Water Stress Alters Morphophysiological, Grain Quality and Vegetation Indices of Soybean Cultivars
Abstract
:1. Introduction
2. Results and Discussion
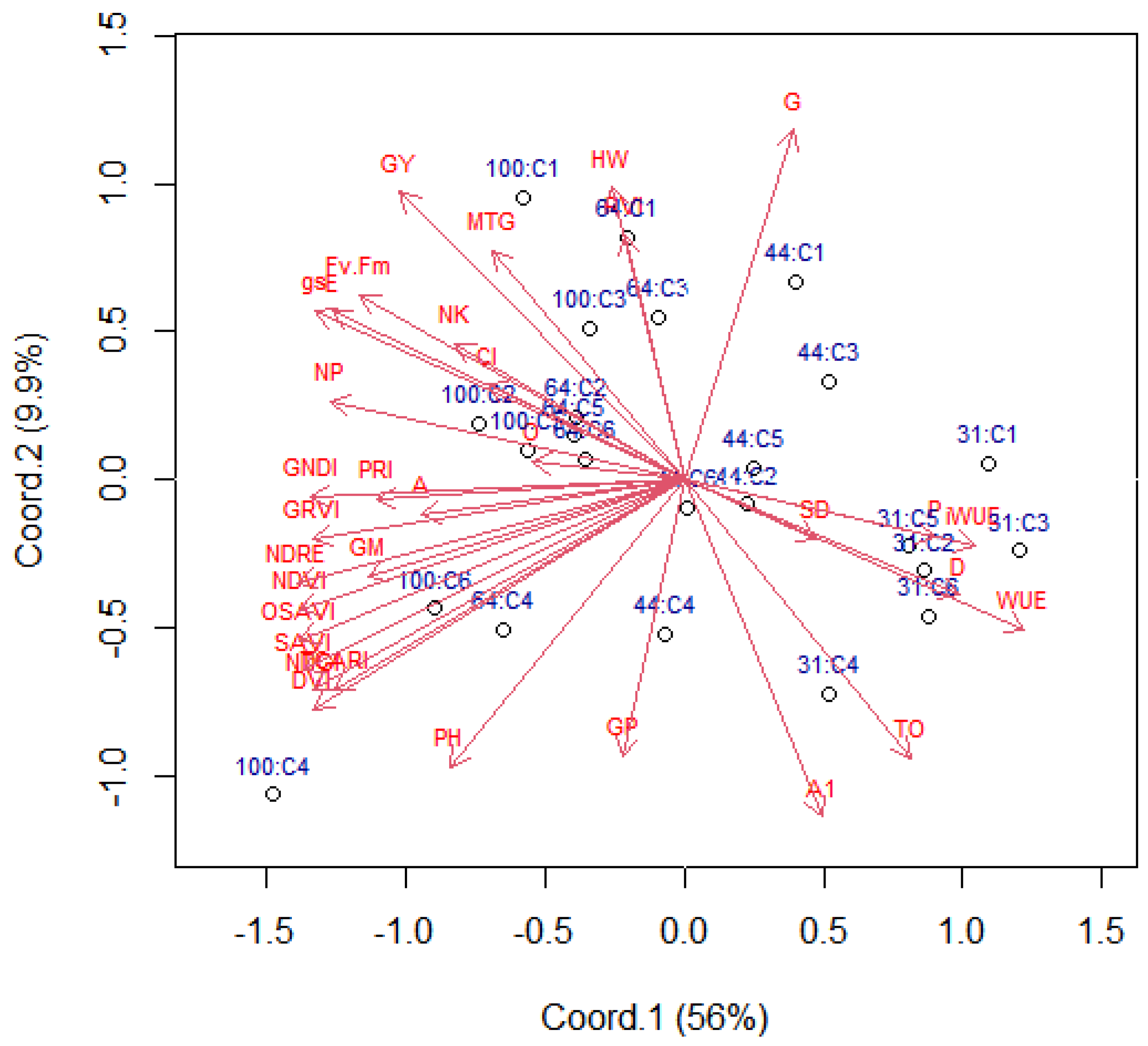
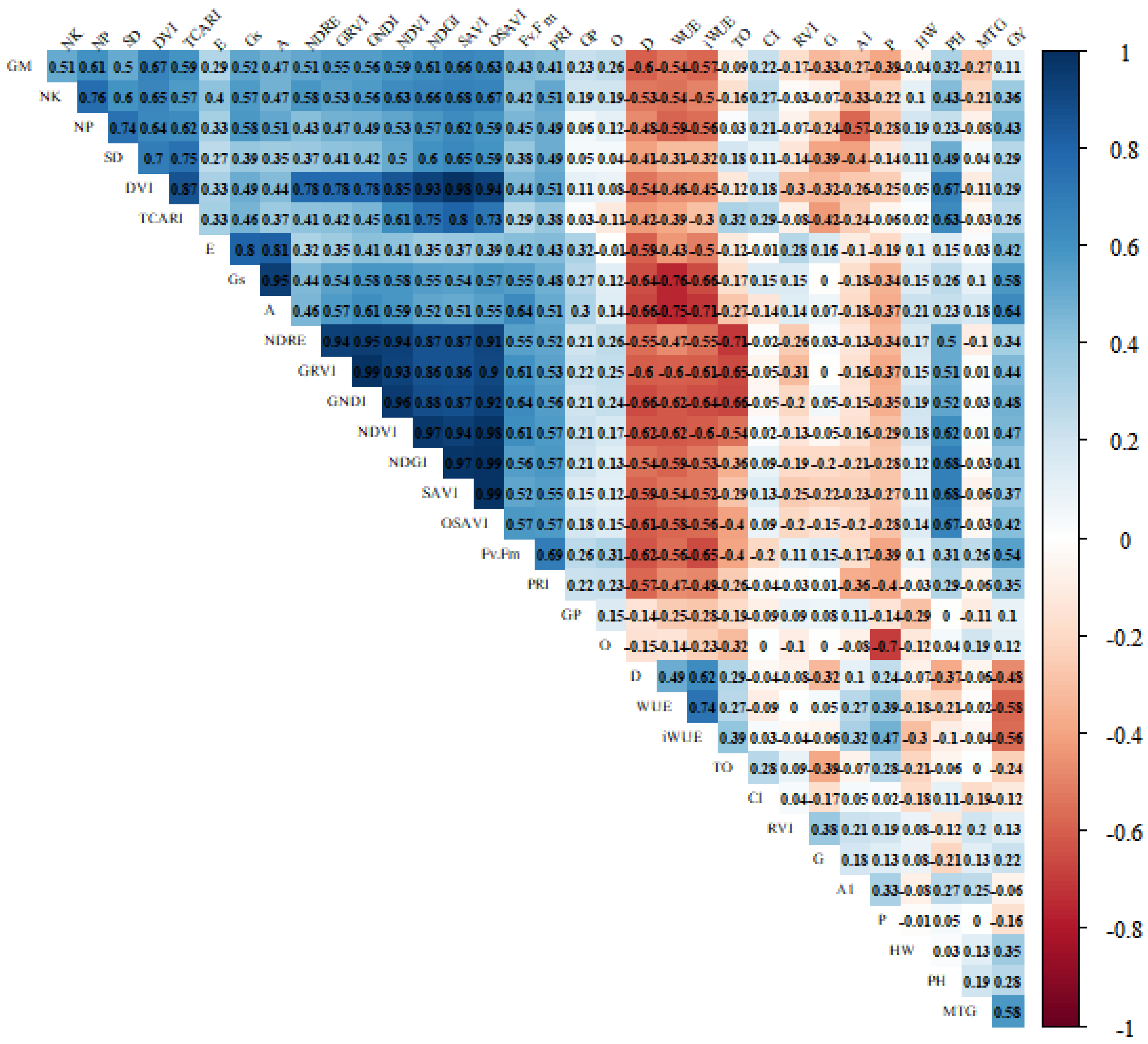
2.1. Variable Contributions in the Multivariate Response
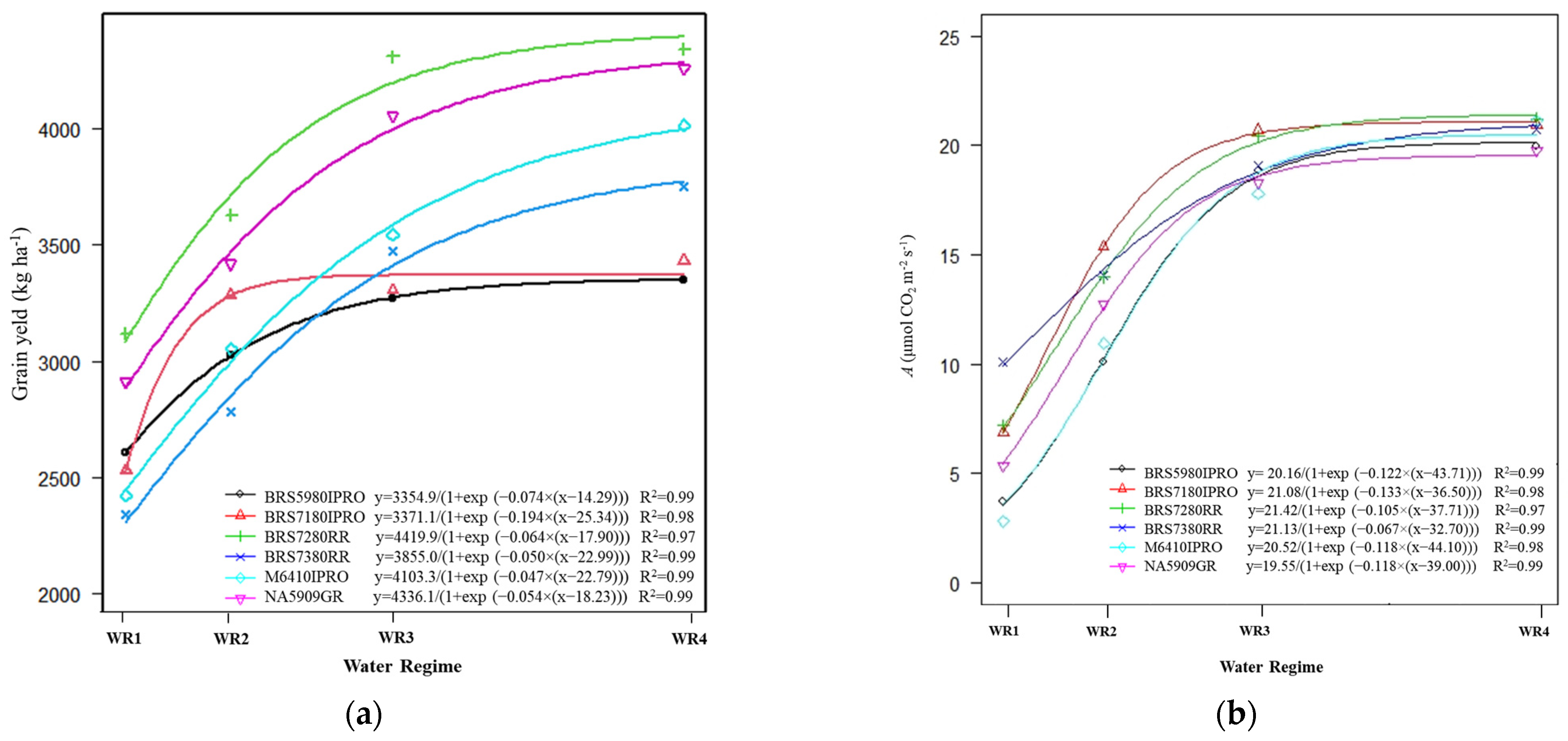
2.2. Grain Yield and Net CO2 Assimilation
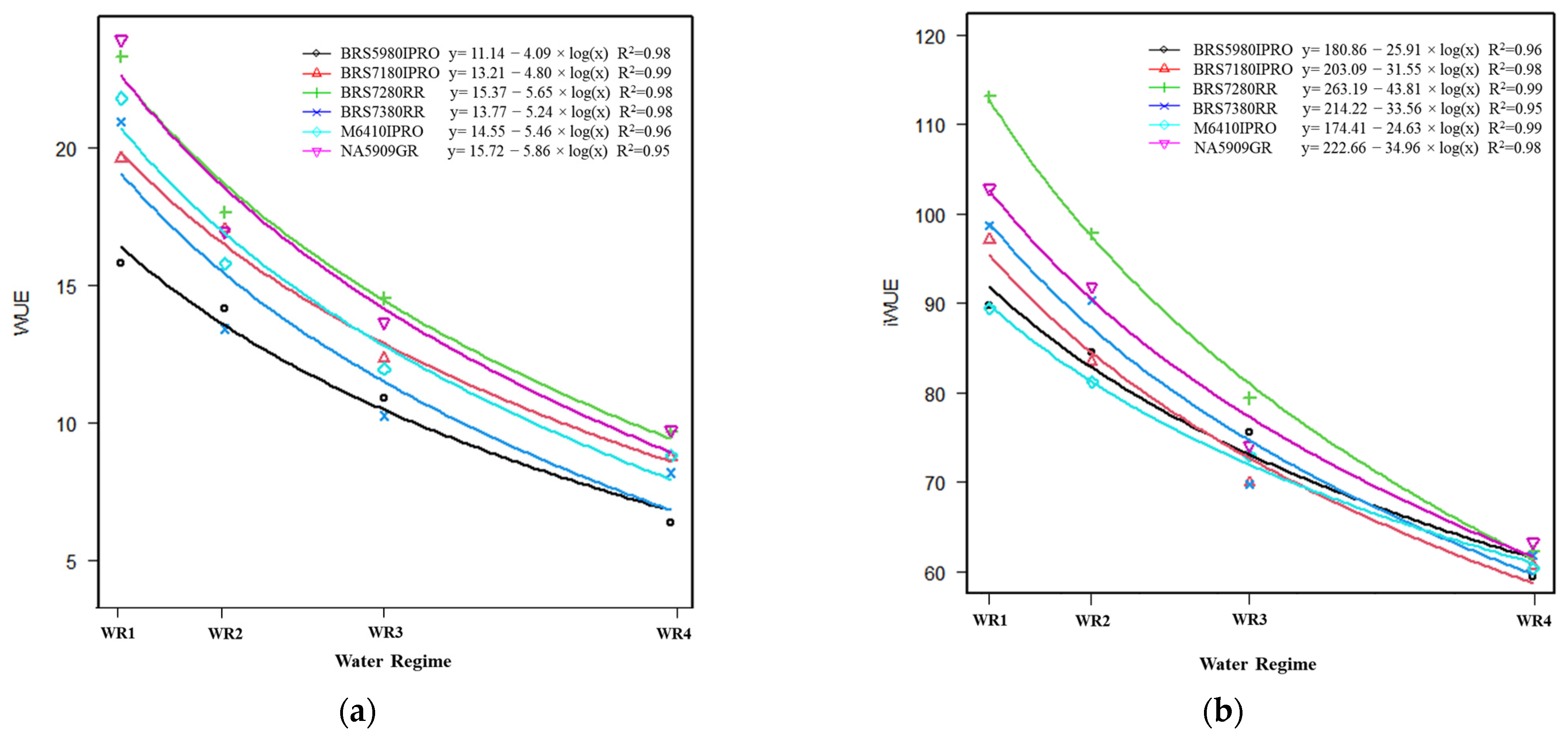
2.3. Water Use Efficiency
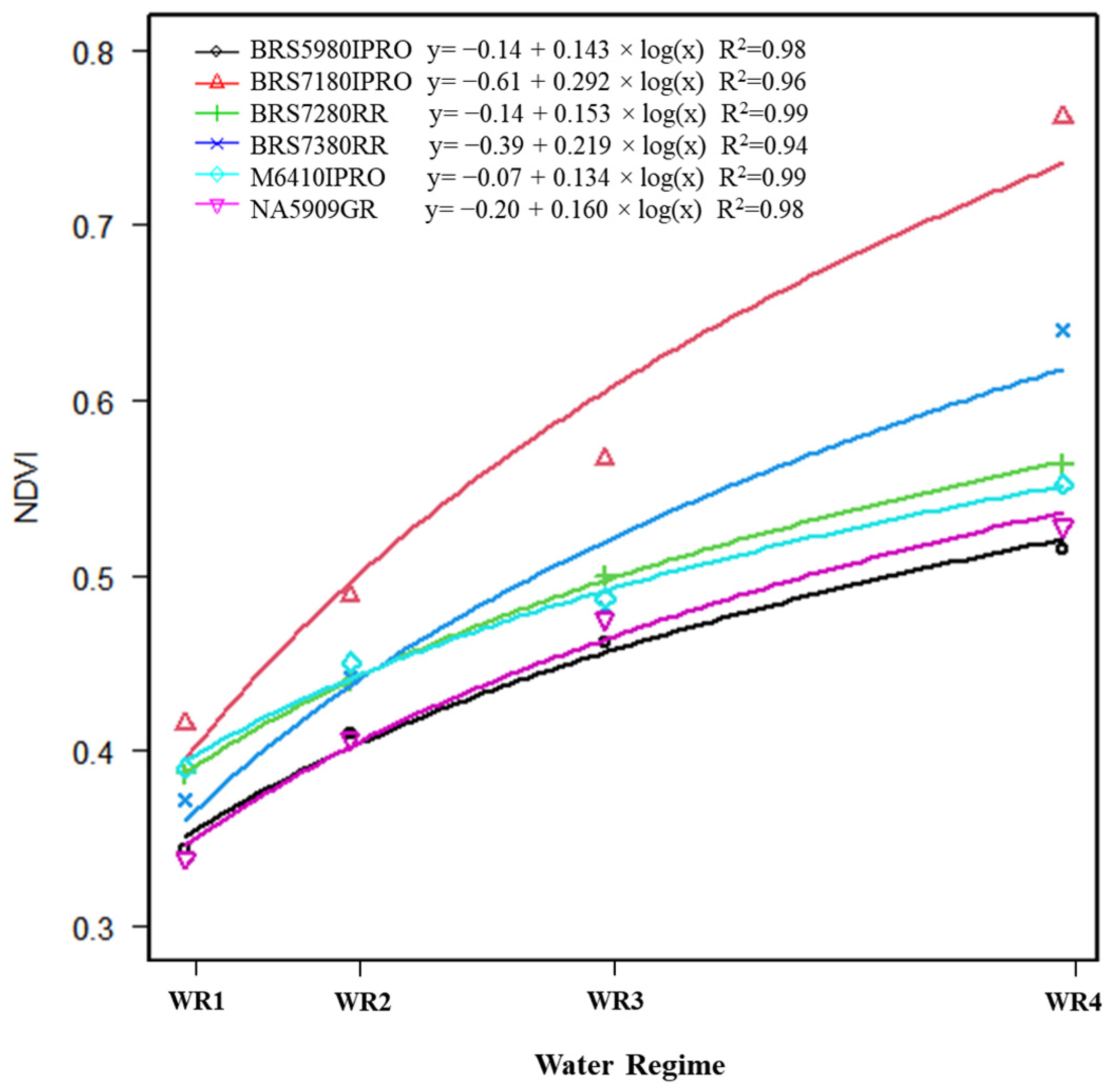
2.4. Normalized Difference Vegetation Index (NDVI)
2.5. Protein Content and Oil Content in the Grains
3. Materials and Methods
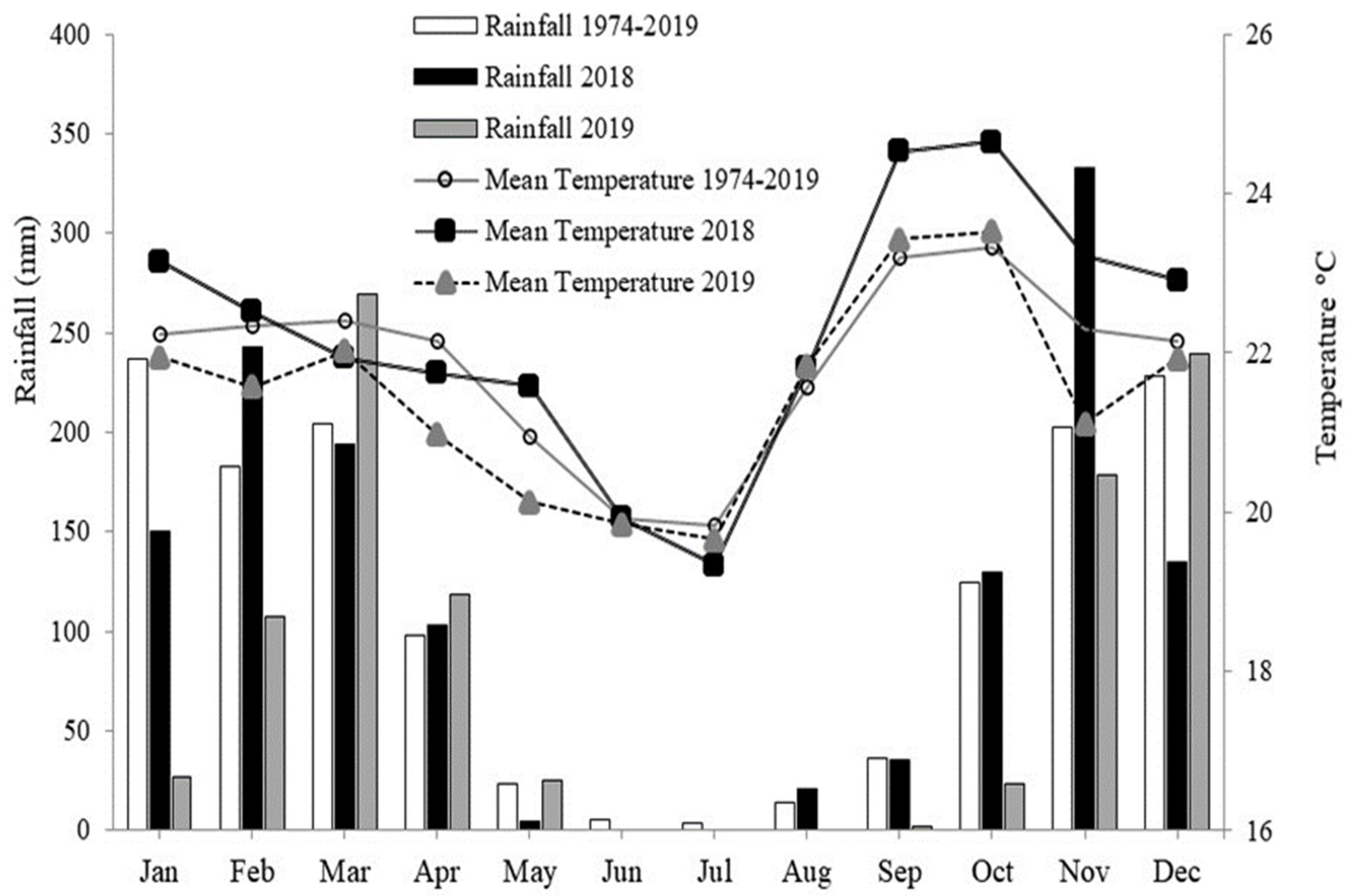
3.1. Experimental Design and Conducting the Experiment
3.2. Gas Exchange and Fluorescence Analysis
3.3. Vegetation Index
3.4. Grain Yield and Quality Components
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, L.F.; Ribeiro Junior, W.Q.; Ramos, M.L.G.; Santos, N.Z.; Soares, G.F.; Casari, R.A.C.N.; Muller, O.; Tavares, C.J.; Martins, É.S.; Rascher, U.; et al. Physiological changes in soybean cultivated with soil remineralizer in the Cerrado under variable water regimes. Pesqui. Agropecu. Bras. 2021, 56, e01455. [Google Scholar] [CrossRef]
- FAO. How to Feed the World—2050: High-Level Expert Forum. Food and Agriculture Organization of the United Nations: Rome, Italy. 2009. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/DD85967BC807184AC125764900433678-Full_Report.pdf (accessed on 20 January 2022).
- Scheelbeek, P.F.; Bird, F.A.; Tuomisto, H.L.; Green, R.; Harris, F.B.; Joy, E.J.M.; Chalabi, Z.; Allemn, E.; Haines, A.; Dangour, A.D. Effect of environmental changes on vegetable and legume yields and nutritional quality. Proc. Natl. Acad. Sci. USA 2018, 115, 6804–6809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, G.F.; Ribeiro Júnior, W.Q.; Pereira, L.F.; Lima, C.A.; Soares, D.D.S.; Muller, O.; Rascher, U.; Ramos, M.L.G. Characterization of wheat genotypes for drought tolerance and water use efficiency. Sci. Agric. 2021, 78, e20190304. [Google Scholar] [CrossRef]
- Cattelan, A.J.; Dall’agnol, A. The rapid soybean growth in Brazil. OCL 2018, 25, D102. [Google Scholar] [CrossRef] [Green Version]
- Lopes, A.S.; Guimarães, G.L.R. A career perspective on soil management in the Cerrado region of Brazil. Adv. Agron. 2016, 137, 1–72. [Google Scholar] [CrossRef]
- Alexander, L.V. Global observed long-term changes in temperature and precipitation extremes, a review of progress and limitations in IPCC assessments and beyond. Weather Clim. Extrem. 2016, 11, 4–16. [Google Scholar] [CrossRef] [Green Version]
- Meena, R.P.; Venkatesh, K.; Sendhil, R.; Khoba, R.; Sharma, R.K.; Tripathi, S.C.; Singh, G.P. Identification of water use efficient wheat genotypes with high yield for regions of depleting water resources in India. Agric. Water Manag. 2019, 223, 105709. [Google Scholar] [CrossRef]
- Fried, H.G.; Narayanan, S.; Fallen, B. Evaluation of soybean [Glycine max (L.) Merr.] genotypes for yield, water use efficiency, and root traits. PLoS ONE 2019, 14, e0212700. [Google Scholar] [CrossRef]
- Braga, P.; Crusiol, L.G.T.; Nanni, M.R.; Caranhato, A.L.H.; Fuhrmann, M.B.; Nepomuceno, A.L.; Neumaier, N.; Farias, J.R.B.; Koltun, A.; Gonçalves, L.S.A.; et al. Vegetation indices and NIR-SWIR spectral bands as a phenotyping tool for water status determination in soybean. Precis. Agric. 2021, 22, 249–266. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, J.; Ye, H.; Ali, M.D.L.; Chen, P.C.; Nguyen, H.T.; Chen, P. Yield estimation of soybean breeding lines under drought stress using unmanned aerial vehicle-based imagery and convolutional neural network. Biosyst. Eng. 2021, 204, 90–103. [Google Scholar] [CrossRef]
- Buezo, J.; Sanz-Saez, A.; Morana, J.F.; Sobaa, D.; Aranjuelo, I.; Esteban, R. Drought tolerance response of high-yielding soybean varieties to mild drought, physiological and photochemical adjustments. Physiol. Plant. 2019, 166, 88–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijewardana, C.; Reddy, K.R.; Bellaloui, N. Soybean seed physiology, quality, and chemical composition under soil moisture stress. Food Chem. 2019, 278, 92–100. [Google Scholar] [CrossRef]
- Qiu, R.C.; Wei, S.; Zhang, M.; Sun, H.; Li, H.; Liu, G.; Li, M. Sensors for measuring plant phenotyping: A review. Int. J. Agric. Biol. Eng. 2018, 11, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.E.; Baio, F.H.R.; Teodoro, L.P.R.; Silva Junior, C.A.; Borges, R.S.; Teodoro, P.E. UAV-multispectral and vegetation indices in soybean grain yield prediction based on in situ observation. Remote Sens. Appl. Soc. Environ. 2020, 18, 100318. [Google Scholar] [CrossRef]
- Sobejano-Paz, V.; Mikkelsen, T.N.; Baum, A.; Mo, X.; Liu, S.; Köppl, C.J.; Johnson, M.S.; Gulyas, L.; García, M. Hyperspectral and Thermal Sensing of Stomatal Conductance, Transpiration, and Photosynthesis for Soybean and Maize under Drought. Remote Sens. 2020, 12, 3182. [Google Scholar] [CrossRef]
- Zhao, Y.; Potgieter, A.B.; Zhang, M.; Wu, B.; Hammer, G.L. Predicting Wheat Yield at the Field Scale by Combining High-Resolution Sentinel-2 Satellite Imagery and Crop Modelling. Remote Sens. 2020, 12, 1024. [Google Scholar] [CrossRef] [Green Version]
- Ballester, C.; Zarco-Tejada, P.J.; Nicolás, E.; Alarcón, J.J.; Fereres, E.; Intrigliolo, D.S.; Gonzalez-Dugo, V. Evaluating the performance of xanthophyll, chlorophyll and structure-sensitive spectral indices to detect water stress in five fruit tree species. Precis. Agric. 2018, 19, 178–193. [Google Scholar] [CrossRef]
- Crusiol, L.G.T.; Carvalho, J.D.F.C.; Sibaldelli, R.N.R.; Neiverth, W.; Rio, A.; Ferreira, L.C.; Procópio, S.O.; Mertz-Henning, L.M.; Nepomuceno, A.L.; Neumaier, N.; et al. NDVI variation according to the time of measurement, sampling size, positioning of sensor and water regime in different soybean cultivars. Precis. Agric. 2017, 18, 470–490. [Google Scholar] [CrossRef] [Green Version]
- Maimaitiyiming, M.; Ghulam, A.; Bozzolo, A.; Wilkins, J.L.; Kwasniewski, M.T. Early detection of plant physiological responses to different levels of water stress using reflectance spectroscopy. Remote Sens. 2017, 9, 745. [Google Scholar] [CrossRef] [Green Version]
- Zarco-Tejada, P.J.; González-Dugo, V.; Berni, J.A. Fluorescence, temperature and narrow-band indices acquired from a UAV platform for water stress detection using a micro-hyperspectral imager and a thermal camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Guillén-Climent, M.L.; Hernández-Clemente, R.; Catalina, A.; González, M.R.; Martín, P. Estimating leaf ca-rotenoid content in vineyards using high resolution hyperspectral imagery acquired from an unmanned aerial vehicle (UAV). Agric. For. Meteorol. 2013, 171, 281–294. [Google Scholar] [CrossRef] [Green Version]
- Peres, D.J.; Cancelliere, A. Analysis of Multi-Spectral Images Acquired by UAVs to Monitor Water Stress of Citrus Orchards in Sicily, Italy. In World Environmental and Water Resources Congress; American Society of Civil Engineers: Reston, VA, USA, 2021; pp. 270–278. [Google Scholar]
- Santesteban, L.G.; Di Gennaro, S.F.; Herrero-Langreo, A.; Miranda, C.; Royo, J.B.; Matese, A. High-resolution UAV-based thermal imaging to estimate the instantaneous and seasonal variability of plant water status within a vineyard. Agric. Water Manag. 2017, 183, 49–59. [Google Scholar] [CrossRef]
- Gurumurthy, S.; Sarkar, B.; Vanaja, M.; Lakshmi, J.; Yadav, S.K.; Maheswari, M. Morpho-physiological and biochemical changes in black gram (Vigna mungo L. Hepper) genotypes under drought stress at flowering stage. Acta Physiol. Plant. 2019, 41, 42. [Google Scholar] [CrossRef]
- Silva, A.N.; Ramos, M.L.G.R.; Ribeiro Júnior, W.Q.R.; Alencar, E.R.; Silva, P.C.; Lima, C.A.; Vinson, C.C.; Silva, M.A.V. Water stress alters physical and chemical quality in grains of common bean, triticale and wheat. Agric. Water Manag. 2020, 231, 106023. [Google Scholar] [CrossRef]
- Gabriel, K.R. The biplot graphical display of matrices with application to principal component analysis. Biometrika 1971, 58, 453–467. [Google Scholar] [CrossRef]
- Rencher, A.C. Methods of Multivariate Analysis, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2002. [Google Scholar]
- Damm, A.; Paul-Limoges, E.; Haghighi, E.; Simmer, C.; Morsdorf, F.; Schneider, F.D.; Van Der Tol, C.; Migliavacca, M.; Rascher, U. Remote sensing of plant-water relations, An overview and future perspectives. J. Plant Physiol. 2018, 227, 3–19. [Google Scholar] [CrossRef]
- Ali, F.; Ahsan, M.; Ali, Q.; Kanwal, N. Phenotypic stability of Zea mays grain yield and its attributing traits under drought stress. Front. Plant Sci. 2017, 8, 1397. [Google Scholar] [CrossRef] [Green Version]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress, Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, N.; Hussain, S.; Zhang, X.W.; Yang, C.Q.; Raza, M.A.; Deng, J.C.; Ahmad, S.; Ashgar, M.A.; Zhang, J.; Yang, W.; et al. Imbalance water deficit improves the seed yield and quality of soybean. Agronomy 2018, 8, 168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Feng, G.; Read, J.J.; Kong, X.; Ouyang, Y.; Adeli, A.; Jenkins, J.N. Simulating soybean productivity under rainfed conditions for major soil types using APEX model in East Central Mississippi. Agric. Water Manag. 2016, 177, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Basal, O.; Szabó, A.; Veres, S. Physiology of soybean as affected by PEG-induced drought stress. Curr. Plant Biol. 2020, 22, 100135. [Google Scholar] [CrossRef]
- Flexas, J.; Diaz-Espejo, A.; Gago, J.; Gallé, A.; Galmés, J.; Gulías, J.; Medrano, H. Photosynthetic limitations in Mediterranean plants: A review. Environ. Exp. Bot. 2014, 103, 12–23. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Identification of drought tolerant genotypes using physiological traits in soybean. Physiol. Mol. Biol. Plants 2019, 25, 697–711. [Google Scholar] [CrossRef]
- Mwamlima, L.H.; Ouma, J.P.; Cheruiyot, E.K. Physiological response of soybean [Glycine max (L) Merrill] to soil moisture stress. Afr. J. Agric. Res. 2019, 14, 729–739. [Google Scholar] [CrossRef]
- Castro, J.N.; Muller, C.; Almeida, G.M.; Costa, A.C. Physiological tolerance to drought under high temperature in soybean cultivars. Aust. J. Crop Sci. 2019, 13, 976–987. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Sauer, T.J.; Prueger, J.H. Managing soils to achieve greater water use efficiency: A review. Agron. J. 2001, 93, 271–280. [Google Scholar] [CrossRef]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of stomatal density and morphology on water-use efficiency in a changing world. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorthi, A.; Volenec, J.J.; Welp, L.R. Stomatal response in soybean during drought improves leaf-scale and field-scale water use efficiencies. Agric. For. Meteorol. 2019, 276, 107629. [Google Scholar] [CrossRef]
- Lopez, M.A.; Xavier, A.; Rainey, K.M. Phenotypic Variation and Genetic Architecture for Photosynthesis and Water Use Efficiency in Soybean (Glycine max L. Merr). Front. Plant Sci. 2019, 10, 680. [Google Scholar] [CrossRef] [Green Version]
- Chacon, D.P.; Barajas, E.M.; Esteva, A.G.; Delgado, R.L.; Shibata, J.K.; Valdivia, C.B.P. Biomass remobilization in two common bean (Phaseolus vulgaris) cultivars under water restriction. S. Afr. J. Bot. 2017, 112, 79–88. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy. In Soil Survey Field and Laboratory Methods Manual; Soil Survey Investigations Report No. 51, Version 2.0; Burt, R., Soil Survey Staff, Eds.; U.S. Department of Agriculture; Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef] [Green Version]
- Hanks, R.J.; Keller, J.; Rasmusse, V.P.; Wilson, G.D. Line source sprinkler for continuous variable irrigation crop production studies. Soil Sci. Soc. Am. J. 1976, 40, 426–429. [Google Scholar] [CrossRef]
- Jayme-Oliveira, A.; Ribeiro Junior, W.Q.; Ramos, M.L.G.; Ziviani, A.C.; Jakelaitis, A. Amaranth, quinoa, and millet growth and development under different water regimes in the Brazilian Cerrado. Pesqui. Agropecu. Bras. 2017, 52, 561–571. [Google Scholar] [CrossRef]
- Embrapa. Programa de Monitoramento da Irrigação; EMBRAPA: Brasília, Brazil, 2011. [Google Scholar]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Micasense. How Do Professional Multispectral Cameras Differ from Single-Imager Multispectral Cameras—RedEdge-MX 2020. Available online: http://www.micasense.com/ (accessed on 15 January 2020).
- Myneni, R.B.; Hall, F.G.; Sellers, P.J.; Marshak, A.L. The interpretation of spectral vegetation indexes. IEEE Trans. Geosci. Remote Sens. 1995, 33, 481–486. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; Colstoun, E.B.; Mcmurtrey, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Sripada, R.P.; Heiniger, R.W.; White, J.G.; Meijer, A.D. Aerial Color Infrared Photography for Determining Early In-season Nitrogen Requirements in Corn. Agron. J. 2006, 98, 968–977. [Google Scholar] [CrossRef]
- Gao, B.C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Quantitative estimation of chlorophyll-a using reflectance spectra, Experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation índices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Gamon, J.A.; Penuelas, J.; Field, C.B.A. Narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Steven, M.D. The sensitivity of the OSAVI vegetation index to Observational Parameters. Remote Sens. Environ. 1998, 63, 49–60. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrowband vegetation indices for prediction of crop chlorophyll content for application to Precis. Agric. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Heil, C. Rapid, Multi-Component Analysis of Soybeans by FT-NIR Spectroscopy; Thermo Fisher Scientific: Madison, WI, USA, 2010; 3p, (Application note, 51954); Available online: https://tools.thermofisher.com/content/sfs/brochures/AN-51954-Rapid-Multi-omponent-Analysis-Soybeans-AN51954-EN.pdf (accessed on 27 January 2020).
- Villasenor-Alva, J.A.; Gonzalez-Estrada, E. A generalization of Shapiro-Wilk’s test for multivariate normality. Commun. Stat. Theory Methods 2009, 38, 1870–1883. [Google Scholar] [CrossRef]
- Box, G.E.P. A general distribution theory for a class of likelihood criteria. Biometrika 1949, 36, 317–346. [Google Scholar] [CrossRef]


| Vegetation Index | Formula | Source |
|---|---|---|
| Normalized Difference Vegetation Index | [51] | |
| Green Normalized Difference Vegetation Index | [52] | |
| Green-Red Vegetation Index | [53] | |
| Difference Vegetation Index | [54] | |
| Normalized Difference Red Edge | [55] | |
| Soil-Adjusted Vegetation Index | [56] | |
| Photochemical l Reflectance Index | [57] | |
| Optimized Soil-Adjusted Vegetation Index | [58] | |
| Chlorophyll Absorption and Reflectance Index | TCARI = 3[(RedEdge-Red) − 0.2(RedEdge-Green) (Red Edge/Red)] | [59] |
| TCARI/OSAVI Ratio | TO = TCARI/OSAVI | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, C.J.; Ribeiro Junior, W.Q.; Ramos, M.L.G.; Pereira, L.F.; Casari, R.A.d.C.N.; Pereira, A.F.; de Sousa, C.A.F.; da Silva, A.R.; Neto, S.P.d.S.; Mertz-Henning, L.M. Water Stress Alters Morphophysiological, Grain Quality and Vegetation Indices of Soybean Cultivars. Plants 2022, 11, 559. https://doi.org/10.3390/plants11040559
Tavares CJ, Ribeiro Junior WQ, Ramos MLG, Pereira LF, Casari RAdCN, Pereira AF, de Sousa CAF, da Silva AR, Neto SPdS, Mertz-Henning LM. Water Stress Alters Morphophysiological, Grain Quality and Vegetation Indices of Soybean Cultivars. Plants. 2022; 11(4):559. https://doi.org/10.3390/plants11040559
Chicago/Turabian StyleTavares, Cássio Jardim, Walter Quadros Ribeiro Junior, Maria Lucrecia Gerosa Ramos, Lucas Felisberto Pereira, Raphael Augusto das Chagas Noqueli Casari, André Ferreira Pereira, Carlos Antonio Ferreira de Sousa, Anderson Rodrigo da Silva, Sebastião Pedro da Silva Neto, and Liliane Marcia Mertz-Henning. 2022. "Water Stress Alters Morphophysiological, Grain Quality and Vegetation Indices of Soybean Cultivars" Plants 11, no. 4: 559. https://doi.org/10.3390/plants11040559
APA StyleTavares, C. J., Ribeiro Junior, W. Q., Ramos, M. L. G., Pereira, L. F., Casari, R. A. d. C. N., Pereira, A. F., de Sousa, C. A. F., da Silva, A. R., Neto, S. P. d. S., & Mertz-Henning, L. M. (2022). Water Stress Alters Morphophysiological, Grain Quality and Vegetation Indices of Soybean Cultivars. Plants, 11(4), 559. https://doi.org/10.3390/plants11040559







