epoxycarotenoid Dioxygenase. Front. Plant Sci. 2017, 8, 357. [Google Scholar] [CrossRef]
- Erb, M.; Glauser, G. Family Business: Multiple Members of Major Phytohormone Classes Orchestrate Plant Stress Responses. Chem. Eur. J. 2010, 16, 10280–10289. [Google Scholar] [CrossRef]
- Kepka, M.; Benson, C.L.; Gonugunta, V.K.; Nelson, K.M.; Christmann, A.; Grill, E.; Abrams, S.R. Action of Natural Abscisic Acid Precursors and Catabolites on Abscisic Acid Receptor Complexes. Plant Physiol. 2011, 157, 2108–2119. [Google Scholar] [CrossRef]
- Taylor, H.F.; Burden, R.S. Xanthoxin, a Recently Discovered Plant Growth Inhibitor. Proc. R. Soc. Lond. B Biol. Sci. 1972, 180, 317–346. [Google Scholar] [CrossRef]
- Milborrow, B.V.; Burden, R.S.; Taylor, H.F. Xanthoxal: A Revision of the Nomenclature of the ABA Precursor Xanthoxin. Phytochemistry 1997, 44, 977–978. [Google Scholar] [CrossRef]
- Xia, Q.; Ponnaiah, M.; Thanikathansubramanian, K.; Corbineau, F.; Bailly, C.; Nambara, E.; Meimoun, P.; El-Maarouf-Bouteau, H. Re-Localization of Hormone Effectors Is Associated with Dormancy Alleviation by Temperature and after-Ripening in Sunflower Seeds. Sci. Rep. 2019, 9, 4861. [Google Scholar] [CrossRef]
- Laspina, N.V.; Batlla, D.; Benech-Arnold, R.L. Dormancy Cycling Is Accompanied by Changes in ABA Sensitivity in Polygonum Aviculare Seeds. J. Exp. Bot. 2020, 71, 5924–5934. [Google Scholar] [CrossRef] [PubMed]
- Zeevaart, J.A.D. Levels of (+)-Abscisic Acid and Xanthoxin in Spinach under Different Environmental Conditions. Plant Physiol. 1974, 53, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Häckl, K.; Kunz, W. Some Aspects of Green Solvents. Comptes Rendus Chim. 2018, 21, 572–580. [Google Scholar] [CrossRef]
- Parry, A.D.; Babiano, M.J.; Horgan, R. The Role of Cis-Carotenoids in Abscisic Acid Biosynthesis. Planta 1990, 182, 118–128. [Google Scholar] [CrossRef]
- Hernandez-Marin, E.; Galano, A.; Martínez, A. Cis Carotenoids: Colorful Molecules and Free Radical Quenchers. J. Phys. Chem. B 2013, 117, 4050–4061. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Xie, Q.; Gong, Z. Abscisic Acid. In Hormone Metabolism and Signaling in Plants; Li, J., Li, C., Smith, S.M., Eds.; Academic Press: London, UK, 2017; pp. 161–202. ISBN 978-0-12-811562-6. [Google Scholar]
- Bruggeman, F.J.; Libbenga, K.R.; Duijn, B. The Diffusive Transport of Gibberellins and Abscisic Acid through the Aleurone Layer of Germinating Barley Grain: A Mathematical Model. Planta 2001, 214, 89–96. [Google Scholar] [CrossRef]
- Boursiac, Y.; Léran, S.; Corratgé-Faillie, C.; Gojon, A.; Krouk, G.; Lacombe, B. ABA Transport and Transporters. Trends Plant Sci. 2013, 18, 325–333. [Google Scholar] [CrossRef]
- Park, J.; Lee, Y.; Martinoia, E.; Geisler, M. Plant Hormone Transporters: What We Know and What We Would like to Know. BMC Biol. 2017, 15, 93. [Google Scholar] [CrossRef]
- Kang, J.; Yim, S.; Choi, H.; Kim, A.; Lee, K.P.; Lopez-Molina, L.; Martinoia, E.; Lee, Y. Abscisic Acid Transporters Cooperate to Control Seed Germination. Nat. Commun. 2015, 6, 8113. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Allelopathic Substances in Pueraria thunbergiana. Phytochemistry 2003, 63, 577–580. [Google Scholar] [CrossRef]
- Kang, J.; Lee, Y.; Martinoia, E. How Can We Interpret the Large Number and Diversity of ABA Transporters? In Progress in Botany; Cánovas, F.M., Lüttge, U., Risueño, M.-C., Pretzsch, H., Eds.; Springer International Publishing: Cham, Switzerland, 2020; Volume 82, pp. 233–257. ISBN 978-3-030-68619-2. [Google Scholar]
- Todoroki, Y. ABA and Its Derivatives: Chemistry and Physiological Functions. In Abscisic Acid: Metabolism, Transport and Signaling; Zhang, D.-P., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–20. ISBN 978-94-017-9423-7. [Google Scholar]
- Parry, A.D.; Neill, S.J.; Horgan, R. Xanthoxin Levels and Metabolism in the Wild-Type and Wilty Mutants of Tomato. Planta 1988, 173, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Gianinetti, A. Anomalous Germination of Dormant Dehulled Red Rice Seeds Provides a New Perspective to Study the Transition from Dormancy to Germination and to Unravel the Role of the Caryopsis Coat in Seed Dormancy. Seed Sci. Res. 2016, 26, 124–138. [Google Scholar] [CrossRef][Green Version]
- Pawela, A.; Banasiak, J.; Biała, W.; Martinoia, E.; Jasiński, M. Mt ABCG 20 Is an ABA Exporter Influencing Root Morphology and Seed Germination of Medicago truncatula. Plant J. 2019, 98, 511–523. [Google Scholar] [CrossRef]
- Srivastava, L.M. Plant Growth and Development: Hormones and Environment; Academic Press: Boston, MA, USA, 2002; ISBN 978-0-12-660570-9. [Google Scholar]
- Dörffling, K. The Possible Rôle of Xanthoxin in Plant Growth and Development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1978, 284, 499–507. [Google Scholar] [CrossRef]
- Ruiz-Partida, R.; Rosario, S.M.; Lozano-Juste, J. An Update on Crop ABA Receptors. Plants 2021, 10, 1087. [Google Scholar] [CrossRef]
- Wang, X.-F.; Zhang, D.-P. ABA Signal Perception and ABA Receptors. In Abscisic Acid: Metabolism, Transport and Signaling; Zhang, D.-P., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 89–116. ISBN 978-94-017-9423-7. [Google Scholar]
- Finkelstein, R.R.; Lynch, T.J. Abscisic Acid Inhibition of Radicle Emergence but Not Seedling Growth Is Suppressed by Sugars. Plant Physiol. 2000, 122, 1179–1186. [Google Scholar] [CrossRef]
- Katayama, H.; Miyahara, M. Liquid−liquid Phase Equilibria of (Ethanol or Methanol + Water) Containing Either Dipotassium Hydrogen Phosphate or Sodium Dihydrogen Phosphate. J. Chem. Eng. Data 2006, 51, 914–918. [Google Scholar] [CrossRef]
- Schertz, F.M. The Extraction and Separation of Chlorophyll (A+β) Carotin and Xanthophyll in Fresh Green Leaves, Preliminary to Their Quantitative Determination. Plant Physiol. 1928, 3, 211–216. [Google Scholar] [CrossRef]
- Bochet, C.G. Photochemical Activation of Functional Groups. Adv. Org. Synth. 2005, 1, 3–23. [Google Scholar] [CrossRef]
- Craft, N.E. Chromatographic Techniques for Carotenoid Separation. Curr. Protoc. Food Anal. Chem. 2001, 00, F2.3.1–F2.3.15. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Tan, B.C.; Gage, D.A.; Zeevaart, J.A.D.; McCarty, D.R. Specific Oxidative Cleavage of Carotenoids by VP14 of Maize. Science 1997, 276, 1872–1874. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. Relationship between the Colour and the Chemical Structure of Carotenoid Pigments. Food Chem. 2007, 101, 1145–1150. [Google Scholar] [CrossRef]
- Gianinetti, A.; Cohn, M.A. Seed Dormancy in Red Rice. XII. Population-Based Analysis of Dry-Afterripening with a Hydrotime Model. Seed Sci. Res. 2007, 17, 253–271. [Google Scholar] [CrossRef]
- Ghotbzadeh, S.; Gianinetti, A. A Response of the Imbibed Dormant Red Rice Caryopsis to Biotic Challenges Involves Extracellular PH Increase to Elicit Superoxide Production. Seed Sci. Res. 2018, 28, 261–271. [Google Scholar] [CrossRef]
- Coyle, J.D. Introduction to Organic Photochemistry; Wiley: Chichester, UK, 1986; ISBN 978-0-471-90974-3. [Google Scholar]
- Zeeshan, M.; Sliwka, H.-R.; Partali, V.; Martínez, A. Electron Uptake by Classical Electron Donators: Astaxanthin and Carotenoid Aldehydes. Tetrahedron Lett. 2012, 53, 4522–4525. [Google Scholar] [CrossRef]
- Denisov, E.T.; Afanasʹev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; Taylor & Francis: Boca Raton, FL, USA, 2005; ISBN 978-0-8247-5356-6. [Google Scholar]
- Counce, P.A.; Keisling, T.C.; Mitchell, A.J. A Uniform, Objective, and Adaptive System for Expressing Rice Development. Crop. Sci. 2000, 40, 436. [Google Scholar] [CrossRef]
- Gianinetti, A.; Laarhoven, L.J.J.; Persijn, S.T.; Harren, F.J.M.; Petruzzelli, L. Ethylene Production Is Associated with Germination but Not Seed Dormancy in Red Rice. Ann. Bot. 2007, 99, 735–745. [Google Scholar] [CrossRef]
- Gianinetti, A. Basic Features of the Analysis of Germination Data with Generalized Linear Mixed Models. Data 2020, 5, 6. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianinetti, A. In Dormant Red Rice Seeds, the Inhibition of Early Seedling Growth, but Not of Germination, Requires Extracellular ABA. Plants 2022, 11, 1023. https://doi.org/10.3390/plants11081023
Gianinetti A. In Dormant Red Rice Seeds, the Inhibition of Early Seedling Growth, but Not of Germination, Requires Extracellular ABA. Plants. 2022; 11(8):1023. https://doi.org/10.3390/plants11081023
Chicago/Turabian StyleGianinetti, Alberto. 2022. "In Dormant Red Rice Seeds, the Inhibition of Early Seedling Growth, but Not of Germination, Requires Extracellular ABA" Plants 11, no. 8: 1023. https://doi.org/10.3390/plants11081023
APA StyleGianinetti, A. (2022). In Dormant Red Rice Seeds, the Inhibition of Early Seedling Growth, but Not of Germination, Requires Extracellular ABA. Plants, 11(8), 1023. https://doi.org/10.3390/plants11081023






