Priming by Insects: Differential Effects of Sympatric and Allopatric Priming upon Plant Performance and Tolerance to Herbivory
Abstract
:1. Introduction
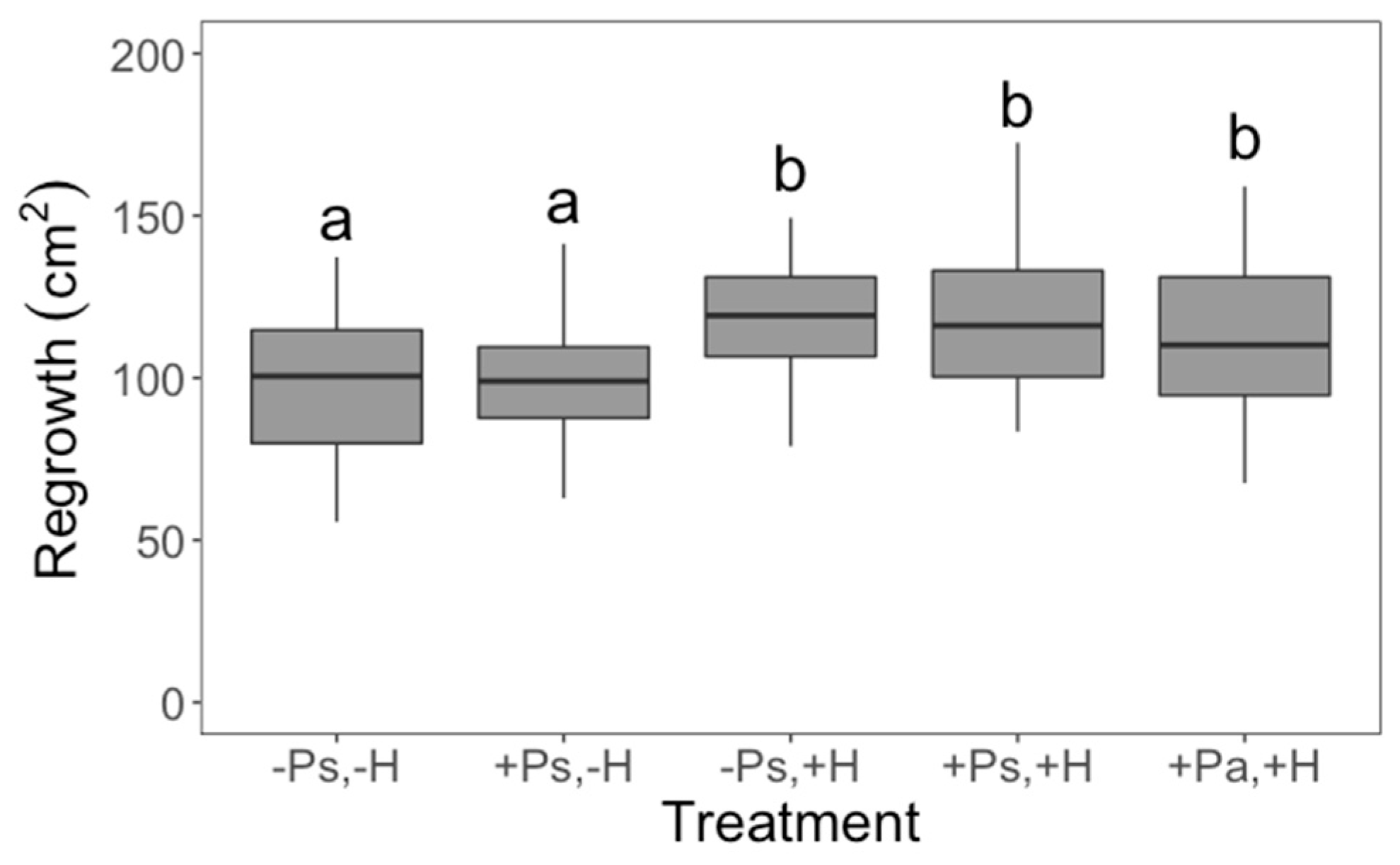
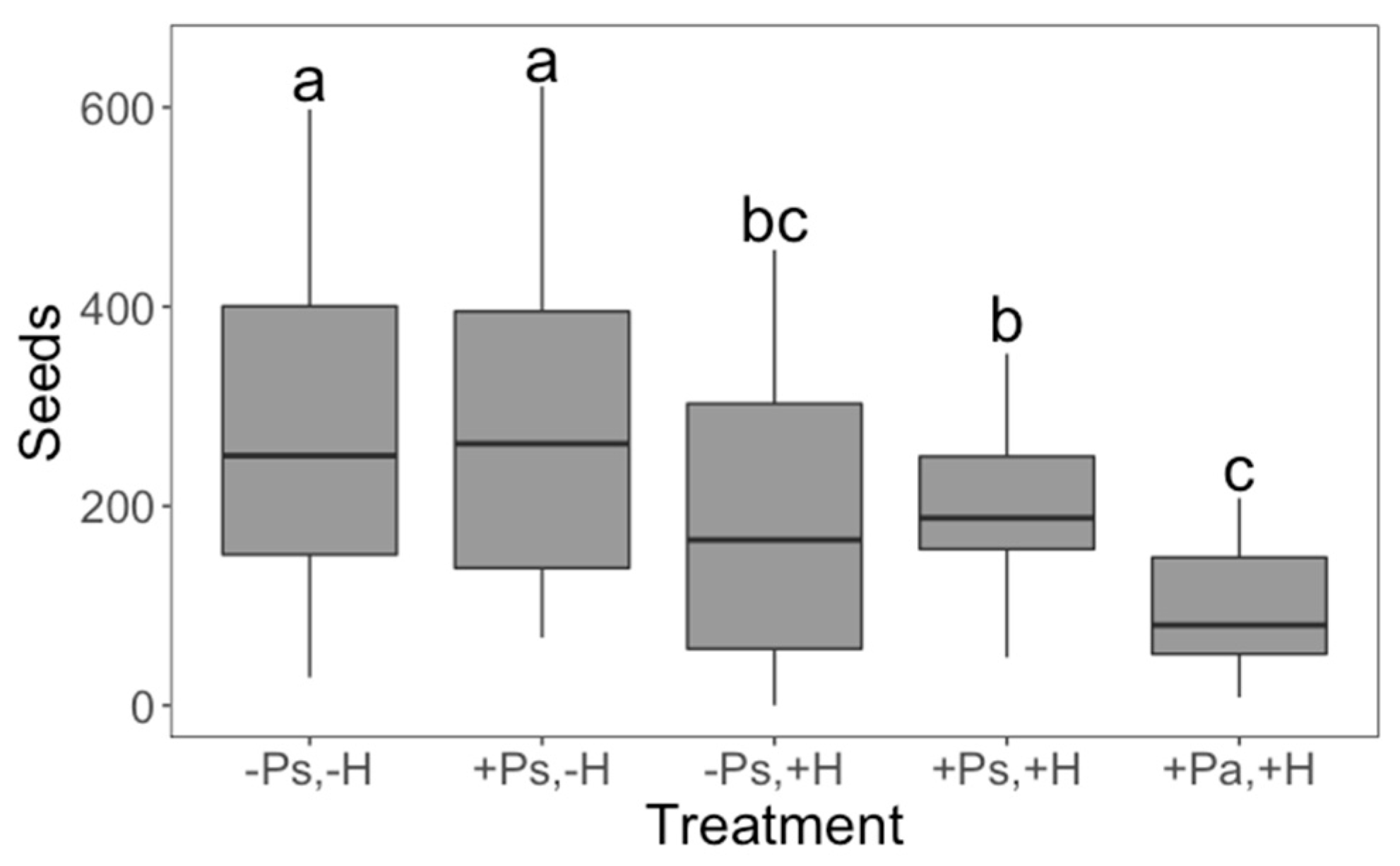
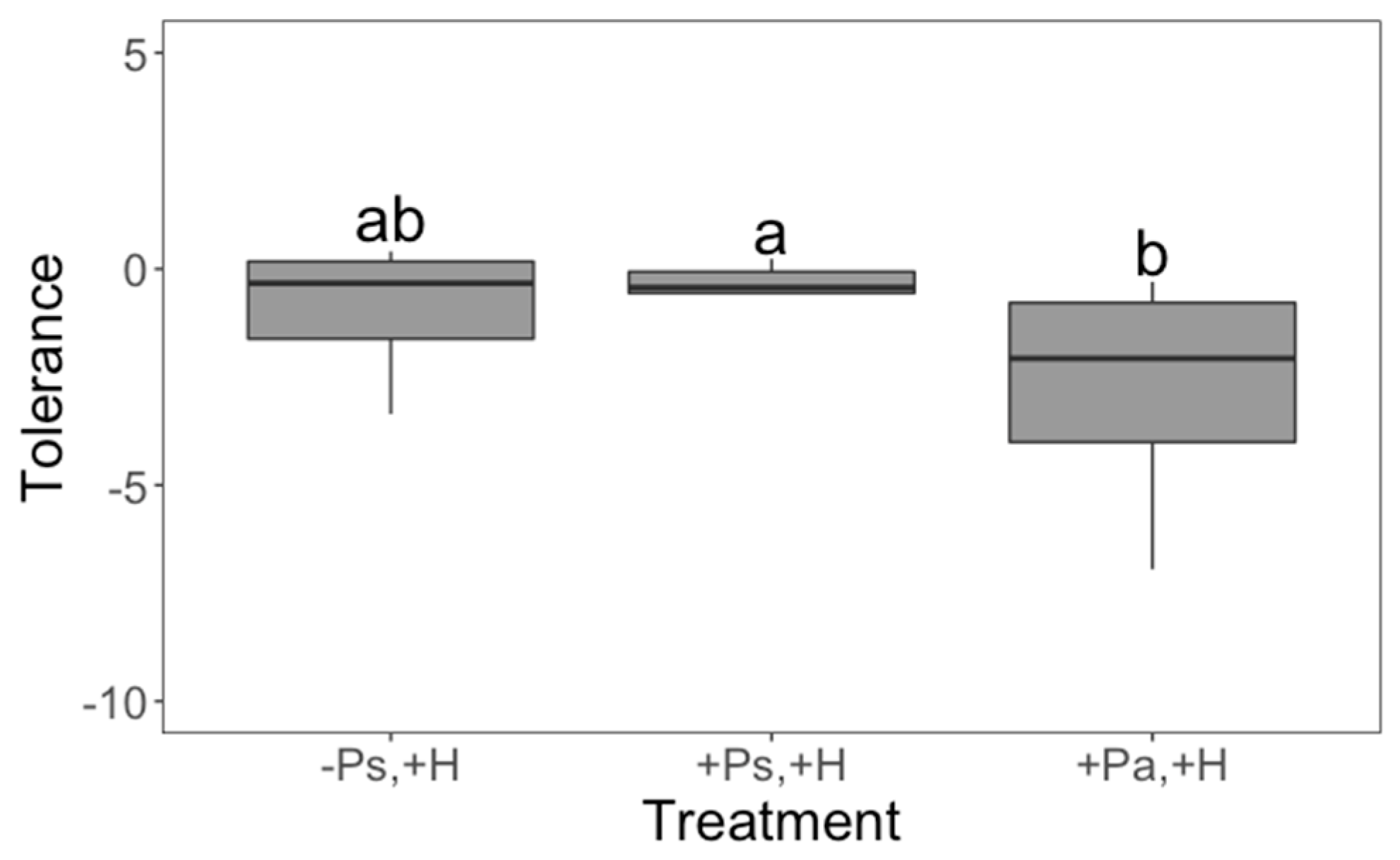
2. Results
3. Discussion
4. Materials and Methods
4.1. Study System
4.2. Experimental Design
4.3. Response Variables
4.4. Statistical Analyses
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.-A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting Ready for Battle. Mol. Plant-Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Futuyma, D.J.; Agrawal, A.A. Macroevolution and the biological diversity of plants and herbivores. Proc. Natl. Acad. Sci. USA 2009, 106, 18054–18061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, C.J.; Mescher, M.C.; Carlson, J.E.; De Moraes, C.M. Plant Defense Priming against Herbivores: Getting Ready for a Different Battle. Plant Physiol. 2008, 146, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. 2015, 91, 1118–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; von Dahl, C.C.; Preston, C.A. Volatile Signaling in Plant-Plant Interactions: “Talking Trees” in the Genomics Era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef] [Green Version]
- van Hulten, M.; Pelser, M.; van Loon, L.C.; Pieterse, C.M.J.; Ton, J. Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 5602–5607. [Google Scholar] [CrossRef] [Green Version]
- Hilker, M.; Schmülling, T. Stress priming, memory, and signalling in plants. Plant Cell Environ. 2019, 42, 753–761. [Google Scholar] [CrossRef]
- Walter, J.; Jentsch, A.; Beierkuhnlein, C.; Kreyling, J. Ecological stress memory and cross stress tolerance in plants in the face of climate extremes. Environ. Exp. Bot. 2013, 94, 3–8. [Google Scholar] [CrossRef]
- Carmona, D.; Fornoni, J. Herbivores can select for mixed defensive strategies in plants. New Phytol. 2012, 197, 576–585. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef]
- Garrido, E.; Andraca-Gómez, G.; Fornoni, J. Local adaptation: Simultaneously considering herbivores and their host plants. New Phytol. 2012, 193, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, D.; Ba, L.; Bai, Y.; Liu, B. Interactions between herbivory and resource availability on grazing tolerance of Leymus chinensis. Environ. Exp. Bot. 2008, 63, 113–122. [Google Scholar] [CrossRef]
- Gavloski, J.E.; Lamb, R.J. Compensation for Herbivory in Cruciferous Plants: Specific Responses to Three Defoliating Insects. Environ. Èntomol. 2000, 29, 1258–1267. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense Priming: An Adaptive Part of Induced Resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, G.A.; Jander, G. Plant Immunity to Insect Herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felton, G.W.; Tumlinson, J.H. Plant–insect dialogs: Complex interactions at the plant–insect interface. Curr. Opin. Plant Biol. 2008, 11, 457–463. [Google Scholar] [CrossRef]
- Selosse, M.-A.; Bessis, A.; Pozo, M.J. Microbial priming of plant and animal immunity: Symbionts as developmental signals. Trends Microbiol. 2014, 22, 607–613. [Google Scholar] [CrossRef]
- Gouhier-Darimont, C.; Schmiesing, A.; Bonnet, C.; Lassueur, S.; Reymond, P. Signalling of Arabidopsis thaliana response to Pieris brassicae eggs shares similarities with PAMP-triggered immunity. J. Exp. Bot. 2013, 64, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Sutter, R.; Müller, C. Mining for treatment-specific and general changes in target compounds and metabolic fingerprints in response to herbivory and phytohormones in Plantago lanceolata. New Phytol. 2011, 191, 1069–1082. [Google Scholar] [CrossRef]
- Korpita, T.; Gómez, S.; Orians, C.M. Cues from a specialist herbivore increase tolerance to defoliation in tomato. Funct. Ecol. 2013, 28, 395–401. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Wang, D.; Bonser, S.P.; Sun, F.; Zhou, Y.; Gao, Y.; Teng, X. Plants Can Benefit from Herbivory: Stimulatory Effects of Sheep Saliva on Growth of Leymus chinensis. PLoS ONE 2012, 7, e29259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, X.; Zas, R.; Sampedro, L. Genetic variation and phenotypic plasticity of nutrient re-allocation and increased fine root production as putative tolerance mechanisms inducible by methyl jasmonate in pine trees. J. Ecol. 2011, 100, 810–820. [Google Scholar] [CrossRef] [Green Version]
- Ferrieri, A.P.; Agtuca, B.; Appel, H.M.; Ferrieri, R.A.; Schultz, J. Temporal Changes in Allocation and Partitioning of New Carbon as 11C Elicited by Simulated Herbivory Suggest that Roots Shape Aboveground Responses in Arabidopsis. Plant Physiol. 2012, 161, 692–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, S.; Steinbrenner, A.D.; Osorio, S.; Schueller, M.; Ferrieri, R.A.; Fernie, A.R.; Orians, C.M. From shoots to roots: Transport and metabolic changes in tomato after simulated feeding by a specialist lepidopteran. Èntomol. Exp. Appl. 2012, 144, 101–111. [Google Scholar] [CrossRef]
- Heidel-Fischer, H.M.; Musser, R.O.; Vogel, H. Plant transcriptomic responses to herbivory. Annu. Plant Rev. Insect Plant Interact. 2014, 47, 155–196. [Google Scholar]
- Hilker, M.; Meiners, T. How do plants “notice” attack by herbivorous arthropods? Biol. Rev. Camb. Philos. Soc. 2010, 85, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silver- leaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne signals prime plants against insect herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kessler, A.; Halitschke, R.; Diezel, C.; Baldwin, I.T. Priming of plant defense responses in nature by airborne signaling between Artemisia tridentata and Nicotiana attenuata. Oecologia 2006, 148, 280–292. [Google Scholar] [CrossRef]
- Crawley, M.J. Herbivores and plant population dynamics. In Plant Population Ecology; Davey, A.J., Hutchings, M.J., Watkinson, A.R., Eds.; Blackwell: Oxford, UK, 1988; pp. 367–392. [Google Scholar]
- Valverde, P.L.; Fornoni, J.; Nunez-Farfan, J. Defensive role of leaf trichomes in resistance to herbivorous insects in Datura stramonium. J. Evol. Biol. 2001, 14, 424–432. [Google Scholar] [CrossRef]
- Espinosa, E.G.; Fornoni, J. Host tolerance does not impose selection on natural enemies. New Phytol. 2006, 170, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Farfán, J.; Dirzo, R. Evolutionary ecology of Datura stramonium L. in central Mexico: Natural selection for resistance to herbivorous insects. Evolution 1994, 48, 423–436. [Google Scholar] [PubMed]
- Strauss, S.Y.; Agrawal, A.A. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 1999, 14, 179–185. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 19 July 2011).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, E.; Boege, K.; Domínguez, C.A.; Fornoni, J. Priming by Insects: Differential Effects of Sympatric and Allopatric Priming upon Plant Performance and Tolerance to Herbivory. Plants 2022, 11, 3567. https://doi.org/10.3390/plants11243567
Garrido E, Boege K, Domínguez CA, Fornoni J. Priming by Insects: Differential Effects of Sympatric and Allopatric Priming upon Plant Performance and Tolerance to Herbivory. Plants. 2022; 11(24):3567. https://doi.org/10.3390/plants11243567
Chicago/Turabian StyleGarrido, Etzel, Karina Boege, César A. Domínguez, and Juan Fornoni. 2022. "Priming by Insects: Differential Effects of Sympatric and Allopatric Priming upon Plant Performance and Tolerance to Herbivory" Plants 11, no. 24: 3567. https://doi.org/10.3390/plants11243567
APA StyleGarrido, E., Boege, K., Domínguez, C. A., & Fornoni, J. (2022). Priming by Insects: Differential Effects of Sympatric and Allopatric Priming upon Plant Performance and Tolerance to Herbivory. Plants, 11(24), 3567. https://doi.org/10.3390/plants11243567






