The Genus Cynometra: A Review of Ethnomedicine, Chemical, and Biological Data
Abstract
1. Introduction
2. Results and Discussions
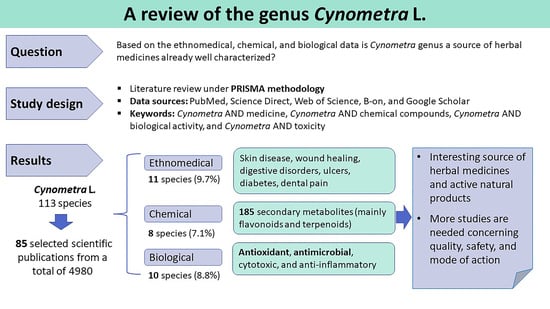
2.1. Selection of the Information
2.2. Ethnomedicinal Data
2.3. Chemical Compounds
| Species | Part Used | Chemical Class | Compounds | Ref. |
|---|---|---|---|---|
| C. anata | leaf | Alkaloids | anantine, cynometrine and cynodine | [44] |
| C. cauliflora | leaf | Flavonoids | xanthotoxin, fraxetin, capensine, naringenin, malvidin, cyanidin, amorphigenin, nobiletin, isorhamnetin, epigallocatechin, gallate, apigenin, and oenin | [41] |
| stem | Flavonoids | apigenin | [53] | |
| twig | Flavonoids | naringenin, eriodictyol, apigenin, acacetin, luteolin, luteolin 3’,5 dimethyl ether, 3’,4’,7-trihydroxyflavone, 4’,7-dihydroxyflavone and 5,7-dihydroxychromone | [54] | |
| leaf | Mono- terpenoids | α-thujene, α-pinene, β-pinene, myrcene, δ-3-carene, α-terpinene, p-cymene, limonene, (z)-β-ocimene, γ-terpinene, terpinolene, linalool, α-terpineol, neo-dihydrocarveol, cis-carvone oxide, trans-dihydro-a-terpinyl acetate | [40] | |
| Sesqui- terpenoids | α-bulnesene, β-chamigrene, α-himachalene, trans-cadin-1,4-diene | |||
| Phenols | p-vinyl guaiacol | |||
| Hydrocarbons | (3E)-2-methyl-octen-5-yne | |||
| twig | Mono- terpenoids | (z)-β-ocimene, santolina alcohol, (E)-cis-jasmonol, cis-verbenol, linalool, geraniol, cis-4-caranone, trans-sabinene hydrate, dihydromyrcenol | ||
| Sesqui- terpenoids | squamulosone, occidol acetate, α-eudesmol acetate | |||
| Fatty acids | octanoic acid, decanoic acid, dodecanoic acid, linoleic acid | |||
| Flavonoids | fragranol | |||
| fruit | Sesqui- terpenoids | β-cubebene, β-elemene, α-guaiene, prezizaene, ishwarane, β-chamigrene, germacrene d, α-muurolene, β-bisabolene, α-bulnesene, γ-cadinene, (E)-γ-bisabolene, γ-cuprenene, trans-cadina-1,4-diene, selina-3,7(11)-diene, 9-epi-(E)-caryophyllene, α-chenopodiol, longiborneol, trans-β-elemenone, α-acorenol, agarospirol, occidenol, cryptomerione, curcumenol, hinesol, nootkatol, sesquisabinene, α-muurolol, β-calacorene, γ-eudesmol, elemol, eremoligenol, (2E,6E)- farnesol, (E)-nuciferol, (z)-lanceol, 11-αH-himachal-4-en-1-β-ol, globulol, cubebol, longipinanol, valerianol, allohimachalol, epi-β-bisabolol, occidol acetate, longiborneol acetate | ||
| Mono- terpenoids | limonene, cis-thujone, trans-pulegol, cis-β-farnesene | |||
| Fatty acids | linoleic acid | |||
| leaf | Condensed tannins | procyanidin trimer, procyanidin tetramer, procyanidin hexamer | [15] | |
| Flavonoids | catechin, taxifolin pentoside, vitexin, isovitexin, kaempferol hexoside, quercetin pentoside, quercetin hexoside, apigenin-6-C-glucoside-8-C-glucoside, kaempferol–coumaroyl hexoside and isorhamnetin hexoside | |||
| C. hankei | Leaf, seed | Flavonoids | cyanidin | [43] |
| stem bark, seed | Alkaloids | N1-demethyl cynometrine, N1-demethyl cynodine, cynometrine, and cynodine | [45] | |
| C. iripa | leaf, seed oil | Fatty acids | leaf—lauric acid, myristic acid, pentadecanoic acid, palmitic acid, stearic acid, arachidic acid, behenic acid, oleic acid and cis-11-eicosenoic acid, linolenic acidseed—caproic acid, lauric acid, myristic acid, palmitic acid, stearic acid, arachidic acid, behenic acid, tricosanoic acid, lignoceric acid, oleic acid, linoleic acid, linolenic acid, cis-8, 11, 14-eicosatrienoic acid, cis-13, 16-docosadienoic acid | [38] |
| seed, seed coat | Triterpenoids | squalene, β-sitosterol, stigmast-4-en-3-one, cholesta-4,6-diene-3-ol (3-beta) | [55] | |
| Tetraterpenoids | β-carotene | |||
| Esters | 1,2-benzenedicarboxylicacid mono (2-ethylexyl) ester, butyric acid 2-pentadecyl ester, 1,2-benzenedicarboxylic acid butyl 2-ethylhexyl ester | |||
| Fatty alcohols | 1-eicosanol, falcarinol | |||
| Quinones | 2,5-di-ter-butyl-1,4-benzoquinone | |||
| Phenolic aldehydes | 3,5-di-ter-butyl-4-hydroxybenzaldehyde | |||
| Vitamins | vitamin E | |||
| Hormones | progesterone | |||
| C. lujae | not indicated | Alkaloids | anantine, cynometrine, isoanantine, isocynometrine, isocynodine noranantine, hydroxyanantine and cynolujine | [44] |
| C. megallophylla | root oil | Mono- terpenoids | α-pinene, α-thujane, sabinene, β-pinene, myrcene, α-phellandrene, α-terpinene, p-cymene, limonene, β-phellandrene, (E)-β-ocimene, γ-terpinene, terpinolene, 1,8-cineole, cis-p-menth-2-en-1-ol, trans-p-menth-2-en-1-ol, borneol, terpinen-4-ol, carvacrol, p -cymen-8-ol, trans-piperitol, terpinen-4-yl acetate | [42] |
| Sesqui- terpenoids | caryophyllene oxide, α-eudesmol, β-eudesmol, hinesol, globulol, β-selinene, germacrene D, allo-aromadendrene, α-humulene, α-guaiene, β-caryophyllene | |||
| Hydrocarbon | decane, dodecane, undecane, N-tridecane, tetradecane, pentadecane, hexadecane, heptadecane, octadecane | |||
| C. ramiflora | leaf | Triterpenoids | glutinol, glutinone, β-sitosterol | [56] |
| Ester | ethyl 4- ethoxy benzoate | |||
| C. vogelii | leaf oil | Sesquiterpenoids | β-caryophyllene, α- and β-selinene | [57] |
| Fatty acid | isopropyl palmitate |
2.4. Biological Studies
2.5. Toxicity
3. Materials and Methods
3.1. Search Strategy
3.2. Data inclusion and Exclusion Criteria
3.2.1. Inclusion Criteria
- Related to Cynometra genus;
- Abstract or full text in English;
- Studies on Cynometra species concerning medicinal importance.
3.2.2. Exclusion Criteria
- Duplicate scientific publications;
- Not directly related to medicinal issues;
- Containing irrelevant or incomplete information.
4. Conclusion and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dwyer, J.D. The new world species of Cynometra. Ann. Mo. Bot. Gard. 1958, 45, 313–345. [Google Scholar] [CrossRef]
- Léonard, J. Notulae systematicae XI: Les Cynometra et les genres voisins en Afrique tropicale. Bull. Jard. Bot. État Brux. 1951, 21, 373–450. [Google Scholar] [CrossRef]
- Knaap, V.M. A revision of four genera of the tribe Leguminosae-Caesalpinioideae-Cynometreae in Indomalesia and the Pacific. Blumea 1970, 8, 1–52. [Google Scholar]
- Bruneau, A.; Forest, F.; Herendeen, P.S.; Klitgaard, B.B.; Lewis, G.P. Phylogenetic relationships in the Caesalpinioideae (Leguminosae) as inferred from chloroplast trnl intron sequences. Syst. Bot. 2001, 26, 487–514. [Google Scholar] [CrossRef]
- Bruneau, A.; Mercure, M.; Lewis, G.P.; Herendeen, P.S. Phylogenetic patterns and diversification in the Caesalpinioid legumes. Botany 2008, 86, 697–718. [Google Scholar] [CrossRef]
- Mackinder, B.A.; Saslis-Lagoudakis, H.; Wieringa, J.J.; Devey, D.; Forest, F.; Bruneau, A. The tropical African legume Scorodophloeus clade includes two undescribed Hymenostegia segregate genera and Micklethwaitia, a rare, monospecific genus from Mozambique. S. Afr. J. Bot. 2013, 89, 156–163. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Herendeen, P.S.; Mackinder, B.A. Phylogeny of the detarioid legume genera Cynometra and Maniltoa (Leguminosae). Syst. Bot. 2017, 42, 670–679. [Google Scholar] [CrossRef]
- De la Estrella, M.; Forest, F.; Klitgård, B.; Lewis, G.P.; Mackinder, B.A.; de Queiroz, L.P.; Wieringa, J.J.; Bruneau, A. A new phylogeny-based tribal classification of subfamily Detarioideae, an early branching clade of florally diverse tropical arborescent legumes. Sci. Rep. 2018, 8, 6884. [Google Scholar] [CrossRef]
- POWO (Plants of the World Online). Facilitated by the Royal Botanic Gardens, Kew. 2017. Available online: https://powo.science.kew.org/ (accessed on 5 November 2021).
- The Plant List. Version 1.1. 2013. Available online: http://www.theplantlist.org/ (accessed on 1 June 2021).
- WFO (World Flora Online). 2022. Available online: http://www.worldfloraonline.org/ (accessed on 1 January 2022).
- GBIF Secretariat. Cynometra L. in GBIF Secretariat. GBIF Backbone Taxonomy. Checkl. Dataset 2021. Available online: https://doi.org/10.15468/39omei (accessed on 1 January 2022).
- Ragavan, P.; Rana, T.S.; Ravichandran, K.; Jayaraj, R.S.C.; Sivakumar, K.; Saxena, A.; Mohan, P.M. Note on identity and distribution of Cynometra iripa Kostel. and C. ramiflora L. (Fabaceae) in the Andaman and Nicobar Islands, India. Check List 2017, 13, 805–812. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. 2022. Available online: https://www.iucnredlist.org/ (accessed on 1 January 2022).
- Ado, M.A.; Abas, F.; Ismail, I.S.; Ghazali, H.M.; Shaari, K. Chemical profile and anti-acetylcholinesterase, the antityrosinase, antioxidant, and α-Glucosidase inhibitory activity of Cynometra cauliflora L. leaves. J. Sci. Food Agric. 2015, 95, 635–642. [Google Scholar] [CrossRef]
- Tiranda, R.; Suseno, A.D. Cynometra cauliflora L. Inform. Singkat. Benih. 2013, 170, 1–2. [Google Scholar]
- Choi, C.W.; Song, S.B.; Oh, J.S.; Kim, Y.H. Antiproliferation effects of selected Tanzania plants. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 96–102. [Google Scholar] [CrossRef]
- Onjalalaina, G.E.; Sattler, C.; Razafindravao, M.B.; Wanga, V.O.; Mkala, E.M.; Mwihaki, J.K.; Ramananirina, B.M.R.; Jeannoda, V.H.; Hu, G. Ethnobotanical survey in Tampolo forest (Fenoarivo atsinanana, Northeastern Madagascar). Forests 2021, 12, 566. [Google Scholar] [CrossRef]
- Abd Aziz, A.F.; Iqbal, M. Antioxidant activity and phytochemical composition of Cynometra cauliflora. J. Exp. Integr. Med. 2013, 3, 337–341. [Google Scholar] [CrossRef]
- Lim, T.K. Cynometra cauliflora. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; pp. 614–616. Available online: https://sci-hub.se/10.1007/978-94-007-1764-0_75 (accessed on 1 January 2022).
- Caruano, J.; Muccioli, G.G.; Robiette, R. Biologically active γ-Lactams: Synthesis and natural sources. Org. Biomol. Chem. 2016, 14, 10134–10156. [Google Scholar] [CrossRef]
- Sen, U.K.; Bhakat, R.K. Assessment of psammophytic medicinal plant diversity used among the rural communities in Coastal East Midnapore, West Bengal, India. J. Herbs Spices Med. Plants 2019, 26, 219–247. [Google Scholar] [CrossRef]
- Pattanaik, C.; Reddy, C.S.; Dhal, N.K.; Das, R. Utilisation of mangrove forests in Bhitarkanika wildlife sanctuary, Orissa. Indian J. Tradit. Knowl. 2008, 7, 598–603. [Google Scholar]
- Lalitha, P.; Sachithanandam, V.; Swarnakumar, N.S.; Sridhar, R. Review on anti-inflammatory properties of mangrove plants. Asian J. Pharm. Res. 2019, 9, 273–288. [Google Scholar] [CrossRef]
- Idu, M.; Erhabor, J.O.; Efijuemue, H.M. Documentation on medicinal plants sold in markets in Abeokuta, Nigeria. Trop. J. Pharm. Res. 2010, 9, 110–118. [Google Scholar] [CrossRef]
- Soladoye, M.O.; Amusa, N.A.; Raji-Esan, S.O.; Chukwuma, E.C.; Taiwo, A.A. Ethnobotanical survey of anti-cancer plants in Ogun state, Nigeria. Ann. Biol. Res. 2010, 1, 261–273. [Google Scholar]
- Adebisi, M.A. Ethnobotany survey of medicinal plants used in the treatment of fibroid in Ogun and Osun states, Southwestern, Nigeria. J. Res. For. Wildl. Environ. 2019, 11, 33–44. [Google Scholar]
- Allabi, A.C.; Busia, K.; Ekanmian, V.; Bakiono, F. The use of medicinal plants in self-care in the Agonlin region of Benin. J. Ethnopharmacol. 2011, 133, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Cock, I.E. Medicinal and Aromatic Plants–Australia. Ethnopharmacology, Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of UNESCO; EOLSS Publishers: Oxford, UK, 2011. [Google Scholar]
- Siddique, H.; Pendry, B.; Rashid, M.A.; Rahman, M.M. Medicinal plants used to treat infectious diseases in the central part and a northern district of Bangladesh–an ethnopharmacological perception. J. Herb. Med. 2021, 29, 100484. [Google Scholar] [CrossRef]
- Breitbach, U.B.; Niehues, M.; Lopes, N.P.; Faria, J.E.Q.; Brandão, M.G.L. Amazonian Brazilian medicinal plants were described by C.F.P. von Martius in the 19th century. J. Ethnopharmacol. 2013, 147, 180–189. [Google Scholar] [CrossRef]
- Oluwafoise, B.G.; Adaramodu, A.A. Phytochemical screening of tannins in selected indigenous plant species used as chewing sticks in Ekiti state, Nigeria. J. Biol. Agric. Healthc. 2019, 9, 33–37. [Google Scholar] [CrossRef]
- Wahab, N.Z.A.; Badya, N.; Ibrahim, N.; Kamarudin, M.K.A. Phytochemistry and antibacterial activity of Cynometra cauliflora. Indian J. Public Health Res. Dev. 2019, 10, 765–769. [Google Scholar] [CrossRef]
- Fajarwati, I. Aktivitas Sitotoksik Fraksi Polar, Semipolar, Dan Non Polar Ekstrak Etanol Daun Tumbuhan Sala Cynometra ramiflora Linn Terhadap Sel T47D. Ph.D. Thesis, Universitas Muhammadiyah Surakarta, Jawa Tengah, Indonesia, 2014. Available online: http://eprints.ums.ac.id/id/eprint/30368 (accessed on 1 January 2022).
- Paguigan, N.D.; Castillo, D.H.B.; Chichioco-Hernandez, C.L. Anti-ulcer activity of Leguminosae plants. Arq. Gastroenterol. 2014, 51, 64–67. [Google Scholar] [CrossRef]
- Ahmat, N.; Kamarozaman, A.S.; Johari, M.S.M.; Abas, F.; Mohamad, S.A.S.; Yunoh, S.M.M. Screening of phytochemicals from the ethanolic extracts of Gnetum gnemon, Gnetum latifolium and Cynometra malaccensis of Kuala Keniam, Pahang. IOP Conf. Ser. Earth Environ. Sci. 2022, 1019, 1–5. [Google Scholar] [CrossRef]
- Bick, I.R.C. Chapter One Alkaloids from Australian Flora. In Alkaloids: Chemical and Biological Perspectives; Pelletier, S.W., Ed.; Pergamon: Oxford, UK, 1996; Volume 10, pp. 1–154. [Google Scholar] [CrossRef]
- Desai, M.N.; Chavan, N.S. Fatty acid profile of Cynometra iripa kostel. from seeds and leaves. Int. J. Pharm. Res. Dev. 2011, 2, 58–62. [Google Scholar]
- Basak, U.C.; Das, A.B.; Das, P. Chlorophylls, carotenoids, proteins and secondary metabolites in leaves of 14 species of mangrove. Bull. Marine Sci. 1996, 58, 654–659. [Google Scholar]
- Samling, B.A.; Assim, Z.; Tong, W.Y.; Leong, C.R.; Ab Rashid, S.; Nik Mohamed Kamal, N.N.S.; Muhamad, M.; Tan, W.N. Cynometra cauliflora L.: An indigenous tropical fruit tree in Malaysia bearing essential oils and their biological activities. Arab. J. Chem. 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Sumarlin, L.O.; Suprayogi, A.; Rahminiwati, M.; Satyaningtijas, A.; Nugraha, A.T.; Sukandar, D.; Pangestika, H.; Pratiwi, L. Identification of compounds flavonoids namnam leaf extract (Cynometra cauliflora) as inhibiting α-glucosidase. J. Phys. Conf. Ser. 2020, 1594, 012005. [Google Scholar] [CrossRef]
- Ogunbinu, A.O.; Okeniyi, S.; Flamini, G.; Cioni, P.L.; Ogunwande, I.A. Monoterpenoid constituents of the volatile oils of Cynometra megalophylla Harms., Caesalpinia Pulcherrima L. Swartz and Pachylobus Edulis G. Don., Growing in Nigeria. J. Essent. Oil Res. 2010, 22, 536–539. [Google Scholar] [CrossRef]
- Gartlan, J.S.; McKey, D.B.; Waterman, P.G.; Struhsaker, T.T. A Comparative study of the phytochemistry of two African rain forests. Biochem. Syst. Ecol. 1980, 8, 401–422. [Google Scholar] [CrossRef]
- Maat, L.; Beyerman, H.C. The Imidazole alkaloids. In The Alkaloids: Chemistry and Pharmacology; Brossi, A., Ed.; Academic Press: Cambridge, MA, USA, 1984; pp. 281–333. [Google Scholar] [CrossRef]
- Waterman, P.G.; Faulkner, D.F. Imidazole alkaloids from Cynometra hankei. Phytochemistry 1981, 20, 2765–2767. [Google Scholar] [CrossRef]
- Wong, P.L.; Fauzi, N.A.; Mohamed Sumarlin, S.N.; Abdul Hamid, N.A.; Abd Ghafar, S.Z.; Azizan, A.; Zolkeflee, N.K.Z.; Abas, F. Biological activities of selected plants and detection of bioactive compounds from Ardisia elliptica using UHPLC-Q-executive orbitrap mass spectrometry. Molecules 2020, 25, 3067. [Google Scholar] [CrossRef]
- Yunus, S.N.M.; Faridah, A.; Jaafar, A.H.; Azizan, A.; Zolkeflee, N.K.Z.; Abd Ghafar, S.Z. Antioxidant and α-glucosidase inhibitory activities of eight neglected fruit extracts and UHPLC-MS/MS profile of the active extracts. Food Sci. Biotechnol. 2021, 30, 195–208. [Google Scholar] [CrossRef]
- Ikram, E.H.K.; Eng, K.H.; Jalil, A.M.M.; Ismail, A.; Idris, S.; Azlan, A.; Nazri, H.S.M.; Diton, N.A.M.; Mokhtar, R.A.M. Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J. Food Compost. Anal. 2009, 22, 388–393. [Google Scholar] [CrossRef]
- Fernando, A.S.K.; Attanayake, A.P.; Jayatilaka, K.A.P.W. Screening of selected Sri lankan seasonal fruit extracts for total antioxidant activity in vitro. Asian J. Pharm. Res. Dev. 2019, 7, 7–12. [Google Scholar] [CrossRef]
- Mallawaarachchi, M.A.L.N.; Madhujith, T.; Suriyagoda, L.D.B.; Pushpakumara, D.K.N.G. Antioxidant efficacy of selected underutilized fruit species grown in Sri Lanka. Tropical Agric. Res. 2021, 32, 68–80. [Google Scholar] [CrossRef]
- Abeysuriya, H.I.; Bulugahapitiya, V.P.; Pulukkuttige, J.L. Total vitamin c, ascorbic acid, dehydroascorbic acid, antioxidant properties, and iron content of underutilized and commonly consumed fruits in Srilanka. Int. J. Food Sci. 2020, 13. [Google Scholar] [CrossRef]
- Afrin, S.; Pervin, R.; Sabrin, F.; Rony, S.R.; Sohrab, M.H.; Islam, M.E.; Islam, K.D.; Billah, M.M. In vitro antioxidant activity, antimicrobial and preliminary cytotoxic activity of Cynometra ramiflora - a mangrove plant. J. Microbiol. Biotechnol. Food Sci. 2016, 6, 844–850. [Google Scholar] [CrossRef]
- Sukandar, D.; Nurbayti, S.; Rudiana, T.; Husna, T.W. Isolation and structure determination of antioxidants active compounds from ethyl acetate extract of Heartwood Namnam (Cynometra cauliflora L.). J. Kim. Terap. Indones. 2017, 19, 11–17. [Google Scholar] [CrossRef]
- Azmin, N.F.; Fatini, N.A. Phytochemical Studies of Gnetum Microcarpum, Gnetum Cuspidatum, Cynometra Cauliflora, Bouea Oppositifolia and Their Biological Activities; IGS Biannual Publication; Institute of Graduate Studies, UiTM: Selangor, Malaysia, 2018. [Google Scholar]
- Desai, M.N.; Chavan, N.S. Bioactive compounds from seed and seed coat of Cynometra iripa a mangrove species. Universal J. Pharm. 2014, 3, 1–5. [Google Scholar]
- Holly, S. Bangladeshi medicinal plants: Ethnopharmacology, Phytochemistry, and Anti-Staphylococcal Activity. Ph.D. Thesis, School of Health, Sport and Bioscience, University of East London, London, UK, 2019. [Google Scholar] [CrossRef]
- Owolabi, M.S.; Ogundajo, L.A.; Solomon, B.O.; Olatunde, L.; Poudel, A.; Powers, C.N.; Setzer, W.N. Chemical constituents and antifungal properties of the essential oils from the stem bark of Mitragyna ciliata aubrév. & pellegr. and leaves of Cynometra vogelii hook. f. from Nigeria. Am. J. Essent. Oil. Nat. Prod. 2020, 8, 1–5. [Google Scholar]
- Graham, J.G.; Pendland, S.L.; Prause, J.L.; Danzinger, L.H.; Schunke Vigo, J.; Cabieses, F.; Farnsworth, N.R. Antimycobacterial evaluation of Peruvian plants. Phytomedicine 2003, 10, 528–535. [Google Scholar] [CrossRef]
- Tajudin, T.S.A.; Mat, N.; Siti-Aishah, A.B.; Yusran, A.A.M.; Alwi, A.; Ali, A.M. Cytotoxicity, antiproliferative effects, and apoptosis induction of methanolic extract of Cynometra cauliflora linn. whole fruit on human promyelocytic leukemia hl-60 cells. Evid. Based Complement. Altern. Med. 2012, 2012, 127373. [Google Scholar] [CrossRef]
- Rabeta, M.S.; Faraniza, R.N. Total phenolic content and ferric reducing antioxidant power of the leaves and fruits of Garcinia Atrovirdis and Cynometra cauliflora. Int. Food Res. J. 2013, 20, 1691–1696. [Google Scholar]
- Ado, M.A.; Abas, F.; Mohammed, A.S.; Ghazali, H.M. Anti- and pro-Lipase activity of selected medicinal, herbal and aquatic plants, and structure elucidation of an anti-lipase compound. Molecules 2013, 18, 14651–14669. [Google Scholar] [CrossRef]
- Sumarlin, L.O.; Suprayogi, A.; Rahminiwati, M.; Tjahja, A.; Sukandar, D. Bioaktivitas ekstrak metanol daun namnam serta kombinasinya dengan madu trigona. J. Teknol. Indust. Pangan. 2015, 26, 144–154. [Google Scholar] [CrossRef]
- Sumarlin, L.O.; Suprayogi, A.; Rahminiwati, M.; Satyaningtijas, A.; Sukandar, D.; Nugraha, A.T.; Amalia, I. Antidiabetic and antidiarrheal activity from extract of namnam (Cynometra cauliflora) leaf. In Proceedings of the International Conference on Global Resource Conservation, Malang, Indonesia, 2–4 November 2016; Volume 1, p. 6. [Google Scholar]
- Perera, H.D.S.M.; Samarasekera, J.K.R.R.; Handunnetti, S.M.; Weerasena, O.V.D.S.J. In vitro anti-inflammatory and antioxidant activities of Sri lankan medicinal plants. Ind. Crops Prod. 2016, 94, 610–620. [Google Scholar] [CrossRef]
- Abd Aziz, A.F.; Bhuiyan, M.S.A.; Iqbal, M. An evaluation of the antioxidant and antidiabetic potential of Cynometra cauliflora (nam-nam, Fabaceae). Trans. Innov. Sci. Technol. 2017, 4, 372–383. [Google Scholar]
- Ong, C.W.; Chan, Y.S.; Khoo, K.S.; Ong, H.C.; Sit, N.W. Antifungal and cytotoxic activities of extracts obtained from underutilized edible tropical fruits. Asian Paci. J. Trop. Biomed. 2018, 8, 313. [Google Scholar] [CrossRef]
- Ulpiyah, Z.; Shita, A.D.P.; Wahyukundari, M.A. Inhibition of namnam (Cynometra cauliflora L.) leaves extract on the growth of Porphyromonas gingivalis. Padjadjaran J. Dent. 2019, 31, 106–111. [Google Scholar] [CrossRef]
- Rawa, M.S.A.; Hassan, Z.; Murugaiyah, V.; Nogawa, T.; Wahab, H.A. Anti-cholinesterase potential of diverse botanical families from malaysia: Evaluation of crude extracts and fractions from liquid-liquid extraction and acid-base fractionation. J. Ethnopharmacol. 2019, 245, 1–17. [Google Scholar] [CrossRef]
- Seyedan, A.; Mohamed, Z.; Alshagga, M.A.; Koosha, S.; Alshawsh, M.A. Cynometra cauliflora Linn. attenuates metabolic abnormalities in high-fat diet-induced obese mice. J. Ethnopharmacol. 2019, 236, 173–182. [Google Scholar] [CrossRef]
- Xin, L.Y.; Min, T.H.; Zin, P.N.L.M.; Pulingam, T.; Appaturi, J.N.; Parumasivam, T. Antibacterial potential of Malaysian ethnomedicinal plants against methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA). Saudi J. Biol. Sci. 2021, 28, 5884–5889. [Google Scholar] [CrossRef]
- Wahab, A.; Zarina, N.; Azizul, A.; Badya, N.; Ibrahim, N. Antiviral activity of an extract from leaves of the tropical plant Cynometra cauliflora. Pharmacog. J. 2021, 13, 752–757. [Google Scholar] [CrossRef]
- Fathoni, A.; Sumarlin, L.O.; Hanief, F.; Sukandar, D. Isolation and cytotoxic activity of the β-carotene combination of trigona honey and namnam leaves extract (Cynometra cauliflora). J. Islam. Sci. Tech. 2021, 7, 52–66. [Google Scholar] [CrossRef]
- Komarudin, D. Uji efektivitas ekstrak dekok daun namnam (Cynometra cauliflora L.) Terhadap penurunan kadar gula darah pada tikus putih jantan (Rattus norvegicus) yang diinduksi aloksan. ION Tech. J. 2021, 2, 12–20. [Google Scholar]
- Anliza, S.; Rachmawati, N. Cytotoxic Activity of Ethanol Extract in Namnam Leaves (Cynometra cauliflora L.) to Hela Cell. WJC. 2021, 4, 107–112. [Google Scholar] [CrossRef]
- Hudson, J.B.; Lee, M.K.; Rasoanaivo, P. Antiviral activities in plants endemic to Madagascar. Pharm. Biol. 2000, 38, 36–39. [Google Scholar] [CrossRef]
- Choudhury, S.; Sree, A.; Mukherjee, S.C.; Pattnaik, P.; Bapuji, M. In vitro antibacterial activity of extracts of selected marine algae and mangroves against fish pathogens. Asian Fish. Sci. 2005, 18, 285–294. [Google Scholar] [CrossRef]
- Desai, M.N.; Chavan, N.S. Antibacterial activity and phytochemical screening of Cynometra iripa kostel. Int. J. Pharma Bio Sci. 2010, 1, 1–4. [Google Scholar]
- Powar, P.S.; Chavan, S.R.; Gaikwad, D.K. Antifungal activity of mangrove bark. Int. J. Pharma Bio Sci. 2011, 2, 484–488. [Google Scholar]
- Bunyapraphatsara, N.; Jutiviboonsuk, A.; Sornlek, P.; Therathanathorn, W.; Aksornkaew, S.; Fong, H.H.; Pezzuto, J.M.; Kosmeder, J. Pharmacological studies of plants in the mangrove forest. J. Phytopharm. 2003, 10, 1–12. [Google Scholar]
- Tiwari, P.; Rahuja, N.; Kumar, R.; Lakshmi, V.; Srivastava, M.N.; Agarwal, S.C.; Raghubir, R.; Srivastava, A.K. Search for anti-hyperglycemic activity in few marine flora and fauna. Indian J. Sci. Technol. 2008, 1, 1–5. [Google Scholar] [CrossRef]
- Uddin, S.J.; Grice, I.D.; Tiralongo, E. Cytotoxic effects of Bangladeshi medicinal plant extracts. Evid. Based Complement Altern. Med. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Subarnas, A.; Diantini, A.; Abdulah, R.; Zuhrotun, A.; Yamazaki, C.; Nakazawa, M.; Koyama, H. Antiproliferative activity of primates-consumed plants against MCF-7 human breast cancer cell lines. E3 J. Med. Res. 2012, 1, 38–43. [Google Scholar]
- Afjalus, S.M.; Malik, S.; Emrul, H.; Sanjana, S.; Farjana, Y. Evaluation of neuropharmacological, antibacterial, and antinociceptive activity of methanolic extract of the bark of Cynometra ramiflora Linn. (Leguminosae). Int. J. Res. Ayurveda Pharm. 2013, 4, 192–197. [Google Scholar] [CrossRef]
- Suhendi, A.; Indrayudha, P.; Azizah, T. Antibacterial activity of ethanol extract of steam bark of Cynometra ramiflora Linn a against Various Bacterial. Open Conf. Proc. J. 2013, 4, 92. [Google Scholar] [CrossRef][Green Version]
- Indrayudha, P.; Suhendi, A.; Azizah, T. Cytotoxic effects of ethanol extract from Cynometra ramiflora Linn leaves on T47D, HeLa, and WiDr cancer cell lines. Open Conf. Proc. J. 2013, 4, 91. [Google Scholar] [CrossRef][Green Version]
- Sookying, S.; Pekthong, D.; Oo-puthinan, A.M.; Xing, J.; Zhan, Z.; Ingkaninan, K. Antioxidant activity of Sala (Cynometra ramiflora Linn) plant extract. Open Conf. Proc. J. 2013, 4, 56. [Google Scholar] [CrossRef]
- Haryoto, H.; Muhtadi, M.; Indrayudha, P.; Azizah, T.; Suhendi, A. The activity of sitotoksik ethanol extract plant growth (Cynometra ramiflora Linn) on heavy cell, T47D, and WiDR. J. Penelit. Saintek 2013, 18. [Google Scholar] [CrossRef]
- Premkaisorn, P.; Wasupongpun, W. Antioxidant activity of fourteen herbal plants in Thailand. APHEIT Conf. 2015, 356–364. [Google Scholar]
- Abdulah, R.; Milanda, T.; Sugijanto, M.; Barliana, M.I.; Diantini, A.; Supratman, U.; Subarnas, A. Antibacterial properties of selected plants consumed by primates against Escherichia coli and Bacillus subtilis. Southeast Asian J. Trop. Med. Public Health 2017, 48, 109. [Google Scholar]
- Meutia, Z.Z.; Dewi, B.E. Effects of C. ramiflora Linn. leaf extract against dengue virus replication in vitro on huh7it-1 cell. Adv. Sci. Lett. 2017, 23, 6834–6837. [Google Scholar] [CrossRef]
- Groiss, S.; Selvaraj, R.; Varadavenkatesan, T.; Vinayagam, R. Structural characterization, antibacterial and catalytic effect of iron oxide nanoparticles synthesized using the leaf extract of Cynometra ramiflora. J. Mol. Struct. 2017, 1128, 572–578. [Google Scholar] [CrossRef]
- Yusuf, A.I.; Dewi, B.E.; Sjatha, F. Antiviral activity of Cynometra ramiflora Linn leaves extract against replication of dengue virus serotype 2 on huh 7.5 cells in vitro. In Proceedings of the BROMO Conference—Symposium on Natural Product and Biodiversity, Surabaya, Indonesia, 11–12 July 2018. [Google Scholar] [CrossRef][Green Version]
- Argulla, L.E.; Chichioco-Hernandez, C.L. Xanthine oxidase inhibitory activity of some leguminosae plants. Asian Pac. J. Trop. Dis. 2014, 4, 438–441. [Google Scholar] [CrossRef]
- Suffredini, I.B.; Paciencia, M.L.B.; Varella, A.D.; Younes, R.N. In vitro cytotoxic activity of brazilian plant extracts against human lung, colon and CNS solid cancers and leukemia. Fitoterapia 2007, 78, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Ozi, J.M.; Suffredini, I.B.; Paciencia, M.; Frana, S.A.; Dib, L.L. In vitro cytotoxic effects of Brazilian plant extracts on squamous cell carcinoma of the oral cavity. Braz. Oral Res. 2011, 25, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Nafar, A.A.; Vijayan, F.P.; Rani, V.K.J.; Padikkala, J. Antioxidant and anti-inflammatory activities of methanolic extract of Cynometra travancorica Bedd leaves. Biomedicine 2010, 30, 480–486. [Google Scholar]
- Suhail, P.T. Comparative evaluation of antioxidant activity of methanolic extract of Saraca asoca and its commonly used substitute plants. Int. J. Res. Rev. 2019, 6, 37–43. [Google Scholar]
- Meera, N.; Divya, M.K.; Silpa, P.; Pareeth, C.M.; Raghavamenon, A.C.; Babu, T.D. Amelioration of sodium fluoride-induced oxidative stress by Cynometra travancorica bedd in mice. J. Complement. Integr. Med. 2021. [Google Scholar] [CrossRef]
- Haryoto, S.; Andi, E.P.; Tanti Azizah Sujono, M. Uji toksisitas subkronis ekstrak etanol daun tumbuhan sala (Cynometra ramiflora linn.) Dengan parameter kimia urin dan histopatologi organ ginjal pada tikus galur wistar. In Proceedings of the 2nd University Research Coloquium, Semarang, Indonesia, 2015; pp. 198–201. Available online: https://jurnal.unimus.ac.id/index.php/psn12012010/article/view/1634 (accessed on 1 January 2022).




| Cynometra Species | ||
|---|---|---|
| C. abrahamii Du Puy & R. Rabev. | C. craibii Gagnep. | C. hemitomophylla (Donn. Sm.) Rose |
| C. alexandri C.H. Wright | C. crassifolia Benth. | C. hondurensis Dwyer |
| C. americana Vogel | C. cubensis A.Rich. | C. hostmanniana Tul. |
| C. ananta Hutch. & Dalziel | C. cuneata Tul. | C. humboldtiana Stergios |
| C. ankaranensis Du Puy & R.Rabev. | C. cynometroides (Merr. & L.M.Perry) Rados. | C. inaequifolia A.Gray |
| C. aurita R.Vig. | C. dauphinensis Du Puy & R. Rabev. | C. insularis A.C.Sm. |
| C. basifoliola (Verdc.) Rados. | C. dongnaiensis Pierre | C. iripa Kostel. |
| C. bauhiniifolia Benth. | C. letestui (Pellegr.) J. Léonard | C. polyandra Roxb. |
| C. beddomei Prain | C. longicuspis Ducke | C. portoricensis Krug & Urb. |
| C. bourdillonii Gamble | C. longifolia Huber | C. psilogyne (Harms) Rados. |
| C. brachymischa Harms | C. longipedicellata Harms | C. ramiflora L. |
| C. brachyrrhachis Harms | C. lujae De Wild. | C. retusa Britton & Rose |
| C. brassii (Merr. & L.M. Perry) Rados. | C. lukei Beentje | C. rosea (K.Schum.) Rados. |
| C. browneoides (Harms) Rados. | C. lyallii Baker | C. roseiflora W.E.Cooper |
| C. capuronii Du Puy & R.Rabev. | C. macrocarpa A.S.Tav. | C. sakalava Du Puy & R.Rabev. |
| C. cauliflora L. | C. madagascariensis Baill. | C. sanagaensis Aubrév. |
| C. cebuensis F.Seid. | C. malaccensis Meeuwen | C. schefferi (K.Schum.) Rados. |
| C. cerebriformis Rados. | C. mannii Oliv. | C. schlechteri Harms |
| C. commersoniana Baill. | C. marginata Benth. | C. schottiana Hochr. |
| C. congensis De Wild. | C. mariettae (Meeuwen) Rados. | C. sessiliflora Harms |
| C. copelandii (Elmer) Merr. | C. marleneae A.S.Tav. | C. simplicifolia Harms |
| C. duckei Dwyer | C. mayottensis Labat & O. Pascal | C. sphaerocarpa Pittier |
| C. dwyeri Rados. | C. megalocephala (Harms) Rados. | C. steenisii (Meeuwen) Rados. |
| C. elmeri Merr. | C. megalophylla Harms | C. stenopetala Dwyer |
| C. engleri Harms | C. michelsonii J.Léonard | C. steyermarkii Rados. |
| C. falcata A.Gray | C. minor (A.C. Sm.) Rados. | C. suaheliensis (Taub.) Baker f. |
| C. filifera Harms | C. minutiflora F.Muell. | C. travancorica Bedd. |
| C. fissicuspis (Pittier) Pittier | C. mirabilis Meeuwen | C. trinitensis Oliv. |
| C. floretii Labat & O.Pascal | C. novoguineensis Merr. & L.M.Perry | C. tumbesiana Rados. |
| C. fortuna-tironis (Verdc.) Rados. | C. nyangensis Pellegr. | C. ulugurensis Harms |
| C. gillmanii J.Léonard | C. oaxacana Brandegee | C. vestita (A.C.Sm.) Rados. |
| C. glomerulata Gagnep. | C. oddonii De Wild. | C. vitiensis Rados. |
| C. grandiflora A.Gray | C. palustris J.Léonard | C. vogelii Hook.f. |
| C. greenwayi Brenan | C. parvifolia Tul. | C. warburgii Harms |
| C. hankei Harms | C. pedicellata De Wild. | C. webberi Baker f. |
| C. katikii Verdc. | C. pervilleana Baill. | C. yokotae Kaneh. |
| C. lenticellata (C.T.White) Rados. | C. phaselocarpa (B.Heyne) J.F.Macbr./C. spruceana Benth. | C. zeylanica Kosterm. |
| C. leonensis Hutch. & Dalziel | C. plurijuga (Merr. & L.M.Perry) Rados. | |
| Species | Part Used | Country | Traditional Uses | Method of Preparation | Refs. |
|---|---|---|---|---|---|
| C. brachyrrhachis | root | Tanzania | fungal infections | not available | [17] |
| C. capuronii | leaf | Madagascar | yellow fever | decoction | [18] |
| C. cauliflora | leaf | Indonesia | diarrhea | not available | [16] |
| leaf | Malaysia | hyperlipidemia and diabetes | decoction | [15,19] | |
| fruit | Malaysia | loss of appetite | not available | [20] | |
| seed oil | India | skin diseases | |||
| C. hankei | stem bark | Africa | dental pain and rheumatism | [21] | |
| C. iripa | leaf, seed, stem | India | wound healing | [22] | |
| leaf | India | ulcers | decoction | [23] | |
| seed oil | India | cholera | not available | [24] | |
| C. manii | stem | Nigeria | to suppress swelling in the cheeks | [25] | |
| bark | Nigeria | cancer | [26] | ||
| C. megalophylla | seed | Nigeria | fibroid treatment | [27] | |
| leaf | Benin | stomach infections | [28] | ||
| C. ramiflora | leaf, root | India | purgative, skin diseases | [29] | |
| whole plant | Bangladesh | skin diseases | powder | [30] | |
| C. spruceana | resin | Brazil | weakness of the lungs, tuberculosis, chronic cough | not available | [31] |
| C. vogelii | stem | Nigeria | oral hygiene | [32] | |
| C. webberi | root | Tanzania | skin diseases | [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabiha, S.; Serrano, R.; Hasan, K.; Moreira da Silva, I.B.; Rocha, J.; Islam, N.; Silva, O. The Genus Cynometra: A Review of Ethnomedicine, Chemical, and Biological Data. Plants 2022, 11, 3504. https://doi.org/10.3390/plants11243504
Sabiha S, Serrano R, Hasan K, Moreira da Silva IB, Rocha J, Islam N, Silva O. The Genus Cynometra: A Review of Ethnomedicine, Chemical, and Biological Data. Plants. 2022; 11(24):3504. https://doi.org/10.3390/plants11243504
Chicago/Turabian StyleSabiha, Shabnam, Rita Serrano, Kamrul Hasan, Isabel B. Moreira da Silva, João Rocha, Nurul Islam, and Olga Silva. 2022. "The Genus Cynometra: A Review of Ethnomedicine, Chemical, and Biological Data" Plants 11, no. 24: 3504. https://doi.org/10.3390/plants11243504
APA StyleSabiha, S., Serrano, R., Hasan, K., Moreira da Silva, I. B., Rocha, J., Islam, N., & Silva, O. (2022). The Genus Cynometra: A Review of Ethnomedicine, Chemical, and Biological Data. Plants, 11(24), 3504. https://doi.org/10.3390/plants11243504