More than What Meets the Eye: Differential Spatiotemporal Distribution of Cryptic Intertidal Bangiales
Abstract
1. Introduction
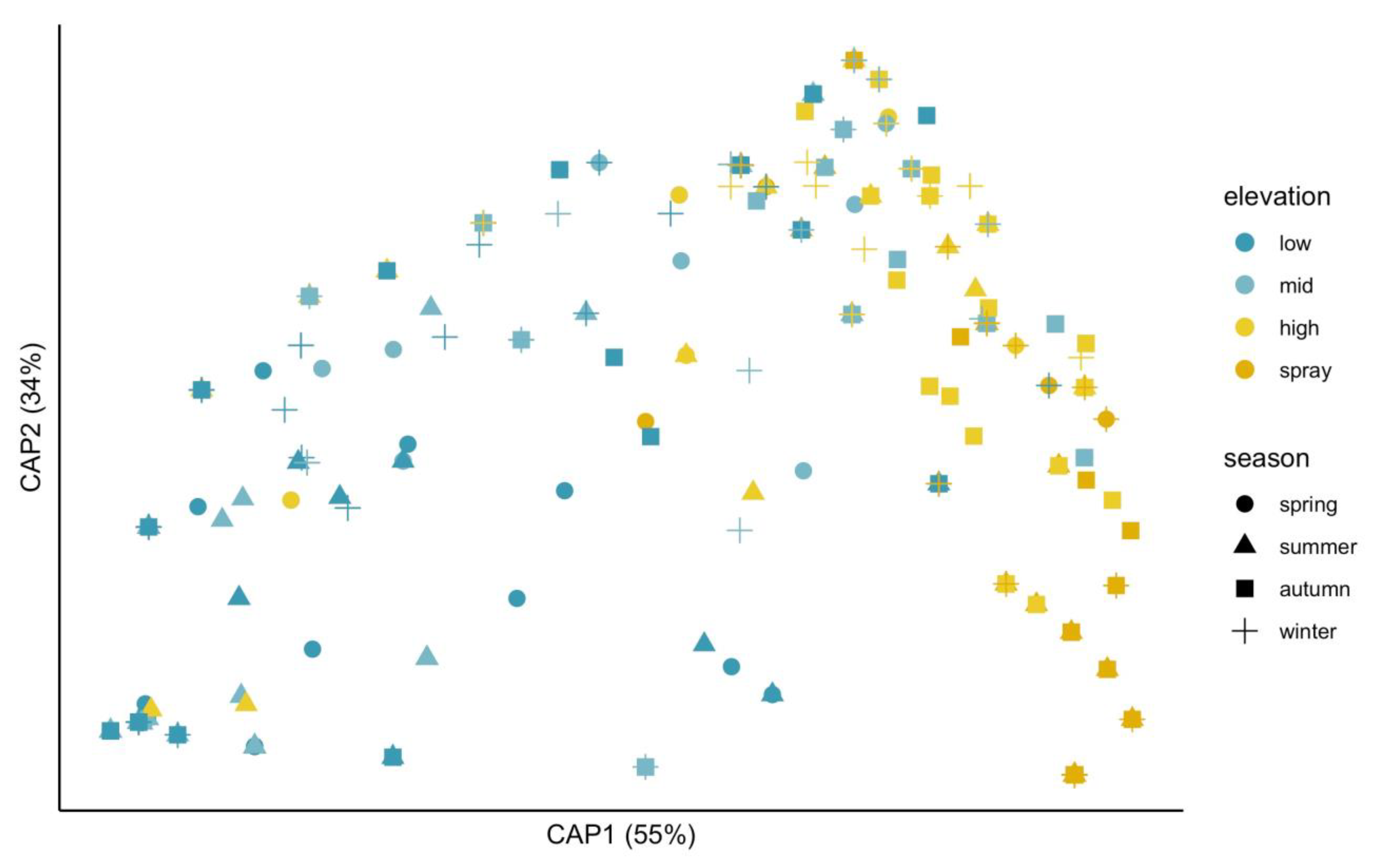
2. Results
3. Discussion
3.1. Responses of Bladed Bangiales to Vertical Stress Gradients in Intertidal Habitats
3.2. Seasonal Variation in Intertidal Species Assemblages
3.3. Diversity and Biogeography of Bladed Bangiales
4. Materials and Methods
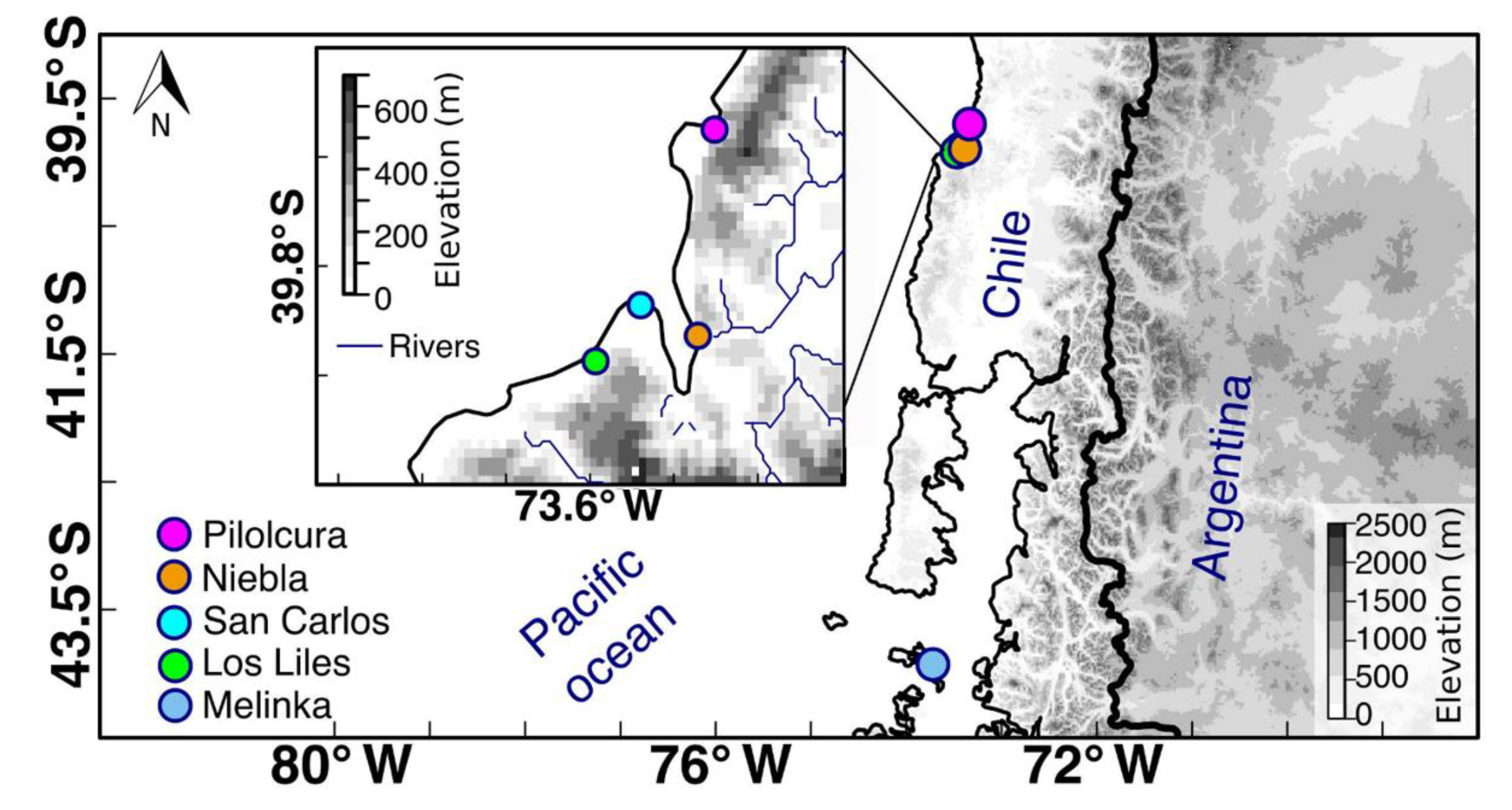
4.1. Study Area
4.2. Bladed Bangiales Sample Collection
4.3. Morphological Traits
4.4. Species Determination Based on Molecular Data
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnston, E.C.; Wyatt, A.S.J.; Leichter, J.J.; Burgess, S.C. Niche differences in co-occurring cryptic coral species (Pocillopora spp.). Coral Reefs 2021, 1–12. [Google Scholar] [CrossRef]
- Bongaerts, P.; Cooke, I.R.; Ying, H.; Wels, D.; Haan, S.D.; Hernandez-Agreda, A.; Brunner, C.A.; Dove, S.; Englebert, N.; Eyal, G.; et al. Morphological stasis masks ecologically divergent coral species on tropical reefs. Curr. Biol. 2021, 31, 2286–2298.e8. [Google Scholar] [CrossRef]
- de Meester, N.; Derycke, S.; Bonte, D.; Moens, T. Salinity effects on the coexistence of cryptic species: A case study on marine nematodes. Mar Biol. 2011, 158, 2717–2726. [Google Scholar] [CrossRef]
- Montero-Pau, J.; Ramos-Rodríguez, E.; Serra, M.; Gómez, A. Long-Term Coexistence of Rotifer Cryptic Species. PLoS ONE 2011, 6, e21530. [Google Scholar] [CrossRef]
- Pardo, C.; Lopez, L.; Peña, V.; Hernandez, J.; Le Gall, L.; Bárbara, I.; Barreiro, R. A Multilocus Species Delimitation Reveals a Striking Number of Species of Coralline Algae Forming Maerl in the OSPAR Maritime Area. PLoS ONE 2014, 9, e104073. [Google Scholar] [CrossRef]
- Guillemin, M.-L.; Contreras-Porcia, L.; Ramírez, M.E.; Macaya, E.; Contador, C.B.; Woods, H.; Wyatt, C.; Brodie, J. The bladed Bangiales (Rhodophyta) of the South Eastern Pacific: Molecular species delimitation reveals extensive diversity. Mol. Phylogenetics Evol. 2016, 94, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Chenuil, A.; Cahill, A.E.; Délémontey, N.; Du Salliant du Luc, E.; Fanton, H. Problems and Questions Posed by Cryptic Species. A Framework to Guide Future Studies. In From Assessing to Conserving Biodiversity: Conceptual and Practical Challenges; Casetta, E., da Silva, J.M., Vecchi, D., Eds.; Springer Nature: Berlin, Germany, 2019; p. 452. [Google Scholar]
- Bickford, D.; Lohman, D.J.; Sodhi, N.S.; Ng, P.K.; Meier, R.; Winker, K.; Ingram, K.K.; Das, I. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007, 22, 148–155. [Google Scholar] [CrossRef]
- Knowlton, N. Molecular genetic analyses of species boundaries in the sea. Hydrobiologia 2000, 420, 73–90. [Google Scholar] [CrossRef]
- Trontelj, P.; Fišer, C. Perspectives: Cryptic species diversity should not be trivialised. Syst. Biodivers. 2009, 7, 1–3. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]
- Van Campenhout, J.; Derycke, S.; Moens, T.; Vanreusel, A. Differences in life-histories refute ecological equivalence of cryptic species and provide clues to the origin of bathyal Halomonhystera (nematoda). PLoS One. 2014, 9, e111889. [Google Scholar] [CrossRef] [PubMed]
- Chesson, P. Mechanisms of Maintenance of Species Diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Spaak, J.W.; Godoy, O.; De Laender, F. Mapping species niche and fitness differences for communities with multiple interaction types. Oikos 2021, 130, 2065–2077. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Lin, K.; Hanski, I. Coexistence of cryptic species. Ecol. Lett. 2004, 7, 165–169. [Google Scholar] [CrossRef]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography. In Monographs in Population Biology Vol. 83; Levin, S.A., Horn, H.S., Eds.; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Shinen, J.L.; Navarrete, S.A. Lottery coexistence on rocky shores: Weak niche differentiation or equal competitors engaged in neutral dynamics? Am. Nat. 2014, 183, 342–362. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.A.; Stephenson, A. The Universal Features of Zonation Between Tide-Marks on Rocky Coasts. J. Ecol. 1949, 37, 289. [Google Scholar] [CrossRef]
- Menge, B.A.; Branch, G.M. Rocky Intertidal Communities. In Marine Community Ecology; Bertness, M.D., Gaines, S.D., Hay, M.E., Eds.; Sinauer Associates: Sunderland, MA, USA, 2001; pp. 221–251. [Google Scholar]
- Branch, G.M.; Moreno, C.A. Intertidal and subtidal grazers. In Rocky Shores: Exploitation in Chile and South Africa Ecological Studies; Siegfried, W.R., Ed.; Springer Verlag: Berlin, Germany, 1994; pp. 75–100. [Google Scholar]
- Aguilera, M.A. The functional roles of herbivores in the rocky intertidal systems in Chile: A review of food preferences and consumptive effects. Rev. Chil. de Hist. Nat. 2011, 84, 241–261. [Google Scholar] [CrossRef]
- Dethier, M.N.; Williams, S.L. Seasonal stresses shift optimal intertidal algal habitats. Mar. Biol. 2009, 156, 555–567. [Google Scholar] [CrossRef]
- Benincà, E.; Ballantine, B.; Ellner, S.P.; Huisman, J. Species fluctuations sustained by a cyclic succession at the edge of chaos. Proc. Natl. Acad. Sci. USA 2015, 112, 6389–6394. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, N.; Aguilera, M.; Navarrete, S.; Broitman, B. Disentangling the effects of propagule supply and environmental filtering on the spatial structure of a rocky shore metacommunity. Mar. Ecol. Prog. Ser. 2015, 538, 67–79. [Google Scholar] [CrossRef]
- Kanamori, Y.; Fukaya, K.; Noda, T. Seasonal changes in community structure along a vertical gradient: Patterns and processes in rocky intertidal sessile assemblages. Popul. Ecol. 2017, 59, 301–313. [Google Scholar] [CrossRef]
- Suárez, J.L.; Hansen, M.; Urtubia, U.; Lenz, M.; Valdivia, N.; Thiel, M. Season-dependent effects of ocean warming on the physiological performance of a native and a non-native sea anemone. J. Exp. Mar. Biol. Ecol. 2019, 522, 151229. [Google Scholar] [CrossRef]
- Vellend, M. The Theory of Ecological Communities (MPB-57); Princeton University Press: Princeton, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Couceiro, L.; Le Gac, M.; Hunsperger, H.M.; Mauger, S.; Destombe, C.; Cock, J.M.; Ahmed, S.; Coelho, S.M.; Valero, M.; Peters, A.F. Data from: Evolution and maintenance of haploid-diploid life cycles in natural populations: The case of the marine brown alga Ectocarpus. Evolution 2015, 69, 1808–1822. [Google Scholar] [CrossRef] [PubMed]
- Montecinos, A.E.; Couceiro, L.; Peters, A.F.; Desrut, A.; Valero, M.; Guillemin, M.L. Species delimitation and phylogeographic analyses in the Ectocarpus subgroup siliculosi (Ectocarpales, Phaeophyceae). J Phycol. 2017, 53, 17–31. [Google Scholar] [CrossRef]
- Schweikert, K.; Sutherland, J.E.; Burritt, D.J.; Hurd, C.L. Analysis of spatial and temporal diversity and distribution of porphyra (rhodophyta) in southeastern new zealand supported by the use of molecular tools1. J. Phycol. 2012, 48, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Meynard, A.; Zapata, J.; Salas, N.; Betancourtt, C.; Pérez-Lara, G.; Castañeda, F.; Ramírez, M.E.; Contador, C.B.; Guillemin, M.L.; Porcia, L.C. Genetic and morphological differentiation of Porphyra and Pyropia species (Bangiales, Rhodophyta) coexisting in a rocky intertidal in Central Chile. J. Phycol. 2019, 55, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Zapata, J.; Meynard, A.; Anguita, C.; Espinoza, C.; Alvear, P.; Kumar, M.; Porcia, L.C. Non-random distribution and ecophysiological differentiation of Pyropia species (Bangiales, Rhodophyta) through environmental gradients. J. Phycol. 2019, 55, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Muangmai, N.; Preuss, M.; Zuccarello, G.C. Comparative physiological studies on the growth of cryptic species of Bostrychia intricata (Rhodomelaceae, Rhodophyta) in various salinity and temperature conditions. Phycol. Res. 2015, 63, 300–306. [Google Scholar] [CrossRef]
- West, A.L.; Mathieson, A.C.; Klein, A.S.; Neefus, C.D.; Bray, T.L. Molecular ecological studies of New England species of Porphyra (Rhodophyta, Bangiales). Nova Hedwig. 2005, 80, 1–24. [Google Scholar] [CrossRef]
- Yang, L.; Lu, Q.; Brodie, J. A review of the bladed Bangiales (Rhodophyta) in China: History, culture and taxonomy. Eur. J. Phycol. 2017, 52, 251–263. [Google Scholar] [CrossRef]
- Avila, M.; Seguel, M. An overview of seaweed resources in Chile. J. Appl. Phycol. 1993, 5, 133–139. [Google Scholar] [CrossRef]
- Yáñez, E.; Lagos, N.A.; Norambuena, R.; Silva, C.; Letelier, J.; Muck, K.-P.; Martin, G.S.; Benítez, S.; Broitman, B.R.; Contreras, H.; et al. Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile. Clim. Change Impacts Fish. Aquac. A Glob. Anal. 2017, 1, 239–332. [Google Scholar] [CrossRef]
- Nelson, W.A.; Broom, J.E.S. The identity of Porphyra columbina (Bangiales, Rhodophyta) originally described from the New Zealand subantarctic islands. Aust. Syst. Bot. 2010, 23, 16–26. [Google Scholar] [CrossRef]
- Ramírez, M.E.; Contreras-Porcia, L.; Guillemin, M.-L.; Brodie, J.; Valdivia, C.; Flores-Molina, M.R.; Núñez, A.; Contador, C.B.; Lovazzano, C. Pyropia orbicularis sp. nov. (Rhodophyta, Bangiaceae) based on a population previously known as Porphyra columbina from the central coast of Chile. Phytotaxa 2014, 158, 133. [Google Scholar] [CrossRef]
- Contreras-Porcia, L.; Callejas, S.; Thomas, D.; Sordet, C.; Pohnert, G.; Contreras, A.; Lafuente, A.; Flores-Molina, M.R.; Correa, J.A. Seaweeds early development: Detrimental effects of desiccation and attenuation by algal extracts. Planta 2011, 235, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; A Berry, J. Recovery of photosynthesis after exposure of intertidal algae to osmotic and temperature stresses: Comparative studies of species with differing distributional limits. Oecologia 1986, 70, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Schonbeck, M.; Norton, T.A. The effects of brief periodic submergence on intertidal fucoid algae. Estuar. Coast. Mar. Sci. 1979, 8, 205–211. [Google Scholar] [CrossRef]
- Mansilla, A.; Werlinger, C.; Palacios, M.; Navarro, N.P.; Cuadra, P. Effects of UVB Radiation on the Initial Stages of Growth of Gigartina Skottsbergii, Sarcothalia Crispata and Mazzaella Laminarioides (Gigartinales, Rhodophyta). J. Appl. Phycol. 2006, 18, 451–459. [Google Scholar] [CrossRef]
- Gómez, I.; Huovinen, P. Morpho-functional patterns and zonation of South Chilean seaweeds: The importance of photosynthetic and bio-optical traits. Mar. Ecol. Prog. Ser. 2011, 422, 77–91. [Google Scholar] [CrossRef]
- Underwood, A.; Jernakoff, P. The effects of tidal height, wave-exposure, seasonality and rock-pools on grazing and the distribution of intertidal macroalgae in New South Wales. J. Exp. Mar. Biol. Ecol. 1984, 75, 71–96. [Google Scholar] [CrossRef]
- Schonbeck, M.W.; Norton, T.A. Factors controlling the lower limits of fucoid algae on the shore. J. Exp. Mar. Biol. Ecol. 1980, 43, 131–150. [Google Scholar] [CrossRef]
- Mangialajo, L.; Chiantore, M.; Susini, M.-L.; Meinesz, A.; Cattaneo-Vietti, R.; Thibaut, T. Zonation patterns and interspecific relationships of fucoids in microtidal environments. J. Exp. Mar. Biol. Ecol. 2012, 412, 72–80. [Google Scholar] [CrossRef]
- Kéfi, S.; Berlow, E.L.; Wieters, E.A.; Joppa, L.N.; Wood, S.A.; Brose, U.; Navarrete, S.A. Network structure beyond food webs: Mapping non-trophic and trophic interactions on Chilean rocky shores. Ecology 2015, 96, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.A.; Jaramillo, E. The Role of Grazers in the Zonation of Intertidal Macroalgae of the Chilean Coast. Oikos 1983, 41, 73. [Google Scholar] [CrossRef]
- Tejada-Martinez, D.; López, D.N.; Bonta, C.C.; Sepúlveda, R.D.; Valdivia, N. Positive and negative effects of mesograzers on early-colonizing species in an intertidal rocky-shore community. Ecol. Evol. 2016, 6, 5761–5770. [Google Scholar] [CrossRef] [PubMed]
- Paine, R.T. Food Webs: Linkage, Interaction Strength and Community Infrastructure. J. Anim. Ecol. 1980, 49, 666. [Google Scholar] [CrossRef]
- Scrosati, R.A.; Heaven, C. Spatial trends in community richness, diversity, and evenness across rocky intertidal environmental stress gradients in eastern Canada. Mar. Ecol. Prog. Ser. 2007, 342, 1–14. [Google Scholar] [CrossRef]
- Valdivia, N.; Segovia-Rivera, V.; Fica, E.; Bonta, C.C.; Aguilera, M.A.; Broitman, B. Context-dependent functional dispersion across similar ranges of trait space covered by intertidal rocky shore communities. Ecol. Evol. 2017, 7, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Muga, P.; Romo, H.; Calderón, C.; Evrard, O.; Díaz, H. Amplificación del gen rbcL revela primer registro de Porphyra mumfordii (Bangiales, Rhodophyta) en la bahía de Valparaíso, Chile central. Rev. Biol. Mar. Oceanogr. 2018, 53, 131–140. [Google Scholar] [CrossRef]
- Lindstrom, S.C.; Cole, K.M. The Porphyra lanceolata–P. pseudolanceolata (Bangiales, Rhodophyta) complex unmasked: Recognition of new species based on isozymes, morphology, chromosomes and distributions. Phycologia 1992, 31, 431–448. [Google Scholar] [CrossRef]
- Lindstrom, S.C.; Hughey, J.R.; Aguilar Rosas, L.E. Four new species of Pyropia (Bangiales, Rhodophyta) from the west coast of North America: The Pyropia lanceolata species complex updated. PhytoKeys. 2015, 52, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Blouin, N.A.; Brodie, J.A.; Grossman, A.C.; Xu, P.; Brawley, S.H. Porphyra: A marine crop shaped by stress. Trends Plant Sci. 2011, 16, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Porcia, L.; Thomas, D.; Flores, V.; Correa, J.A. Tolerance to oxidative stress induced by desiccation in Porphyra columbina (Bangiales, Rhodophyta). J. Exp. Bot. 2010, 62, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Porcia, L.; López-Cristoffanini, C.; Meynard, A.; Kumar, M. Tolerance Pathways to Desiccation Stress in Seaweeds. In Systems Biology of Marine Ecosystems; Springer: Cham, Switzerland, 2017; pp. 13–33. [Google Scholar] [CrossRef]
- Kim, J.K.; Kraemer, G.P.; Yarish, C. Physiological activity of Porphyra in relation to eulittoral zonation. J. Exp. Mar. Biol. Ecol. 2008, 365, 75–85. [Google Scholar] [CrossRef]
- Kim, J.K.; Kraemer, G.P.; Yarish, C. Comparison of growth and nitrate uptake by New England Porphyra species from different tidal elevations in relation to desiccation. Phycol. Res. 2009, 57, 152–157. [Google Scholar] [CrossRef]
- Abe, S.; Kurashima, A.; Yokohama, Y.; Tanaka, J. The Cellular Ability of Desiccation Tolerance in Japanese Intertidal Seaweeds. Bot. Mar. 2001, 44, 125–131. [Google Scholar] [CrossRef]
- Boedeker, C.; Farr, T.J.; Nelson, W.A. Comparative culture experiments with filamentous members of the Bangiales (Rhodophyta) from New Zealand: Insights into ecologic adaptation and biogeography. Phycol. Res. 2008, 56, 183–192. [Google Scholar] [CrossRef]
- Royer, C.J.; Redmond, S.; Lai, C.S.; Brawley, S.H. Porphyra umbilicalis in applied and basic research: Reproductive phenology, development, seed stock culture, and a field trial for aquaculture. J. Appl. Phycol. 2018, 31, 547–560. [Google Scholar] [CrossRef]
- Holmes, M.J.; Brodie, J. Morphology, seasonal phenology and observations on some aspects of the life history in culture of Porphyra dioica (Bangiales, Rhodophyta) from Devon, UK. Phycologia 2004, 43, 176–188. [Google Scholar] [CrossRef]
- Broom, J.E.; Nelson, W.A.; Yarish, C.; Jones, W.A.; Rosas, R.A.; Rosas, L.E.A. A reassessment of the taxonomic status of Porphyra suborbiculata, Porphyra carolinensis and Porphyra lilliputiana (Bangiales, Rhodophyta) based on molecular and morphological data. Eur. J. Phycol. 2002, 37, 227–235. [Google Scholar] [CrossRef]
- Lindstrom, S.C. Cryptic diversity, biogeography and genetic variation in Northeast Pacific species of Porphyra sensu lato (Bangiales, Rhodophyta). J. Appl. Phycol. 2008, 20, 951–962. [Google Scholar] [CrossRef]
- Sutherland, J.E.; Lindstrom, S.C.; Nelson, W.A.; Brodie, J.; Lynch, M.D.J.; Hwang, M.S.; Choi, H.-G.; Miyata, M.; Kikuchi, N.; Oliveira, M.C.; et al. A new look at an ancient order: Generic revision of the bangiales (rhodophyta)1. J. Phycol. 2011, 47, 1131–1151. [Google Scholar] [CrossRef] [PubMed]
- Kucera, H.; Saunders, G.W. A a survey of bangiales (rhodophyta) based on multiple molecular markers reveals cryptic diversity1. J. Phycol. 2012, 48, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Egea, E.; David, B.; Choné, T.; Laurin, B.; Féral, J.P.; Chenuil, A. Morphological and genetic analyses reveal a cryptic species complex in the echinoid Echinocardium cordatum and rule out a stabilizing selection explanation. Mol. Phylogenetics Evol. 2016, 94, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Pfingstl, T.; Lienhard, A.; Baumann, J.; Koblmüller, S. A taxonomist‘s nightmare – Cryptic diversity in Caribbean intertidal arthropods (Arachnida, Acari, Oribatida). Mol. Phylogenet. Evol. 2021, 163, 107240. [Google Scholar] [CrossRef] [PubMed]
- Calderon, M.S.; Bustamante, D.E.; Boo, S.M. Red algal diversity (Rhodophyta) from Peru based on molecular analysis. Phytotaxa 2020, 454, 1–23. [Google Scholar] [CrossRef]
- Stegenga, H.; Bolton, J.J.; Anderson, R.J. Seaweeds of the South African West Coast. In Contributions from the Bolus Herbarium 18; Hall, A.V., Ed.; Bolus Herbarium, University of Cape Town: Cape Town, South Africa, 1997; pp. 1–655. [Google Scholar]
- Jones, W.A.; Griffin, N.J.; Jones, D.T.; Nelson, W.A.; Farr, T.J.; Broom, J.E. Phylogenetic diversity in South African Porphyra (Bangiales, Rhodophyta) determined by nuclear SSU sequence analyses. Eur. J. Phycol. 2004, 39, 197–211. [Google Scholar] [CrossRef]
- Reddy, M.M.; De Clerck, O.; Leliaert, F.; Anderson, R.J.; Bolton, J.J. An appraisal of the genus Pyropia (Bangiales, Rhodophyta) from southern Africa based on a multi-gene phylogeny, morphology and ecology, including the description of Pyropia meridionalis sp. nov. South Afr. J. Bot. 2020, 131, 18–32. [Google Scholar] [CrossRef]
- Reddy, M.M. Taxonomy and Systematics of the Bangiales (Rhodophyta) in South Africa using an Integrative Approach. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2018. [Google Scholar]
- Hommersand, M.H.; Fredericq, S. Biogeography of the marine red algae of the South African West Coast: A molecular approach. In Proceedings of the XVIIth International Seaweed Symposium, Cape Town, South Africa, 28 January–2 February 2001; pp. 325–336. [Google Scholar]
- Fraser, C.I.; Nikula, R.; Spencer, H.G.; Waters, J.M. Kelp genes reveal effects of subantarctic sea ice during the Last Glacial Maximum. Proc. Natl. Acad. Sci. USA 2009, 106, 3249–3253. [Google Scholar] [CrossRef]
- Broom, J.E.S.; Nelson, W.A.; Farr, T.J.; Phillips, L.E.; Clayton, M. Relationships of the Porphyra (Bangiales, Rhodophyta) flora of the Falkland Islands: A molecular survey using rbcL and nSSU sequence data. Aust. Syst. Bot. 2010, 23, 27–37. [Google Scholar] [CrossRef]
- Tyberghein, L.; Verbruggen, H.; Pauly, K.; Troupin, C.; Mineur, F.; De Clerck, O. Bio-ORACLE: A global environmental dataset for marine species distribution modelling. Glob. Ecol. Biogeogr. 2011, 21, 272–281. [Google Scholar] [CrossRef]
- Assis, J.; Tyberghein, L.; Bosch, S.; Verbruggen, H.; Serrão, E.A.; De Clerck, O.; Tittensor, D. Bio-ORACLE v2.0: Extending marine data layers for bioclimatic modelling. Glob. Ecol. Biogeogr. 2017, 27, 277–284. [Google Scholar] [CrossRef]
- Atkinson, L.P.; Valle-Levinson, A.; Figueroa, D.; De Pol-Holz, R.; Gallardo, V.A.; Schneider, W.; Blanco, J.L.; Schmidt, M. Oceanographic observations in Chilean coastal waters between Valdivia and Concepción. J. Geophys. Res. Earth Surf. 2002, 107, 18-1–18-13. [Google Scholar] [CrossRef]
- Pérez, C.A.; Lagos, N.A.; Saldías, G.S.; Waldbusser, G.; Vargas, C.A. Riverine discharges impact physiological traits and carbon sources for shell carbonate in the marine intertidal mussel Perumytilus purpuratus. Limnol. Oceanogr. 2016, 61, 969–983. [Google Scholar] [CrossRef]
- Moreno, C.A. Community patterns generated by human harvesting on Chilean shores: A review. Aquat. Conserv. Mar. Freshw. Ecosyst. 2001, 11, 19–30. [Google Scholar] [CrossRef]
- Häussermann, V.; Försterra, G. Marine Benthic Fauna of Chilean Patagonia; Nature in Focus: Puerto Montt, Chile, 2009; p. 1000. [Google Scholar]
- Velásquez, C.; Jaramillo, E.; A Camus, P.; Manzano, M.; Sánchez, R. Biota del intermareal rocoso expuesto de la Isla Grande de Chiloé, Archipiélago de Chiloé, Chile: Patrones de diversidad e implicancias ecológicas y biogeográficas. Rev. De Biol. Mar. Oceanogr. 2016, 51, 33–50. [Google Scholar] [CrossRef]
- Jara, F.H.; Moreno, C.A. Herbivory and Structure in a Midlittorial Rocky Community: A Case in Southern Chile. Ecol. Soc. Am. 1984, 65, 28–38. [Google Scholar] [CrossRef]
- Saunders, G.W. Applying DNA barcoding to red macroalgae: A preliminary appraisal holds promise for future applications. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011. [Google Scholar]
- Johnson, P.C.D. Extension of Nakagawa & Schielzeth’s R2GLMM to random slopes models. Methods Ecol. Evol. 2014, 5, 944–946. [Google Scholar] [CrossRef]
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The coefficient of determination R 2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 16 August 2021).
- Neuwirth, E. RColorBrewer: ColorBrewer Palettes. Package version 1. Available online: https://CRAN.R-project.org/package=RColorBrewer (accessed on 16 August 2021).
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for ggplot2. R Package Version 100. Available online: https://cran.r-project.org/package=cowplot (accessed on 16 August 2021).
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R. J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Ram, K.; Wickham, H. Wesanderson: A Wes Anderson Palette Generator. R Package Version 036. Available online: https://rdrr.io/cran/wesanderson (accessed on 16 August 2021).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Packag Version 2. Available online: https://CRAN.R-project.org/package=vegan (accessed on 16 August 2021).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference. R package version 1.43.17. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 16 August 2021).
- Luo, Q.; Zhu, Z.; Zhu, Z.; Yang, R.; Qian, F.; Chen, H.; Yan, X. Different Responses to Heat Shock Stress Revealed Heteromorphic Adaptation Strategy of Pyropia haitanensis (Bangiales, Rhodophyta). PLoS ONE 2014, 9, e94354. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yamada, H.; Mine, T.; Kawamura, Y.; Nishihara, G.N.; Terada, R. Photosynthetic responses of Pyropia yezoensis f. narawaensis (Bangiales, Rhodophyta) to a thermal and PAR gradient vary with the life-history stage. Phycologia 2016, 55, 665–672. [Google Scholar] [CrossRef]
- Santelices, B.; Ugarte, R. Algal life-history strategies and resistance to digestion. Mar. Ecol. Prog. Ser. 1987, 35, 267–275. [Google Scholar] [CrossRef]
- Steneck, R.S.; Dethier, M.N. A Functional Group Approach to the Structure of Algal-Dominated Communities. Oikos 1994, 69, 476. [Google Scholar] [CrossRef]



| Pyropia sp. CHJ | Pyropia orbicularis | Porphyra mumfordii | Porphyra sp. FIH | Pyropia sp. CHH | Porphyra longissima | Porphyra luchea | Fuscifolium sp. CHA | Pyropia saldanhae | Ʃ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Autumn | Pilolcura | 28 | 23 | 2 | 27 | 4 | 0 | 0 | 0 | 0 | 84 |
| Niebla | 48 | 11 | 8 | 0 | 2 | 3 | 0 | 0 | 0 | 72 | |
| San Carlos | 15 | 3 | 42 | 18 | 0 | 0 | 0 | 0 | 0 | 78 | |
| Los Liles | 44 | 3 | 23 | 19 | 0 | 0 | 0 | 0 | 0 | 89 | |
| Melinka | 16 | 13 | 2 | 13 | 0 | 0 | 0 | 0 | 0 | 44 | |
| Winter | Pilolcura | 42 | 20 | 0 | 7 | 1 | 3 | 0 | 0 | 0 | 73 |
| Niebla | 39 | 18 | 31 | 0 | 1 | 3 | 0 | 0 | 0 | 92 | |
| San Carlos | 53 | 5 | 15 | 4 | 0 | 7 | 0 | 0 | 1 | 85 | |
| Los Liles | 55 | 2 | 30 | 8 | 0 | 0 | 0 | 0 | 0 | 95 | |
| Melinka | 10 | 30 | 45 | 3 | 0 | 0 | 6 | 0 | 0 | 94 | |
| Spring | Pilolcura | 29 | 48 | 0 | 11 | 1 | 0 | 0 | 0 | 0 | 89 |
| Niebla | 55 | 11 | 0 | 0 | 21 | 5 | 0 | 0 | 0 | 92 | |
| San Carlos | 33 | 28 | 27 | 0 | 0 | 2 | 0 | 1 | 2 | 93 | |
| Los Liles | 40 | 8 | 25 | 17 | 0 | 0 | 0 | 1 | 0 | 91 | |
| Melinka | 0 | 58 | 37 | 2 | 0 | 0 | 0 | 0 | 0 | 97 | |
| Summer | Pilolcura | 12 | 76 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 92 |
| Niebla | 23 | 25 | 1 | 0 | 35 | 0 | 0 | 0 | 0 | 84 | |
| San Carlos | 4 | 64 | 7 | 20 | 0 | 0 | 0 | 0 | 0 | 95 | |
| Los Liles | 31 | 21 | 14 | 20 | 0 | 1 | 0 | 1 | 0 | 88 | |
| Melinka | 2 | 56 | 42 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | |
| Ʃ | 579 | 523 | 351 | 170 | 66 | 24 | 7 | 4 | 3 | 1727 | |
| Effect | df | F | R2 |
|---|---|---|---|
| Elevation | 3 | 42.91 ** | 0.19 |
| Season | 3 | 15.82 ** | 0.07 |
| Elevation: season | 9 | 3.59 ** | 0.05 |
| Residuals | 464 | 0.68 | |
| Total | 479 | 1 | |
| Pairwise test | |||
| Season | Intertidal elevation | ||
| Spring | Mid ** | ||
| Spring | High ** | ||
| Spring | Spray ** | ||
| Summer | Mid | ||
| Summer | High ** | ||
| Summer | Spray ** | ||
| Autumn | Mid ** | ||
| Autumn | High ** | ||
| Autumn | Spray ** | ||
| Winter | Mid ** | ||
| Winter | High ** | ||
| Winter | Spray ** |
| Season | Intertidal Elevation | Pyropia sp. CHJ | Pyropia orbicularis | Porphyra mumfordii | Porphyra sp. FIH | Pyropia sp. CHH |
|---|---|---|---|---|---|---|
| Spring | Mid | 2.40 | −1.5 | <−0.01 | <−0.01 | −1.5 |
| Spring | High | 3.16 ** | −6.2 *** | <−0.01 | <−0.01 | −2.5 |
| Spring | Spray | −1.31 | −4.3 *** | <−0.01 | <−0.01 | <−0.01 |
| Summer | Mid | 2.3 | −0.31 | <−0.01 | <−0.01 | −0.9 |
| Summer | High | 3.49 ** | −4.9 *** | 3.9 ** | <−0.01 | <−0.01 |
| Summer | Spray | 0.04 | <−0.01 | 1.5 | <−0.01 | <−0.01 |
| Autumn | Mid | 1.25 | −4.1 *** | 1.6 | 1.7 | <−0.01 |
| Autumn | High | −0.35 | <−0.01 | 0.9 | 3.9 ** | <−0.01 |
| Autumn | Spray | −4.41 *** | <−0.01 | −0.9 | 2.7 * | <−0.01 |
| Winter | Mid | 1.24 | −3.8 ** | 2.4 | 1.2 | <−0.01 |
| Winter | High | −0.33 | −4.5 *** | 5.0 *** | 1.6 | <−0.01 |
| Winter | Spray | −4.31 *** | <−0.01 | 2.7 * | 1.9 | <−0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cid Alda, F.P.; Valdivia, N.; Guillemin, M.-L. More than What Meets the Eye: Differential Spatiotemporal Distribution of Cryptic Intertidal Bangiales. Plants 2022, 11, 605. https://doi.org/10.3390/plants11050605
Cid Alda FP, Valdivia N, Guillemin M-L. More than What Meets the Eye: Differential Spatiotemporal Distribution of Cryptic Intertidal Bangiales. Plants. 2022; 11(5):605. https://doi.org/10.3390/plants11050605
Chicago/Turabian StyleCid Alda, Fernanda P., Nelson Valdivia, and Marie-Laure Guillemin. 2022. "More than What Meets the Eye: Differential Spatiotemporal Distribution of Cryptic Intertidal Bangiales" Plants 11, no. 5: 605. https://doi.org/10.3390/plants11050605
APA StyleCid Alda, F. P., Valdivia, N., & Guillemin, M.-L. (2022). More than What Meets the Eye: Differential Spatiotemporal Distribution of Cryptic Intertidal Bangiales. Plants, 11(5), 605. https://doi.org/10.3390/plants11050605






