The Potential of Cold Plasma and Electromagnetic Field as Stimulators of Natural Sweeteners Biosynthesis in Stevia rebaudiana Bertoni
Abstract
:1. Introduction
2. Results
2.1. Effects on Germination In Vitro
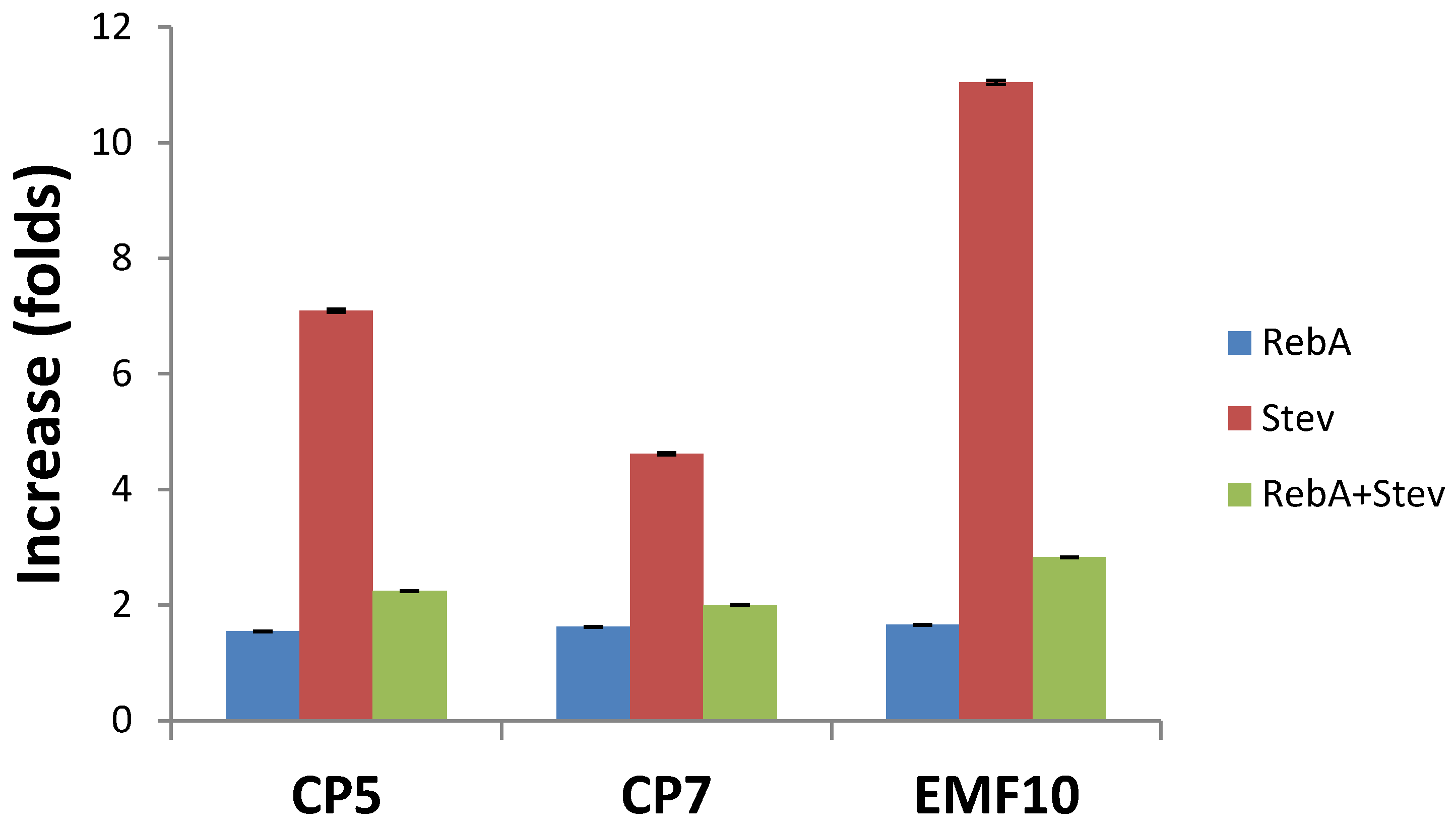
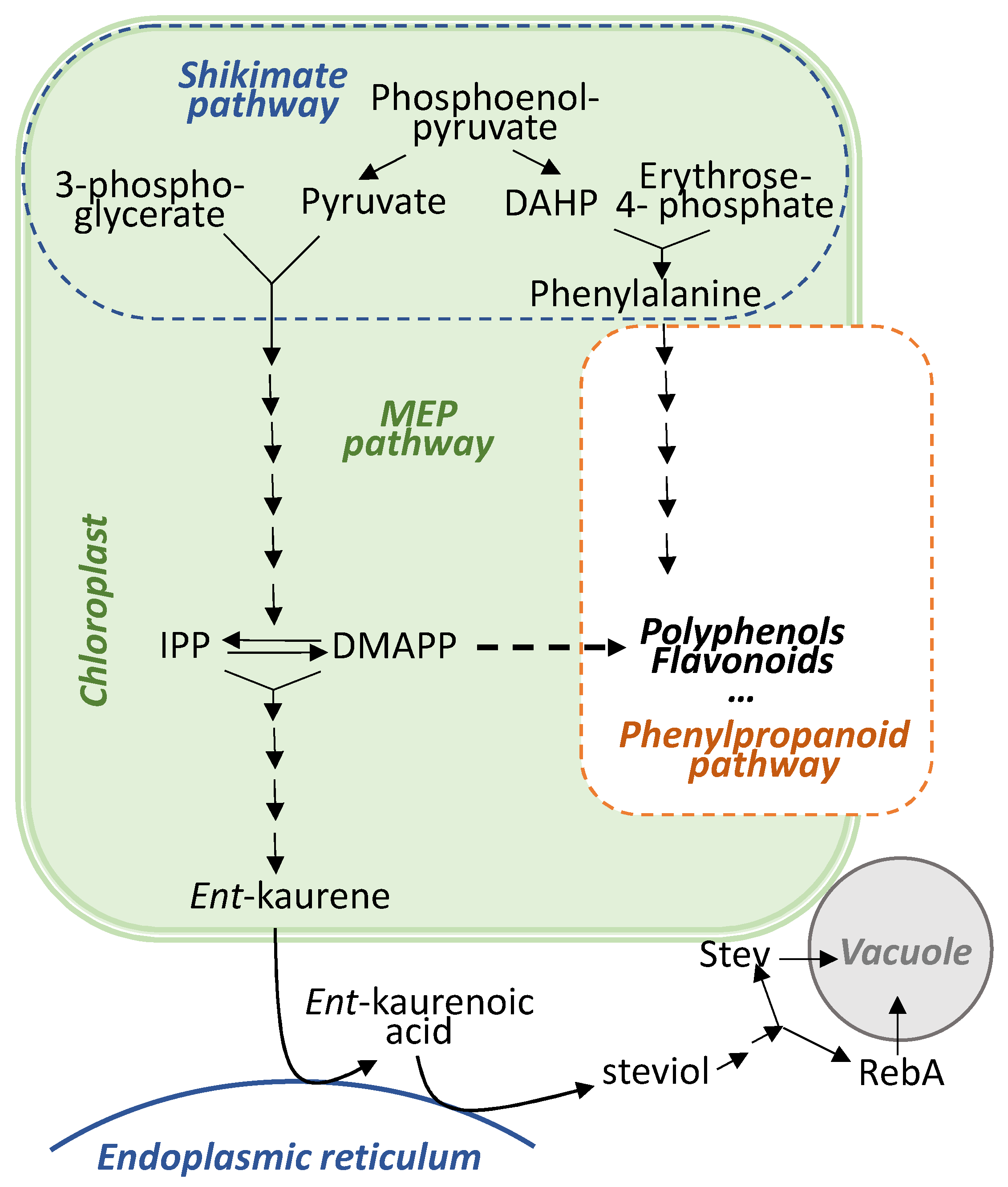
2.2. Effects on Concentrations of Steviol Glycosides
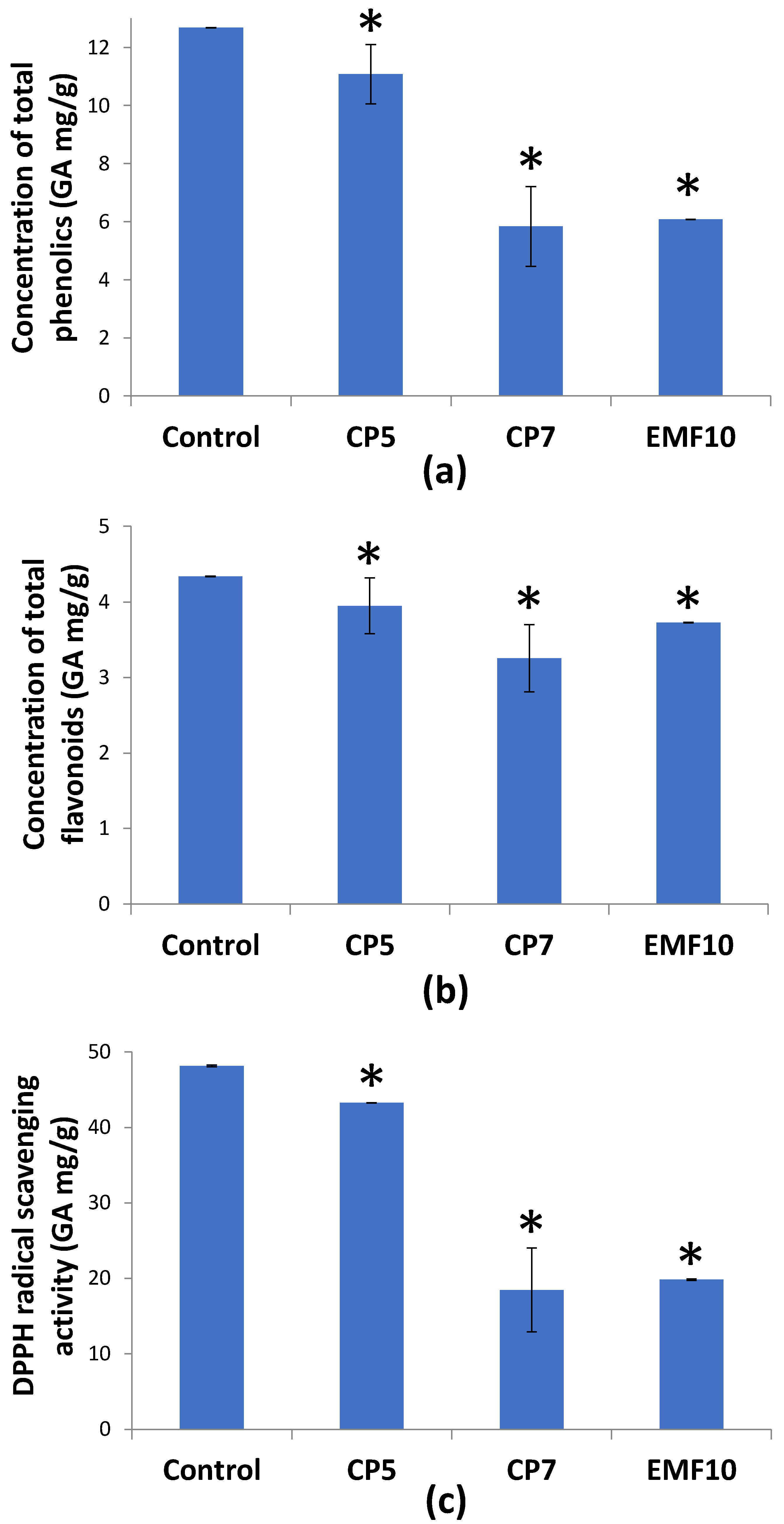
2.3. Effects on Total Phenolic Content, Flavonoid Content, and Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Seed Treatment with CP and EMF
4.4. Seed Germination Test
4.5. Plant Cultivation
4.6. Extract Preparation
4.7. HPLC Analysis of Steviol Glycosides
4.8. Determination of Total Phenolic Content
4.9. Determination of Total Flavonoid Content
4.10. Determination of Antioxidant Activity
4.11. Statistical Analysis
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gantait, S.; Das, A.; Banerjee, J. Geographical Distribution, Botanical Description and Self-Incompatibility Mechanism of Genus Stevia. Sugar Tech 2018, 20, 1–10. [Google Scholar] [CrossRef]
- Verified Market Research. Global Stevia Market Size By Form, By Application, By Geographic Scope and Forecast; Verified Market Research: New York, NY, USA, 2021; p. 202. Available online: https://www.verifiedmarketresearch.com (accessed on 31 January 2022).
- Libik-Konieczny, M.; Capecka, E.; Tuleja, M.; Konieczny, R. Synthesis and production of steviol glycosides: Recent research trends and perspectives. Appl. Microbiol. Biotechnol. 2021, 105, 3883–3900. [Google Scholar] [CrossRef] [PubMed]
- Tavarini, S.; Angelini, L.G. Stevia rebaudiana Bertoni as a source of bioactive compounds: The effect of harvest time, experimental site and crop age on Steviol glycoside content and antioxidant properties. J. Sci. Food Agric. 2012, 93, 2121–2129. [Google Scholar] [CrossRef] [PubMed]
- Lemus-Mondaca, R.; Vega-Galvez, A.; Zura-Bravo, L.; Ah-Hen, K. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 2012, 132, 1121–1132. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, S.; Dhyani, D.; Ahuja, P. A review on the improvement of stevia [Stevia rebaudiana (Bertoni)]. Can. J. Plant Sci. 2011, 91, 1–27. [Google Scholar] [CrossRef]
- Testai, L.; Calderone, V. Stevia rebaudiana Bertoni: Beyond Its Use as a Sweetener. Pharmacological and Toxicological Profile of Steviol Glycosides of Stevia rebaudiana Bertoni. In Steviol Glycosides: Cultivation, Processing, Analysis and Applications in Food, 2nd ed.; Wölwer-Rieck, U., Ed.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 148–161. [Google Scholar] [CrossRef]
- Ceunen, S.; Geuns, J.M.C. Steviol Glycosides: Chemical Diversity, Metabolism, and Function. J. Nat. Prod. 2013, 76, 1201–1228. [Google Scholar] [CrossRef]
- Tavarini, S.; Clemente, C.; Bender, C.; Angelini, L.G. Health-promoting compounds in stevia: The effect of mycorrhizal symbiosis, phosphorus supply and harvest time. Molecules 2020, 25, 5399. [Google Scholar] [CrossRef]
- Javed, R.; Usman, M.; Yücesan, B.; Zia, M.; Gürel, E. Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micropropagated shoots of Stevia rebaudiana Bertoni. Plant Physiol. Biochem. 2017, 110, 94–99. [Google Scholar] [CrossRef]
- Majlesi, Z.; Ramezani, M.; Gerami, M. Investigation on some main glycosides content of Stevia rebaudiana B. under different concentrations of commercial and synthesized silver nanoparticles. Pharm. Biomed. Res. 2018, 4, 8–14. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Ah-Hen, K.; Vega-Gálvez, A.; Honores, C.; Moraga, N.O. Stevia rebaudiana leaves: Effect of drying process temperature on bioactive components, antioxidant capacity and natural sweeteners. Plant Foods Hum. Nutr. 2016, 71, 49–56. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Maras, M.; Barba, F.J.; Granato, D.; Roohinejad, S.; Mallikarjunan, K.; Montesano, D.; Lorenzo, J.M.; Putnik, P. Innovative technologies for the recovery of phytochemicals from Stevia rebaudiana Bertoni leaves: A review. Food Chem. 2018, 268, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cai, R.; Weng, J.; Li, Y.; Jia, H.; Chen, K.; Yan, M.; Ouyang, P. Production of rebaudioside D from stevioside using a UGTSL2 Asn358Phe mutant in a multi-enzyme system. Microb. Biotechnol. 2020, 13, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of plasma-seed treatments as a potential seed processing technology. Front. Phys. 2021, 9, 617345. [Google Scholar] [CrossRef]
- Misra, N.; Schlutter, O.; Cullen, P. Plasma in Food and agriculture. In Cold Plasma in Food and Agriculture: Fundamentals and Applications, 3rd ed.; Patrick, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–16. [Google Scholar]
- Kaur, S.; Vian, A.; Chandel, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Sensitivity of plants to high frequency electromagnetic radiation: Cellular mechanisms and morphological changes. Rev. Environ. Sci. Biotechnol. 2021, 20, 55–74. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma agriculture from laboratory to farm: A review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Staric, P.; Vogel-Mikuš, K.; Mozetic, M.; Junkar, I. Effects of nonthermal plasma on morphology, genetics and physiology of seeds: A review. Plants 2020, 9, 1736. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Park, G. The effects of plasma on plant growth, development, and sustainability. Appl. Sci. 2020, 10, 6045. [Google Scholar] [CrossRef]
- Song, J.-S.; Kim, S.B.; Ryu, S.; Oh, J.; Kim, D.-S. Emerging plasma technology that alleviates crop stress during the early growth stages of plants: A Review. Front. Plant Sci. 2020, 11, 988. [Google Scholar] [CrossRef]
- Holubová, L.; Kyzek, S.; Durovcová, I.; Fabová, J.; Horváthová, E.; Ševcovicová, A.; Gálová, E. Non-thermal plasma—a new green priming agent for plants? Int. J. Mol. Sci. 2020, 21, 9466. [Google Scholar] [CrossRef]
- Mildaziene, V.; Pauzaite, G.; Naucienė, Z.; Malakauskiene, A.; Zukiene, R.; Januskaitiene, I.; Jakstas, V.; Ivanauskas, L.; Filatova, I.; Lyushkevich, V. Pre-sowing Seed Treatment with Cold Plasma and Electromagnetic Field Increases Secondary Metabolite Content in Purple Coneflower (Echinacea purpurea) Leaves. Plasma Process. Polym. 2018, 15, 1700059. [Google Scholar] [CrossRef]
- Ivankov, A.; Nauciene, Z.; Zukiene, R.; Degutyte-Fomins, L.; Malakauskiene, A.; Kraujalis, P.; Venskutonis, P.R.; Filatova, I.; Lyushkevich, V.; Mildaziene, V. Changes in Growth and Production of Non-Psychotropic Cannabinoids Induced by Pre-Sowing Treatment of Hemp Seeds with Cold Plasma, Vacuum and Electromagnetic Field. Appl. Sci. 2020, 10, 8519. [Google Scholar] [CrossRef]
- Mildaziene, V.; Paužaitė, G.; Naučienė, Z.; Zukiene, R.; Malakauskienė, A.; Norkeviciene, E.; Slepetiene, A.; Stukonis, V.; Olauskaite, V.; Padarauskas, A.; et al. Effect of Seed Treatment with Cold Plasma and Electromagnetic Field on Red Clover Germination, Growth and Content of Major Isoflavones. J. Phys. D 2020, 53, 264001. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Pauzaite, G.; Naucienė, Z.; Zukiene, R.; Degutyte-Fomins, L.; Pukalskas, A.; Venskutonis, P.R.; Filatova, I.; Lyushkevich, V. Seed Treatment with Cold Plasma and Electromagnetic Field Induces Changes in Red Clover Root Growth Dynamics, Flavonoid Exudation, and Activates Nodulation. Plasma Process. Polym. 2020, 18, 2000160. [Google Scholar] [CrossRef]
- Ivankov, A.; Naučienė, Z.; Degutytė-Fomins, L.; Žūkienė, R.; Januškaitienė, I.; Malakauskienė, A.; Jakštas, V.; Ivanauskas, L.; Romanovskaja, D.; Šlepetienė, A.; et al. Changes in Agricultural Performance of Common Buckwheat Induced by Seed Treatment with Cold Plasma and Electromagnetic Field. Appl. Sci. 2021, 11, 4391. [Google Scholar] [CrossRef]
- Ivankov, A.; Zukiene, R.; Nauciene, Z.; Degutyte-Fomins, L.; Filatova, I.; Lyushkevich, V.; Mildaziene, V. The effects of red clover seed treatment with cold plasma and electromagnetic field on germination and seedling growth are dependent on seed color. Appl. Sci. 2021, 11, 4676. [Google Scholar] [CrossRef]
- Degutytė-Fomins, L.; Paužaitė, G.; Žukienė, R.; Mildažienė, V.; Koga, K.; Shiratani, M. Relationship between cold plasma treatment-induced changes in radish seed germination and phytohormone balance. Jpn. J. Appl. Phys. 2020, 59, SH1001. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Types of Seeds and Kinds of Seed Dormancy. In Seeds, 2nd ed.; Baskin, C.C., Baskin, J.M., Eds.; Academic Press: Waltham, MA, USA, 2014; pp. 37–77. [Google Scholar] [CrossRef]
- Aman, N.; Hadi, F.; Khalil, S.A.; Zamir, R.; Ahmad, N. Efficient regeneration for enhanced steviol glycosides production in Stevia rebaudiana (Bertoni). Comptes Rendus Biol. 2013, 336, 486–492. [Google Scholar] [CrossRef]
- Zukiene, R.; Nauciene, Z.; Januskaitiene, I.; Pauzaite, G.; Mildaziene, V.; Koga, K.; Shiratani, M. Dielectric barrier discharge plasma treatment induced changes in sunflower seed germination, phytohormone balance, and seedling growth. Appl. Phys. Express 2019, 12, 126003. [Google Scholar] [CrossRef]
- Varnagiris, S.; Vilimaite, S.; Mikelionyte, I.; Urbonavicius, M.; Tuckute, S.; Milcius, D. The Combination of Simultaneous Plasma Treatment with Mg Nanoparticles Deposition Technique for Better Mung Bean Seeds Germination. Processes 2020, 8, 1575. [Google Scholar] [CrossRef]
- Holc, M.; Mozetič, M.; Recek, N.; Primc, G.; Vesel, A.; Zaplotnik, R.; Gselman, P. Wettability Increase in Plasma-Treated Agricultural Seeds and Its Relation to Germination Improvement. Agronomy 2021, 11, 1467. [Google Scholar] [CrossRef]
- Mildaziene, V.; Pauzaite, G.; Malakauskiene, A.; Zukiene, R.; Nauciene, Z.; Filatova, I.; Azharonok, V.; Lyushkevich, V. Response of perennial woody plants to seed treatment by electromagnetic field and low-temperature plasma. Bioelectromagnetics 2016, 37, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Piel, A. Plasma Physics. An Introduction to Laboratory, Space, and Fusion Plasmas, 2nd ed.; Springer International Publishing AG: Cham, Switzerland, 2017. [Google Scholar]
- Bera, K.; Dutta, P.; Sadhukhan, S. Seed priming with non-ionizing physical agents: Plant responses and underlying physiological mechanisms. Plant Cell Rep. 2022, 41, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Paužaitė, G.; Malakauskienė, A.; Naučienė, Z.; Žūkienė, R.; Filatova, I.; Lyushkevich, V.; Azarko, I.; Mildažienė, V. Changes in Norway spruce germination and growth induced by pre-sowing seed treatment with cold plasma and electromagnetic field: Short-term versus long-term effects. Plasma Process. Polym. 2018, 15, 1700068. [Google Scholar] [CrossRef]
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of Common sunflower (Helianthus annus L.) seeds with radio-frequency electromagnetic field and cold plasma induces changes in seed phytohormone balance, seedling development and leaf protein expression. Sci. Rep. 2019, 9, 6437. [Google Scholar] [CrossRef]
- Lucho, S.R.; do Amaral, M.N.; Milech, C.; Ferrer, M.Á.; Calderón, A.A.; Bianchi, V.J.; Braga, E.J.B. Elicitor-Induced Transcriptional Changes of Genes of the Steviol Glycoside Biosynthesis Pathway in Stevia rebaudiana Bertoni. J. Plant Growth Regul. 2018, 37, 971–985. [Google Scholar] [CrossRef]
- Kumar, H.; Kaul, K.; Bajpai-Gupta, S.; Kaul, V.K.; Kumar, S. A comprehensive analysis of fifteen genes of steviol glycosides biosynthesis pathway in Stevia rebaudiana (Bertoni). Gene 2012, 492, 276–284. [Google Scholar] [CrossRef]
- Saifi, M.; Nasrullah, N.; Ahmad, M.M.; Ali, A.; Khan, J.A.; Abdin, M.Z. In silico analysis and expression profiling of miRNAs targeting genes of steviol glycosides biosynthetic pathway and their relationship with steviol glycosides content in different tissues of Stevia rebaudiana. Plant Physiol. Biochem. 2015, 94, 57–64. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Javed, R.; Adeel, M.; Rizwan, M.; Yang, Y. PEG 6000-Stimulated Drought Stress Improves the Attributes of In Vitro Growth, Steviol Glycosides Production, and Antioxidant Activities in Stevia rebaudiana Bertoni. Plants 2020, 9, 1552. [Google Scholar] [CrossRef]
- Hill, C.B.; Roessner, U. Advances in high-throughput untargeted LC-MS analysis for plant metabolomics. In Advanced LC-MS Applications for Metabolomics, 2nd ed.; De Vooght-Johnson, R., Ed.; Future Medicine Ltd.: London, UK, 2015; pp. 58–71. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Libik-Konieczny, M.; Capecka, E.; Kąkol, E.; Dziurka, M.; Grabowska-Joachimiak, A.; Sliwinska, E.; Pistellie, L. Growth, development and steviol glycosides content in the relation to the photosynthetic activity of several Stevia rebaudiana Bertoni strains cultivated under temperate climate conditions. Sci. Hortic. 2018, 234, 10–18. [Google Scholar] [CrossRef]
- Haslam, E. Plant polyphenols (syn. vegetable tannins) and chemical defense—A reappraisal. J. Chem. Ecol. 1988, 14, 1789–1805. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Natural Polyphenols (Vegetable Tannins) as Drugs: Possible Modes of Action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, N.; Karaköse, H. Presentation and Analysis of Other Constituents in the Leaves: Polyphenolics in Stevia rebaudiana Leaves. In Steviol Glycosides: Cultivation, Processing, Analysis and Applications in Food, 2nd ed.; Wölwer-Rieck, U., Ed.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 113–124. [Google Scholar] [CrossRef]
- Kang, J.H.; McRoberts, J.; Shi, F.; Moreno, J.E.; Jones, A.D.; Howe, G.A. The flavonoid biosynthetic enzyme chalcone isomerase modulates terpenoid production in glandular trichomes of tomato. Plant Physiol. 2014, 164, 1161–1174. [Google Scholar] [CrossRef] [Green Version]
- Nagel, J.; Culley, L.K.; Lu, Y.P.; Liu, E.W.; Matthews, P.D.; Stevens, J.F.; Page, J.E. EST analysis of hop glandular trichomes identifies an O-methyltransferase that catalyzes the biosynthesis of xanthohumol. Plant Cell 2008, 20, 186–200. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.D.; Tian, L.; Aziz, N.; Broun, P.; Dai, X.B.; He, J.; King, A.; Zhao, P.X.; Dixon, R.A. Terpene biosynthesis in glandular trichomes of hop. Plant Physiol. 2008, 148, 1254–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, G.A.; Huhman, D.; Lei, Z.T.; Snyder, J.; Sumner, L.W.; Dixon, R.A. Characterization of an isoflavonoid-specific prenyltransferase from Lupinus albus. Plant Physiol. 2012, 159, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voo, S.S.; Grimes, H.D.; Lange, B.M. Assessing the biosynthetic capabilities of secretory glands in Citrus peel. Plant Physiol. 2012, 159, 81–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedon, F.; Bomal, C.; Caron, S.; Levasseur, C.; Boyle, B.; Mansfield, S.D.; Schmidt, A.; Gershenzon, J.; Grima-Pettenati, J.; Séguin, A.; et al. Subgroup 4 R2R3-MYBs in conifer trees: Gene family expansion and contribution to the isoprenoid- and flavonoid-oriented responses. J. Exp. Bot. 2010, 61, 3847–3864. [Google Scholar] [CrossRef]
- Ben Zvi, M.M.; Shklarman, E.; Masci, T.; Kalev, H.; Debener, T.; Shafir, S.; Ovadis, M.; Vainstein, A. PAP1 transcription factor enhances production of phenylpropanoid and terpenoid scent compounds in rose flowers. New Phytol. 2012, 195, 335–345. [Google Scholar] [CrossRef]
- Britun, N.; Gaillard, M.; Ricard, A.; Kim, Y.M.; Kim, K.S.; Han, J.G. Determination of the vibrational, rotational and electron temperatures in N2 and Ar–N2 rf discharge. J. Phys. D Appl. Phys. 2007, 40, 1022. [Google Scholar] [CrossRef]
- Filatova, I.I.; Azharonok, V.; Lushkevich, V.; Zhukovsky, A.; Gadzhieva, G.; Spaisić, K.; Spasić, K.; Živković, S.; Puać, N.; Lasović, S.; et al. Plasma seeds treatment as a promising technique for seed germination improvement. In Proceedings of the 31st International Conference on Phenomena in Ionized Gases, ICPIG (International Conference on Phenomena in Ionized Gases), Granada, Spain, 14–19 July 2013; pp. 4–7. [Google Scholar]
- Filatova, I.; Lyushkevich, V.; Goncharik, S.; Zhukovsky, A.; Krupenko, N.; Kalatskaja, J. The effect of low-pressure plasma treatment of seeds on the plant resistance to pathogens and crop yields. J. Phys. D Appl. Phys. 2020, 53, 244001. [Google Scholar] [CrossRef]
- Richards, F.J. A flexible growth function for empirical use. J. Exp. Bot. 1959, 10, 290–300. [Google Scholar] [CrossRef]
- Hara, Y. Calculation of population parameters using Richards function and application of indices of growth and seed vigor to rice plants. Plant Prod. Sci. 1999, 2, 129–135. [Google Scholar] [CrossRef]
- Blinstrubienė, A.; Burbulis, N.; Juškevičiūtė, N.; Vaitkevičienė, N.; Žūkienė, R. Effect of growth regulators on Stevia rebaudiana Bertoni callus genesis and influence of auxin and proline to steviol glycosides, phenols, flavonoids accumulation, and antioxidant activity in vitro. Molecules 2020, 25, 2759. [Google Scholar] [CrossRef] [PubMed]
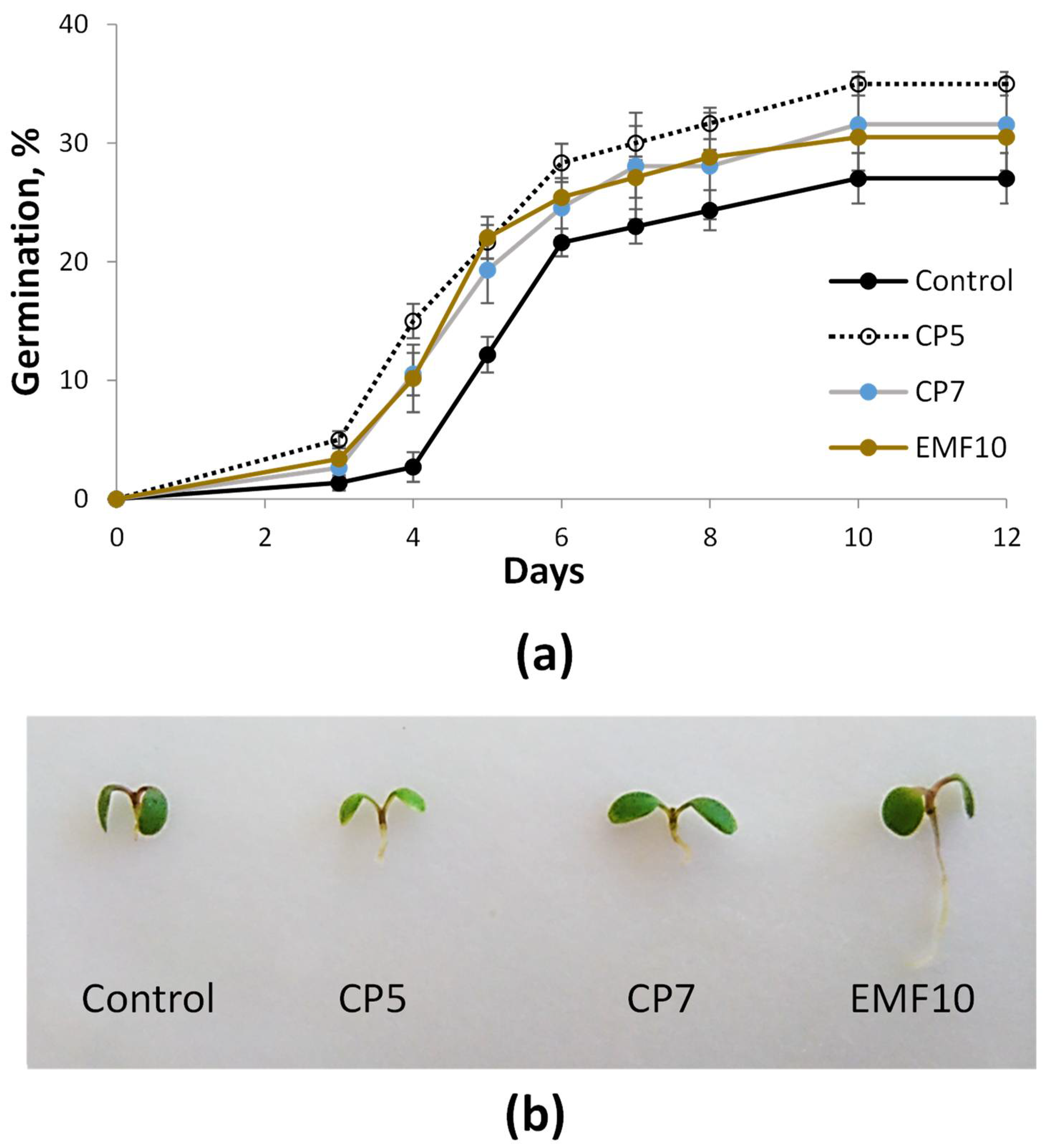
| Group | Vi, % | Me, Days | Qu, Days |
|---|---|---|---|
| Control | 27.03 ± 2.13 | 5.06 ± 0.12 | 0.68 ± 0.10 |
| CP5 | 35.00 ± 1.01 * | 4.39 ± 0.18 * | 1.14 ± 0.08 * |
| CP7 | 31.58 ± 3.90 * | 4.59 ± 0.09 * | 0.98 ± 0.17 * |
| EMF10 | 30.51 ± 0.26 * | 4.36 ± 0.19 * | 0.79 ± 0.09 |
| RebA | Stev | RebA+Stev | RebA/Stev | RebA/(RebA+Stev) | Stev/(RebA+Stev) | |
|---|---|---|---|---|---|---|
| Control | 36.71 ± 3.10 | 5.27 ± 1.63 | 41.98 ± 4.71 | 8.35 ± 1.62 | 0.88 ± 0.02 | 0.12 ± 0.02 |
| CP5 | 56.63 ± 9.07 * | 37.35 ± 8.83 * | 93.99 ± 17.89 * | 1.86 ± 0.24 * | 0.64 ± 0.03 * | 0.36 ± 0.03 * |
| CP7 | 59.58 ± 9.12 * | 24.35 ± 4.14 * | 83.93 ± 13.25 * | 2.50 ± 0.07 * | 0.71 ± 0.01 * | 0.29 ± 0.01 * |
| EMF10 | 60.77 ± 0.33 * | 58.15 ± 0.15 * | 118.93 ± 0.18 * | 1.05 ± 0.01 * | 0.51 ± 0.00 * | 0.49 ± 0.00 * |
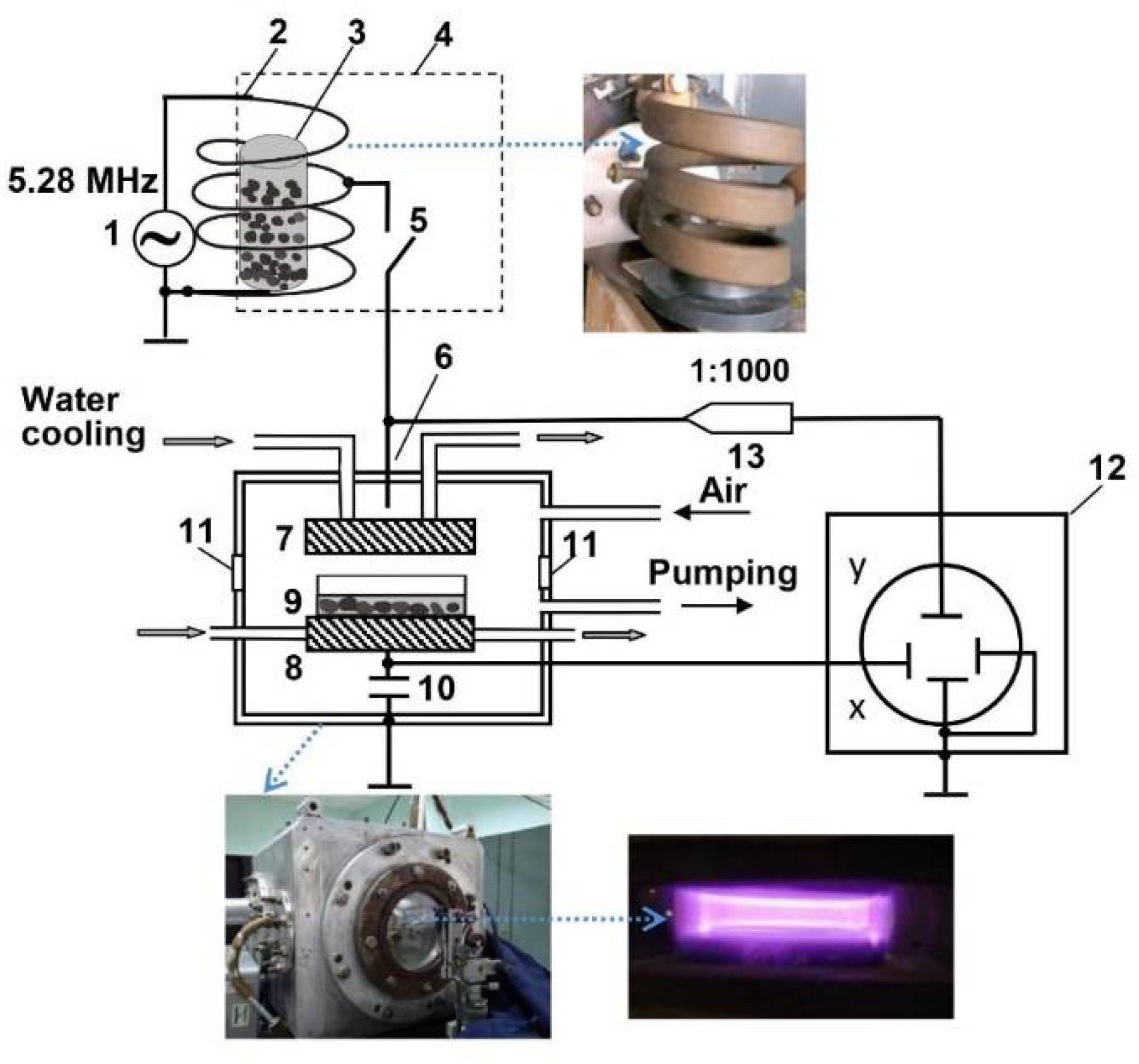
| Parameter | Value |
|---|---|
| CP treatment | |
| Discharge frequency | 5.28 MHz |
| Pressure | 200 Pa |
| Input power | ~8.4W |
| Effective electron temperature (Te) | ~2.3 eV |
| Effective electron density (ne) | ~5 × 108 cm−3 |
| Electrode diameter | 120 mm |
| Distance between electrodes | 20 mm |
| EMF treatment | |
| Frequency | 5.28 MHz |
| Pressure | Atmospheric |
| Amplitude value of the magnetic component | 835 A/m (B ≈ 1 mT) |
| Amplitude value of the electric component | 17.96 kV/m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judickaitė, A.; Lyushkevich, V.; Filatova, I.; Mildažienė, V.; Žūkienė, R. The Potential of Cold Plasma and Electromagnetic Field as Stimulators of Natural Sweeteners Biosynthesis in Stevia rebaudiana Bertoni. Plants 2022, 11, 611. https://doi.org/10.3390/plants11050611
Judickaitė A, Lyushkevich V, Filatova I, Mildažienė V, Žūkienė R. The Potential of Cold Plasma and Electromagnetic Field as Stimulators of Natural Sweeteners Biosynthesis in Stevia rebaudiana Bertoni. Plants. 2022; 11(5):611. https://doi.org/10.3390/plants11050611
Chicago/Turabian StyleJudickaitė, Augustė, Veronika Lyushkevich, Irina Filatova, Vida Mildažienė, and Rasa Žūkienė. 2022. "The Potential of Cold Plasma and Electromagnetic Field as Stimulators of Natural Sweeteners Biosynthesis in Stevia rebaudiana Bertoni" Plants 11, no. 5: 611. https://doi.org/10.3390/plants11050611
APA StyleJudickaitė, A., Lyushkevich, V., Filatova, I., Mildažienė, V., & Žūkienė, R. (2022). The Potential of Cold Plasma and Electromagnetic Field as Stimulators of Natural Sweeteners Biosynthesis in Stevia rebaudiana Bertoni. Plants, 11(5), 611. https://doi.org/10.3390/plants11050611







