Genome-Wide Identification and Expression Analysis of the 14-3-3 (TFT) Gene Family in Tomato, and the Role of SlTFT4 in Salt Stress
Abstract
1. Introduction
2. Results
2.1. Identification and Characterization of SlTFTs
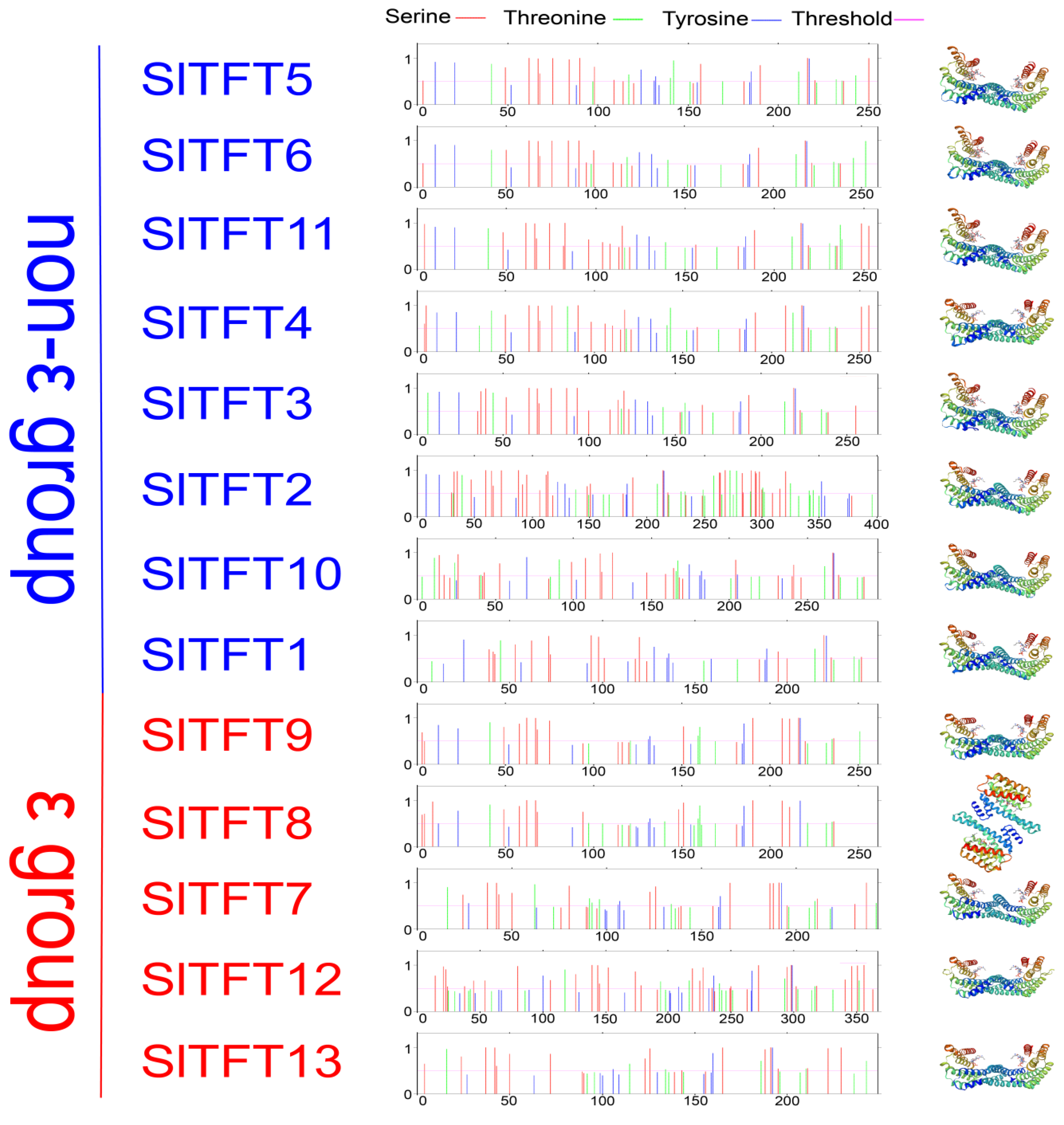
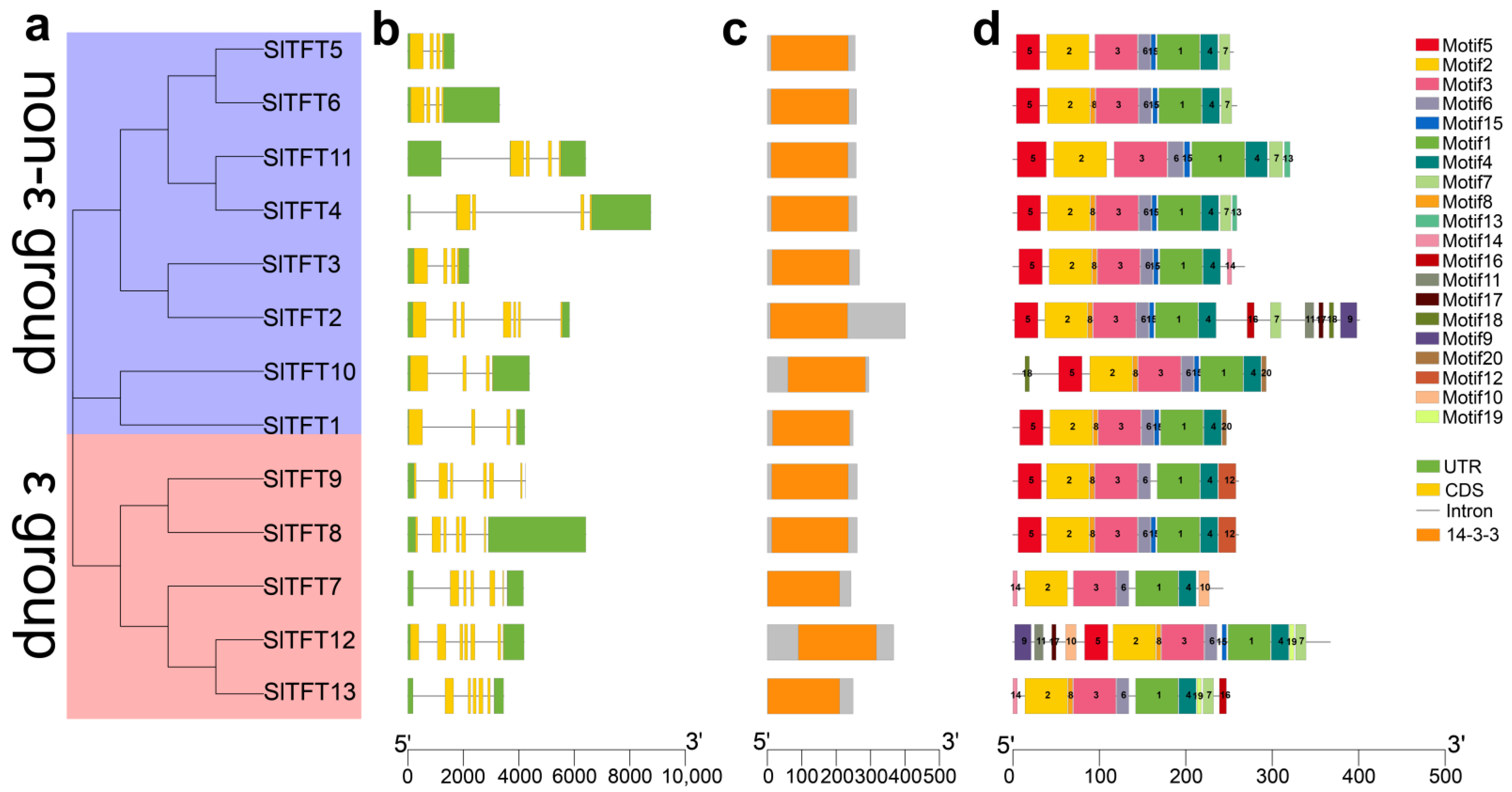
2.2. Phylogeny, Gene Structure, Conserved Domains, and Motif Analysis of SlTFTs
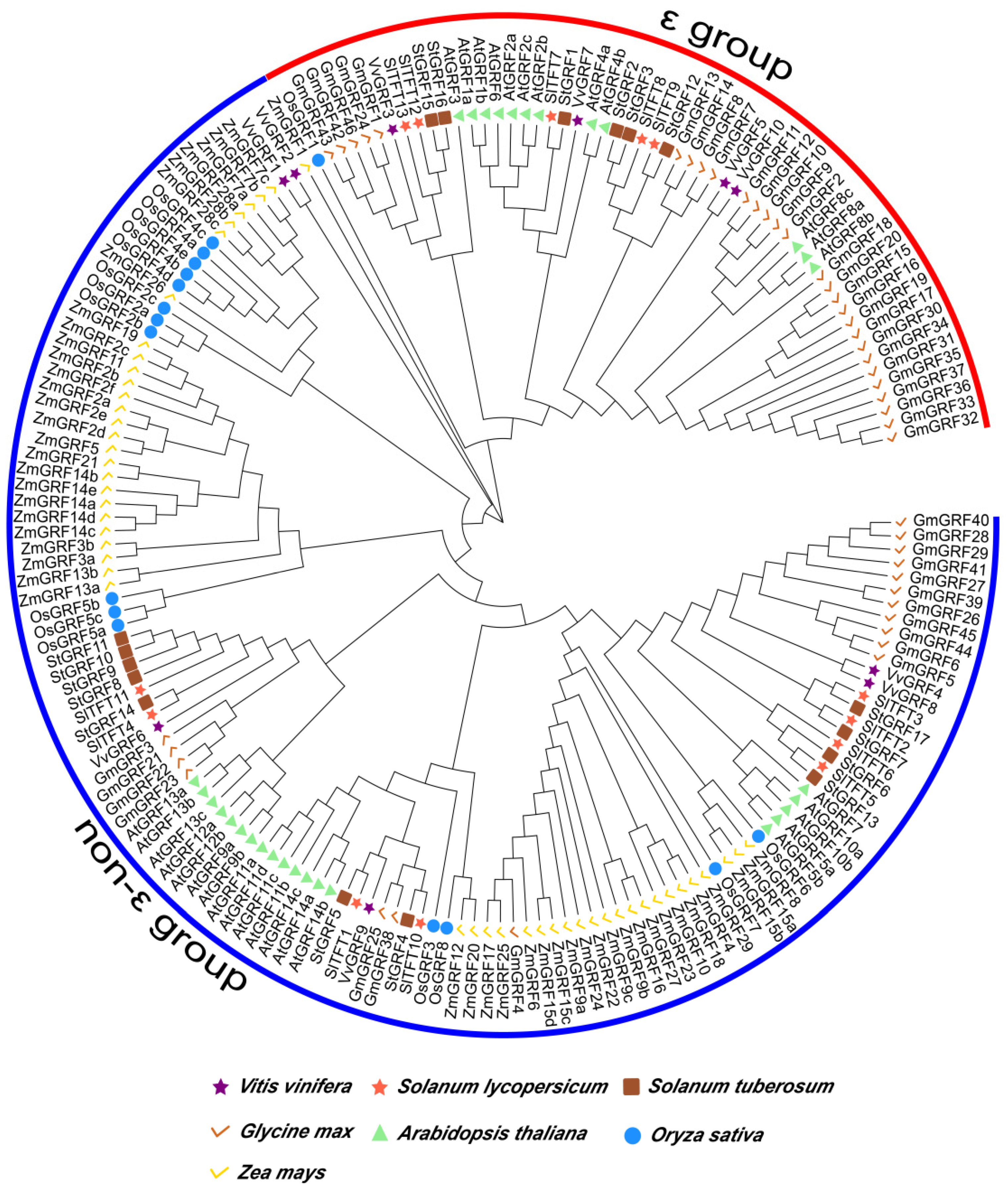
2.3. Phylogenetic Tree Analysis of 14-3-3 Proteins among Different Model Species
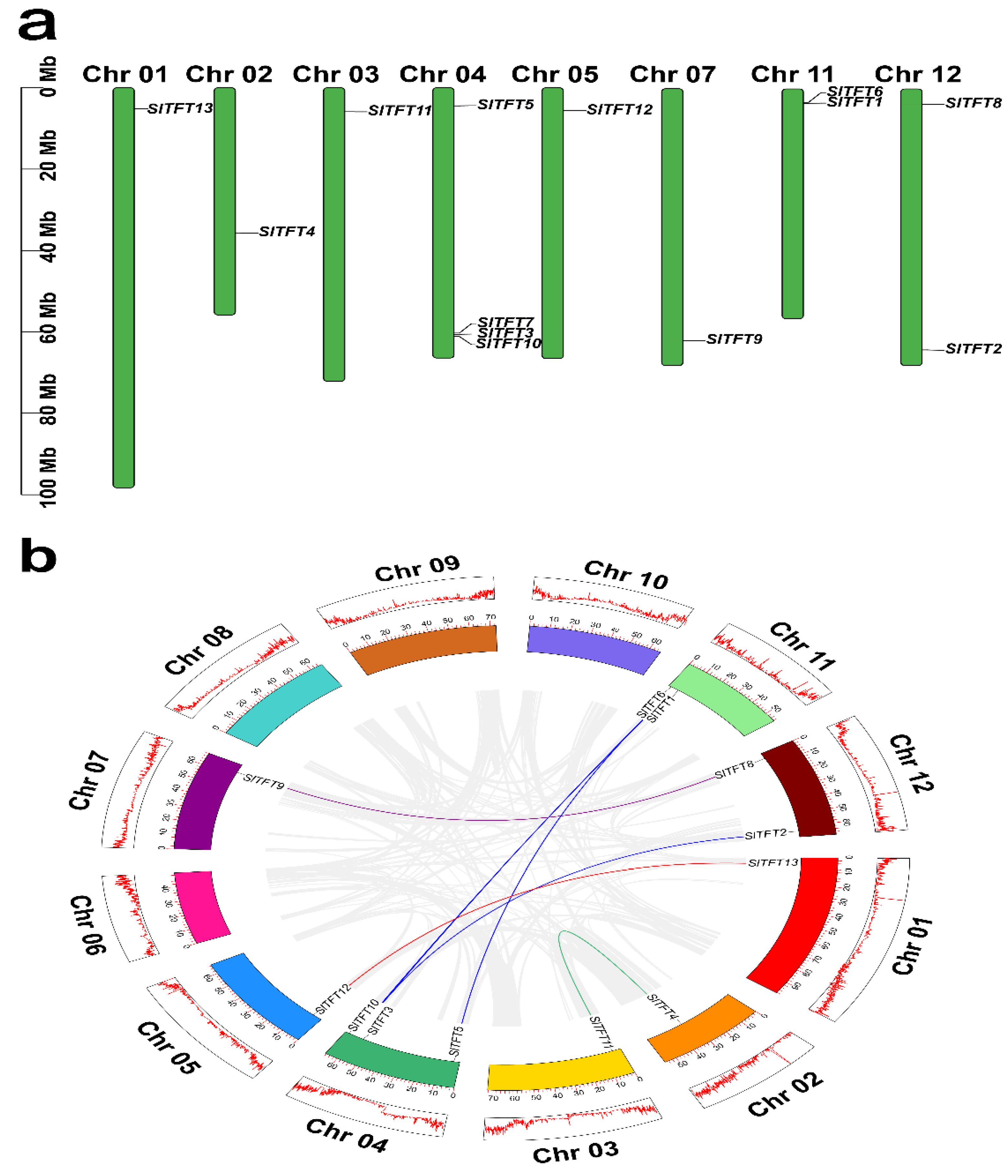
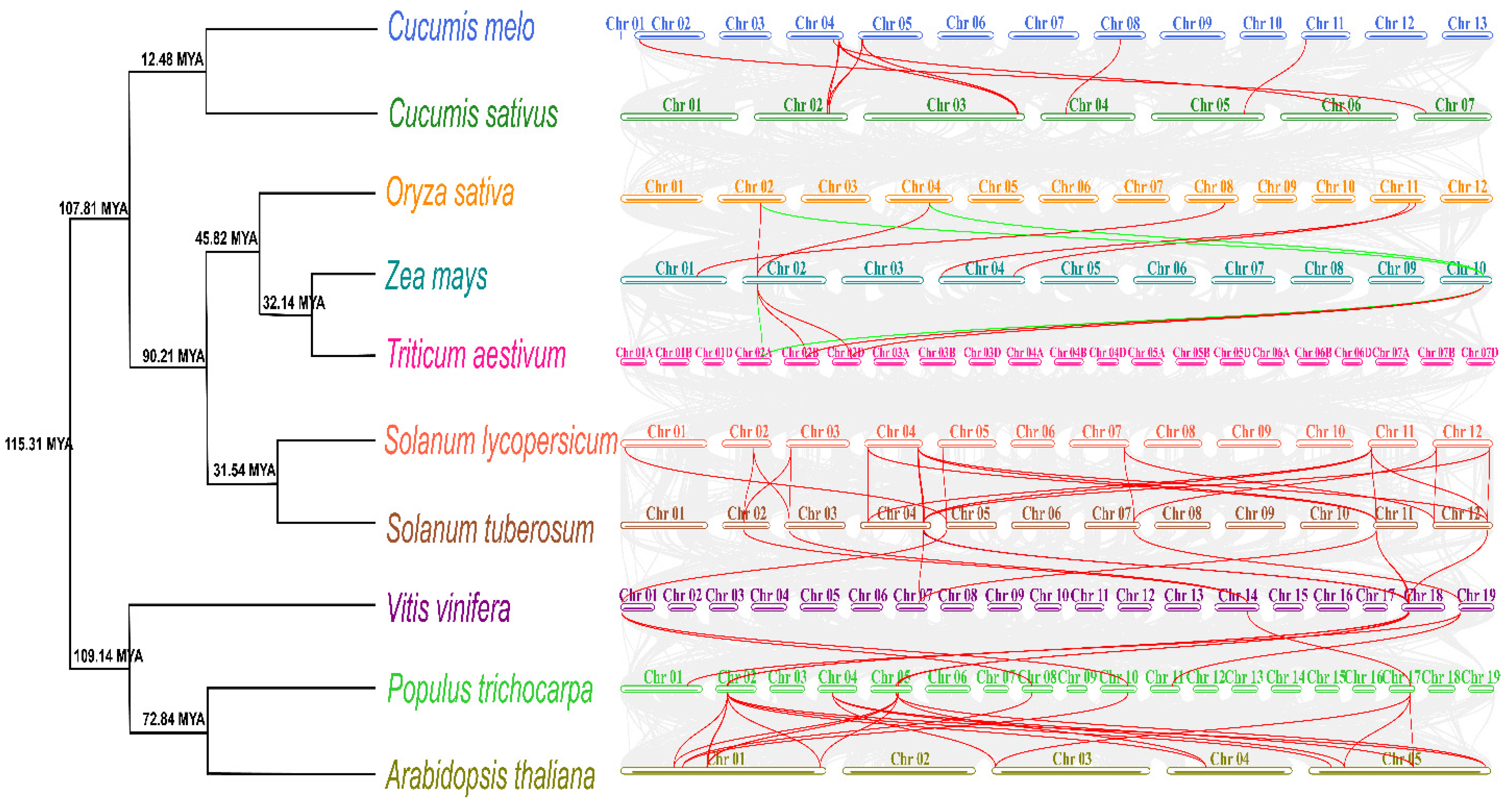
2.4. Chromosome Distribution, Replication Events, Divergence Time, and Synteny Analysis of SlTFTs
2.5. Analysis of Cis-Acting Elements of SlTFTs
2.6. Transcription Profiling of SlTFTs
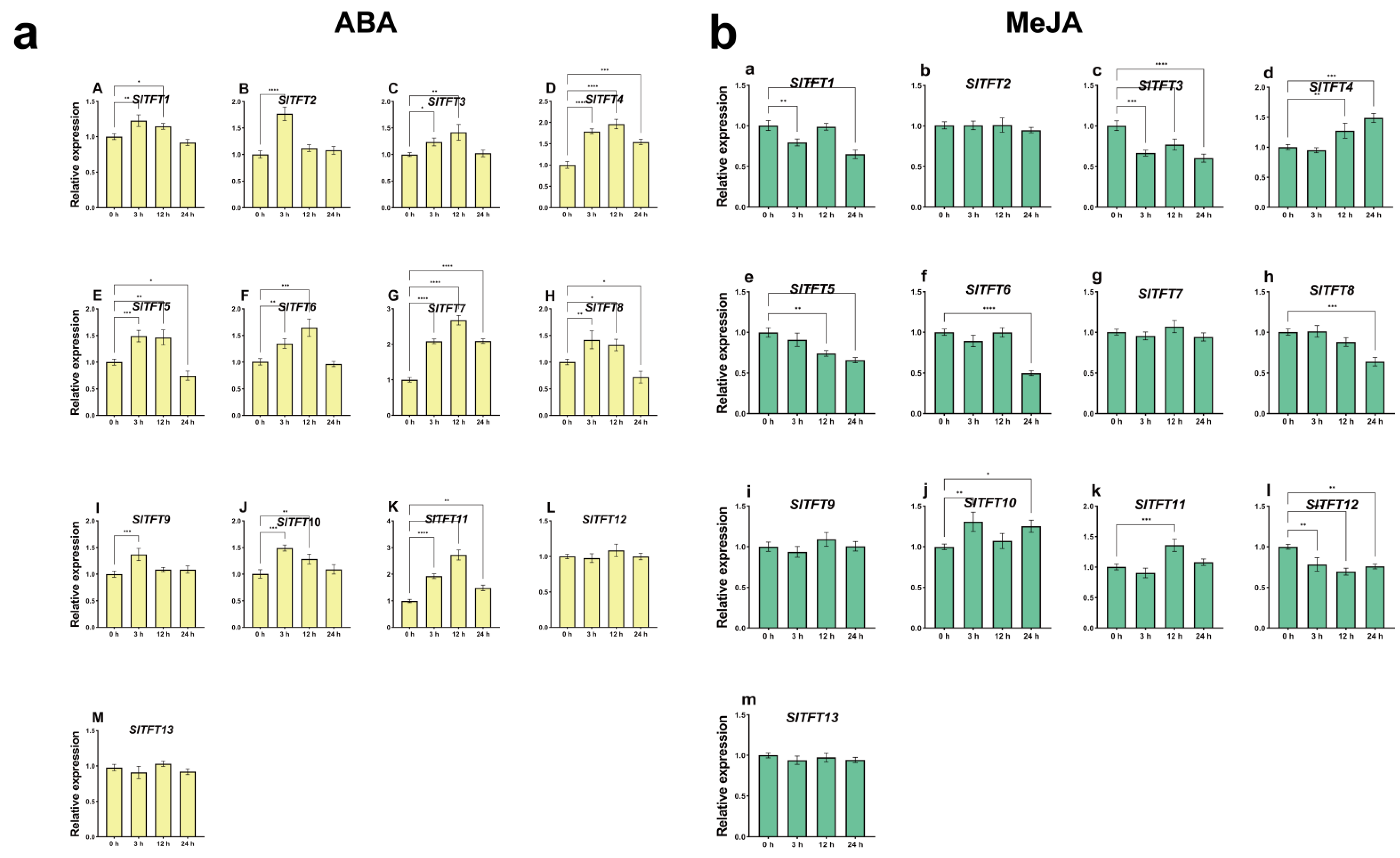
2.7. Analysis of Expression Patterns of SlTFTs in Response to Abiotic Stresses and Phytohormone Treatments
2.8. Correlation between Cis-Acting Elements and Expression Patterns under Different Abiotic Stresses or Phytohormone Treatments
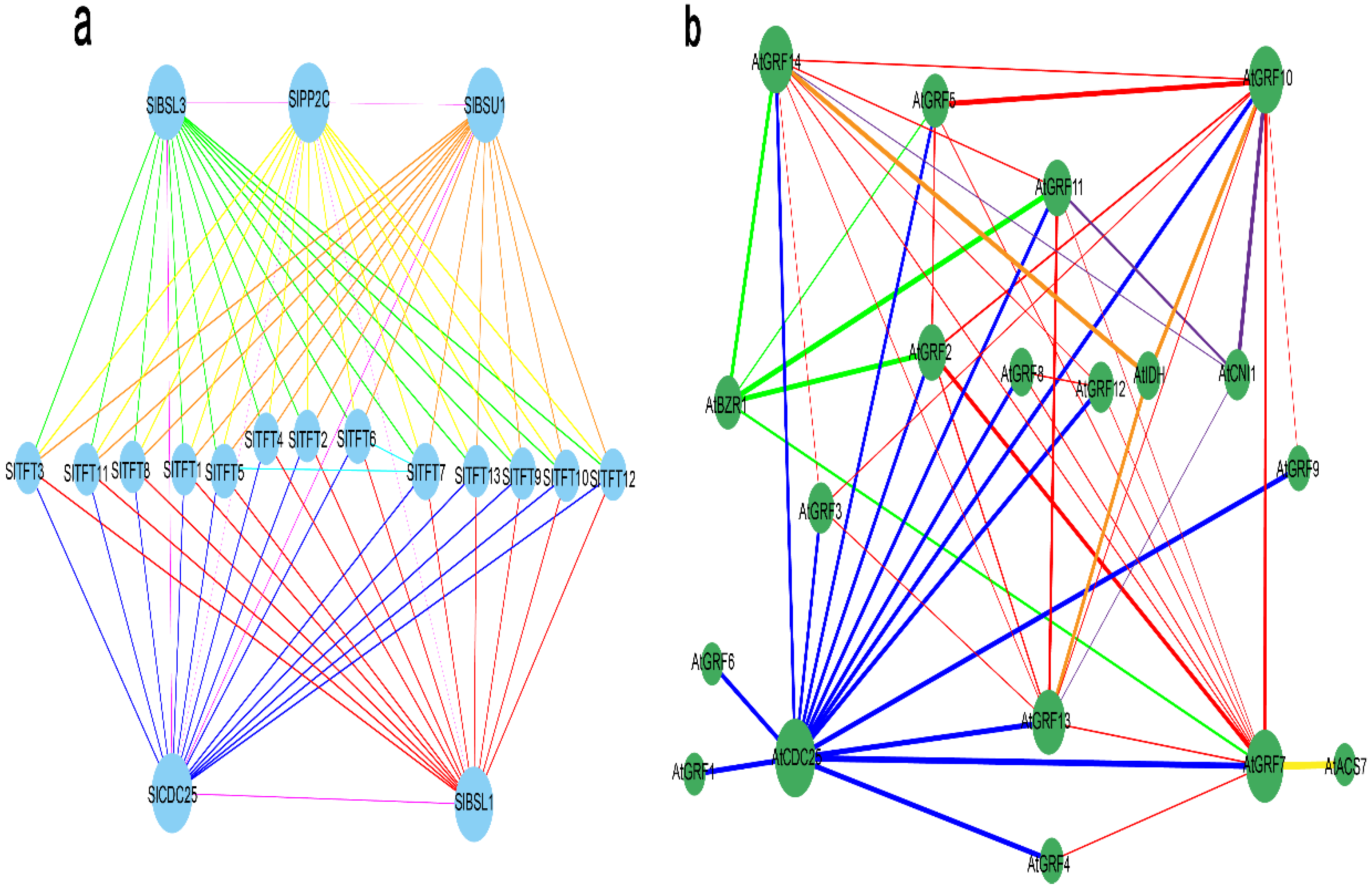
2.9. Interaction Network Analysis of 14-3-3 Proteins
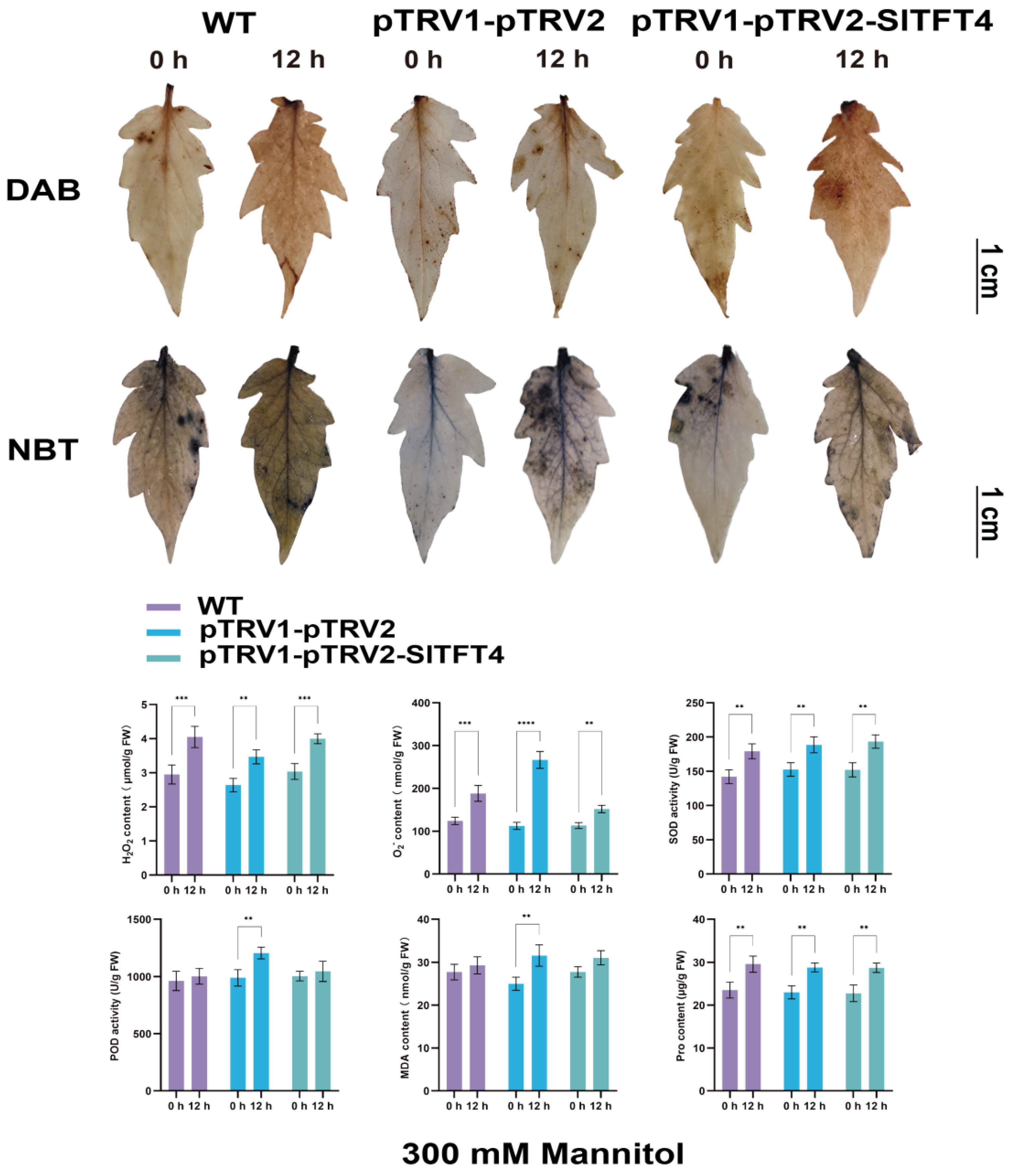
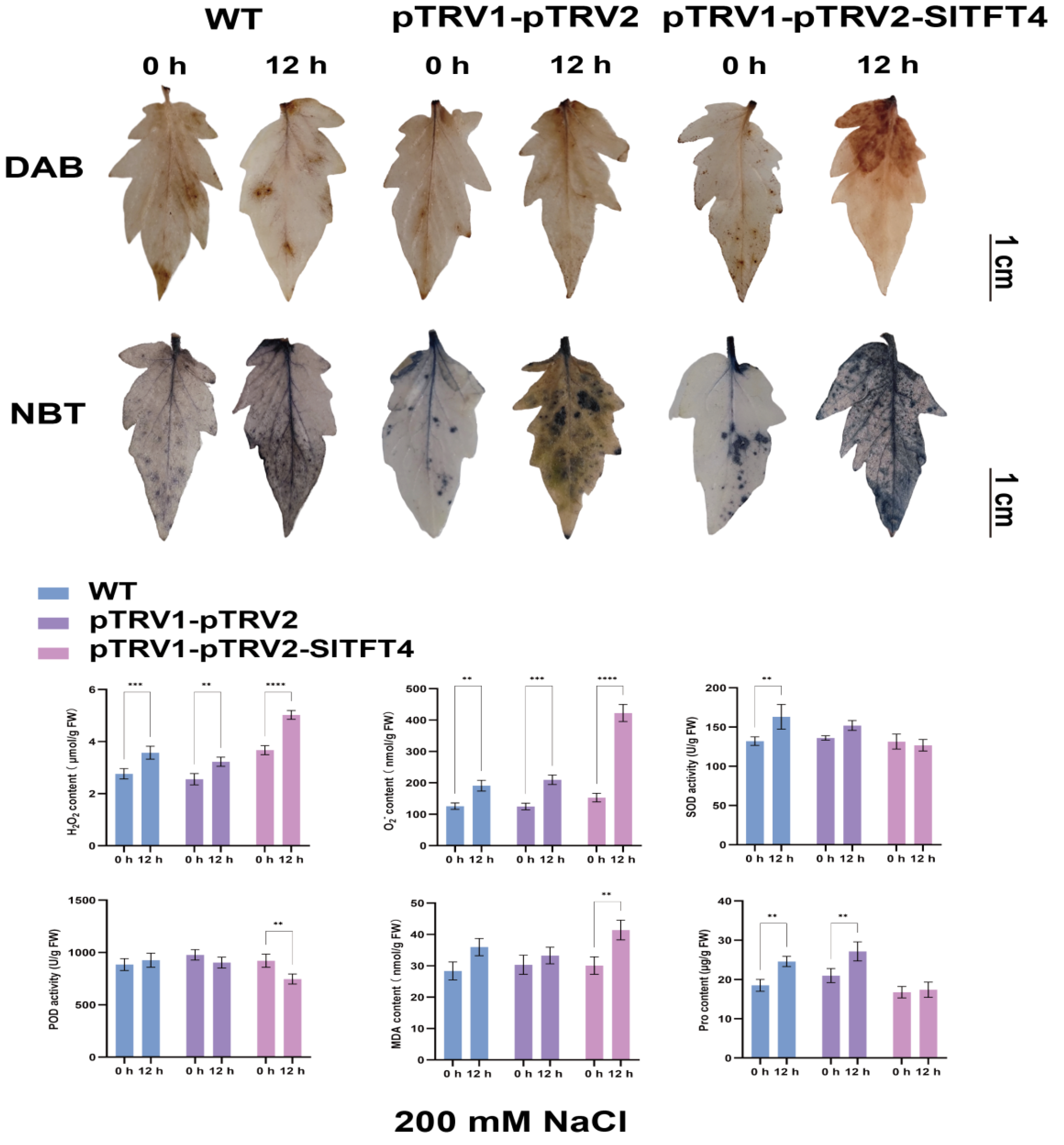
2.10. Subcellular Localization and Gene Silencing of SlTFT4
3. Discussion
3.1. Significance of the Study of SlTFTs and Its Structural Characterization
3.2. Evolutionary Characterization of SlTFTs
3.3. Analysis of Cis-Acting Elements of SlTFTs and Correlation with Expression Patterns
3.4. Unique Transcriptional Profiles and Expression Patterns of SlTFTs
3.5. The Constructed Protein Network That Interacts with SlTFTs Deepens the Knowledge
3.6. Functional Analysis Revealed That SlTFT4 Is a Candidate Gene for Salt Tolerance
4. Materials and Methods
4.1. Identification and Characterization of SlTFT Family
4.2. Phylogenetic Tree, Gene Structure, Conserved Domains, and Motif Analysis of SlTFTs
4.3. Phylogenetic Tree Analysis of SlTFTs among Different Species
4.4. Chromosome Distribution, Replication Events, and Synteny Analysis of SlTFTs
4.5. Analysis of Cis-Acting Elements in the Promoter Region of SlTFTs
4.6. Transcriptional Profiling of SlTFTs
4.7. Plant Material and Treatments
4.8. Total RNA Extraction, Quantitative Real-Time PCR (qRT-PCR), and Statistical Analysis
4.9. Protein Interaction Network Analysis
4.10. Subcellular Localization
4.11. VIGS Vector Construction and the Process of Gene Silencing and Physiological Indices Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, D.; Guthridge, M.; Woodcock, J.; Lopez, A. 14-3-3 protein signaling in development and growth factor responses. Curr. Top. Dev. Biol. 2005, 67, 285–303. [Google Scholar]
- Rooney, M.F.; Ferl, R.J. Sequences of three Arabidopsis general regulatory factor genes encoding GF14 (14-3-3) proteins. Plant Physiol. 1995, 107, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, C.J.; Rooney, M.F.; Miller, P.W.; Ferl, R.J. Molecular organization and tissue-specific expression of an Arabidopsis 14-3-3 gene. Plant Cell 1996, 8, 1239–1248. [Google Scholar]
- Wu, K.; Rooney, M.F.; Ferl, R.J. The Arabidopsis 14-3-3 multigene family. Plant Physiol. 1997, 114, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, M.B.; Rittinger, K.; Volinia, S.; Caron, P.R.; Aitken, A.; Leffers, H.; Gamblin, S.J. The structural basisfor 14-3-3:phosphopeptide binding specificity. Cell 1997, 91, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Ottmann, C.; Marco, S.; Jaspert, N.; Marcon, C.; Schauer, N.; Weyand, M.; Vandermeeren, C.; Duby, G.; Boutry, M.; Wittinghofer, A.; et al. Structure of a 14-3-3 coordinated hexamer of the plant plasma membrane H+ -ATPase by combining X-ray crystallography and electron cryomicroscopy. Mol. Cell 2007, 25, 427–440. [Google Scholar] [CrossRef]
- Taoka, K.; Ohki, I.; Tsuji, H.; Furuita, K.; Hayashi, K.; Yanase, T.; Yamaguchi, M.; Nakashima, C.; Purwestri, Y.A.; Tamaki, S.; et al. 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 2011, 476, 332–335. [Google Scholar] [CrossRef]
- Delille, J.M.; Sehnke, P.C.; Ferl, R.J. The Arabidopsis 14-3-3 family of signaling regulators. Plant Physiol. 2001, 126, 35–38. [Google Scholar] [CrossRef]
- Rosenquist, M.; Alsterfjord, M.; Larsson, C.; Sommarin, M. Data mining the Arabidopsis genome reveals fifteen 14-3-3 genes. Expression is demonstrated for two out of five novel genes. Plant Physiol. 2001, 127, 142–149. [Google Scholar] [CrossRef]
- Tian, F.; Wang, T.; Xie, Y.; Zhang, J.; Hu, J. Genome-wide identification, classification, and expression analysis of 14-3-3 gene family in Populus. PLoS ONE 2015, 10, e0123225. [Google Scholar] [CrossRef]
- Chen, F.; Li, Q.; Sun, L.; He, Z. The rice 14-3-3 gene family and its involvement in responses to biotic and abiotic stress. DNA Res. 2006, 13, 53–63. [Google Scholar] [CrossRef]
- Xu, W.F.; Shi, W.M. Expression profiling of the 14-3-3 gene family in response to salt stress and potassium and iron deficiencies in young tomato (Solanum lycopersicum) roots: Analysis by real-time RT-PCR. Ann. Bot. 2006, 98, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Cotelle, V.; Leonhardt, N. 14-3-3 proteins in guard cell signaling. Front. Plant Sci. 2015, 6, 1210. [Google Scholar] [CrossRef] [PubMed]
- Camoni, L.; Visconti, S.; Aducci, P.; Marra, M. 14-3-3 proteins in plant hormone signaling: Doing several things at once. Front. Plant Sci. 2018, 9, 297. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, S.; Liu, B. 14-3-3 proteins: Macro-regulators with great potential for improving abiotic stress tolerance in plants. Biochem. Biophys. Res. Commun. 2016, 477, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, J.D.; Folta, K.M.; Paul, A.L.; Ferl, R.J. The 14-3-3 Proteins μ and ν influence transition to flowering and early phytochrome response. Plant Physiol. 2007, 145, 1692–1702. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Y.; Zhang, Y.X. Pear 14-3-3a gene (Pp14-3-3a) is regulated during fruit ripening and senescense, and involved in response to salicylic acid and ethylene signalling. J. Genet. 2014, 93, 747–753. [Google Scholar] [CrossRef]
- Li, M.; Ren, L.; Xu, B.; Yang, X.; Xia, Q.; He, P.; Xiao, S.; Guo, A.; Hu, W.; Jin, Z. Genome-wide identification, phylogeny, and expression analyses of the 14-3-3 family reveal their involvement in the development, ripening, and abiotic stress response in banana. Front. Plant Sci. 2016, 7, 1442. [Google Scholar] [CrossRef]
- He, Y.; Wu, J.; Lv, B.; Li, J.; Gao, Z.; Xu, W.; Baluska, F.; Shi, W.; Shaw, P.C.; Zhang, J. Involvement of 14-3-3 protein GRF9 in root growth and response under polyethylene glycol-induced water stress. J. Exp. Bot. 2015, 66, 2271–2281. [Google Scholar] [CrossRef]
- Yan, J.; He, C.; Jing, W.; Mao, Z.; Holaday, S.A.; Allen, R.D.; Zhang, H. Overexpression of the Arabidopsis 14-3-3 protein GF14λ in cotton leads to a "stay-green" phenotype and improves stress tolerance under moderate drought conditions. Plant Cell Physiol. 2004, 45, 1007–1014. [Google Scholar] [CrossRef]
- Campo, S.; Peris-Peris, C.; Montesinos, L.; Penas, G.; Messeguer, J.; San Segundo, B. Expression of the maize ZmGF14-6 gene in rice confers tolerance to drought stress while enhancing susceptibility to pathogen infection. J. Exp. Bot. 2012, 63, 983–999. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.L.; Huang, L.F.; Lu, C.A.; He, S.L.; Wang, C.C.; Yu, S.P.; Chen, J.; Yu, S.M. Sugar starvation- and GA-inducible calcium-dependent protein kinase 1 feedback regulates GA biosynthesis and activates a 14-3-3 protein to confer drought tolerance in rice seedlings. Plant Mol. Biol. 2013, 81, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Luo, X.; Sun, M.; Chen, C.; Ding, X.; Wang, X.; Yang, S.; Yu, Q.; Jia, B.; Ji, W.; et al. A Glycine soja 14-3-3 protein GsGF14o participates in stomatal and root hair development and drought tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 99–118. [Google Scholar] [CrossRef]
- Zhou, H.; Lin, H.; Chen, S.; Becker, K.; Yang, Y.; Zhao, J.; Kudla, J.; Schumaker, K.S.; Guo, Y. Inhibition of the Arabidopsis salt overly sensitive pathway by 14-3-3 proteins. Plant Cell 2014, 26, 1166–1182. [Google Scholar] [CrossRef] [PubMed]
- Catala, R.; Lopez-Cobollo, R.; Mar Castellano, M.; Angosto, T.; Alonso, J.M.; Ecker, J.R.; Salinas, J. The Arabidopsis 14-3-3 protein RARE COLD INDUCIBLE 1A links low-temperature response and ethylene biosynthesis to regulate freezing tolerance and cold acclimation. Plant Cell 2014, 26, 3326–3342. [Google Scholar] [CrossRef]
- Vercruyssen, L.; Tognetti, V.B.; Gonzalez, N.; Van Dingenen, J.; De Milde, L.; Bielach, A.; De Rycke, R.; Van Breusegem, F.; Inze, D. GROWTH REGULATING FACTOR5 stimulates Arabidopsis chloroplast division, photosynthesis, and leaf longevity. Plant Physiol. 2015, 167, 817–832. [Google Scholar] [CrossRef]
- Cao, A.; Jain, A.; Baldwin, J.C.; Raghothama, K.G. Phosphate differentially regulates 14-3-3 family members and GRF9 plays a role in Pi-starvation induced responses. Planta 2007, 226, 1219–1230. [Google Scholar] [CrossRef]
- Yang, J.L.; Chen, W.W.; Chen, L.Q.; Qin, C.; Jin, C.W.; Shi, Y.Z.; Zheng, S.J. The 14-3-3 protein GENERAL REGULATORY FACTOR11 (GRF11) acts downstream of nitric oxide to regulate iron acquisition in Arabidopsis thaliana. New Phytol. 2013, 197, 815–824. [Google Scholar] [CrossRef]
- Radwan, O.; Wu, X.; Govindarajulu, M.; Libault, M.; Neece, D.J.; Oh, M.H.; Berg, R.H.; Stacey, G.; Taylor, C.G.; Huber, S.C.; et al. 14-3-3 proteins SGF14c and SGF14l play critical roles during soybean nodulation. Plant Physiol. 2012, 160, 2125–2136. [Google Scholar] [CrossRef]
- Yao, Y.; Du, Y.; Jiang, L.; Liu, J.Y. Molecular analysis and expression patterns of the 14-3-3 gene family from Oryza Sativa. J. Biochem. Mol. Biol. 2007, 40, 349–357. [Google Scholar] [CrossRef]
- Denison, F.C.; Paul, A.L.; Zupanska, A.K.; Ferl, R.J. 14-3-3 proteins in plant physiology. Semin. Cell Dev. Biol. 2011, 22, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Swatek, K.N.; Thelen, J.J. Regulation of the regulators: Post-translational modifications, subcellular, and spatiotemporal distribution of plant 14-3-3 proteins. Front. Plant Sci. 2016, 7, 611. [Google Scholar] [CrossRef] [PubMed]
- Ormancey, M.; Thuleau, P.; Mazars, C.; Cotelle, V. CDPKs and 14-3-3 proteins: Emerging duo in signaling. Trends Plant Sci. 2017, 22, 263–272. [Google Scholar] [CrossRef]
- Kong, Q.; Ma, W. WRINKLED1 as a novel 14-3-3 client: Function of 14-3-3 proteins in plant lipid metabolism. Plant Signal. Behav. 2018, 13, e1482176. [Google Scholar] [CrossRef]
- Sato, T.; Maekawa, S.; Yasuda, S.; Domeki, Y.; Sueyoshi, K.; Fujiwara, M.; Fukao, Y.; Goto, D.B.; Yamaguchi, J. Identification of 14-3-3 proteins as a target of ATL31 ubiquitin ligase, a regulator of the C/N response in Arabidopsis. Plant J. 2011, 68, 137–146. [Google Scholar] [CrossRef]
- Yao, Y.; Du, Y.; Jiang, L.; Liu, J.Y. Interaction between ACC synthase 1 and 14-3-3 proteins in rice: A new insight. Biochemistry 2007, 72, 1003–1007. [Google Scholar] [CrossRef]
- Schoonheim, P.J.; Sinnige, M.P.; Casaretto, J.A.; Veiga, H.; Bunney, T.D.; Quatrano, R.S.; de Boer, A.H. 14-3-3 adaptor proteins are intermediates in ABA signal transduction during barley seed germination. Plant J. 2007, 49, 289–301. [Google Scholar] [CrossRef]
- Park, S.J.; Jiang, K.; Tal, L.; Yichie, Y.; Gar, O.; Zamir, D.; Eshed, Y.; Lippman, Z.B. Optimization of crop productivity in tomato using induced mutations in the florigen pathway. Nat. Genet. 2014, 46, 1337–1342. [Google Scholar] [CrossRef]
- Song, J.; Zhang, S.; Wang, X.; Sun, S.; Liu, Z.; Wang, K.; Wan, H.; Zhou, G.; Li, R.; Yu, H.; et al. Variations in both FTL1 and SP5G, two tomato FT paralogs, control day-neutral flowering. Mol. Plant 2020, 13, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, F.; Li, K. The 14-3-3 proteins: Regulators of plant metabolism and stress responses. Plant Biol. 2021, 23, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, W.; Yu, H.; Peng, J.; Hu, Z.; Chen, L. The role of 14-3-3 proteins in plant growth and response to abiotic stress. Plant Cell Rep. 2022, 41, 833–852. [Google Scholar] [CrossRef]
- Xu, L.; Li, T.; Wu, Z.; Feng, H.; Yu, M.; Zhang, X.; Chen, B. Arbuscular mycorrhiza enhances drought tolerance of tomato plants by regulating the 14-3-3 genes in the ABA signaling pathway. Appl. Soil Ecol. 2018, 125, 213–221. [Google Scholar] [CrossRef]
- Xu, W.; Jia, L.; Shi, W.; Baluska, F.; Kronzucker, H.J.; Liang, J.; Zhang, J. The tomato 14-3-3 protein TFT4 modulates H+ efflux, basipetal auxin transport, and the PKS5-J3 pathway in the root growth response to alkaline stress. Plant Physiol. 2013, 163, 1817–1828. [Google Scholar] [CrossRef]
- Xu, W.; Shi, W.; Jia, L.; Liang, J.; Zhang, J. TFT6 and TFT7, two different members of tomato 14-3-3 gene family, play distinct roles in plant adaption to low phosphorus stress. Plant Cell Environ. 2012, 35, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Peng, H.C.; He, J.; MacWilliams, J.; Teixeira, M.; Tsuchiya, T.; Chesnais, Q.; Mudgett, M.B.; Kaloshian, I. Aphid effector Me10 interacts with tomato TFT7, a 14-3-3 isoform involved in aphid resistance. New Phytol. 2019, 221, 1518–1528. [Google Scholar] [CrossRef]
- Taylor, K.W.; Kim, J.G.; Su, X.B.; Aakre, C.D.; Roden, J.A.; Adams, C.M.; Mudgett, M.B. Tomato TFT1 is required for PAMP-triggered immunity and mutations that prevent T3S effector XopN from binding to TFT1 attenuate Xanthomonas virulence. PLoS Pathog. 2012, 8, e1002768. [Google Scholar] [CrossRef]
- Shao, W.; Chen, W.; Zhu, X.; Zhou, X.; Jin, Y.; Zhan, C.; Liu, G.; Liu, X.; Ma, D.; Qiao, Y. Genome-wide identification and characterization of wheat 14-3-3 genes unravels the role of TaGRF6-A in salt stress tolerance by binding MYB transcription factor. Int. J. Mol. Sci. 2021, 22, 1904. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, C.; Kang, L.; Zhang, H.; Song, Y.; Zou, Z.; Zheng, W. Over-expression of a 14-3-3 protein from foxtail millet improves plant tolerance to salinity stress in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 449. [Google Scholar] [CrossRef]
- Wang, Y.; Ling, L.; Jiang, Z.; Tan, W.; Liu, Z.; Wu, L.; Zhao, Y.; Xia, S.; Ma, J.; Wang, G.; et al. Genome-wide identification and expression analysis of the 14-3-3 gene family in soybean (Glycine max). PeerJ 2019, 7, e7950. [Google Scholar] [CrossRef] [PubMed]
- Sang, N.; Liu, H.; Ma, B.; Huang, X.; Zhuo, L.; Sun, Y. Roles of the 14-3-3 gene family in cotton flowering. BMC Plant Biol. 2021, 21, 162. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, Y.; Chai, F.; Li, S.; Xin, H.; Liang, Z. Genome-wide identification and characterization of the 14-3-3 family in Vitis vinifera L. during berry development and cold- and heat-stress response. BMC Genom. 2018, 19, 579. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Wang, S.; Xiang, W.; Yang, H.; Tahir, M.M.; Zheng, S.; An, N.; Han, M.; Zhao, C.; Zhang, D. Genome-wide identification of the 14-3-3 gene family and its participation in floral transition by interacting with TFL1/FT in apple. BMC Genom. 2021, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; He, X.; Huang, X.; Yu, H.; Lu, T.; Xie, X.; Zeng, X.; Zhu, J.; Luo, C. Genome-wide identification and expression analysis of the 14-3-3 gene family in mango (Mangifera indica L.). Int. J. Mol. Sci. 2022, 23, 1593. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.P.; Li, H.L.; Guo, D.; Tang, X.; Peng, S.Q. Identification and characterization of the 14-3-3 gene family in Hevea brasiliensis. Plant Physiol. Biochem. 2014, 80, 121–127. [Google Scholar] [CrossRef]
- Qin, C.; Cheng, L.; Shen, J.; Zhang, Y.; Cao, H.; Lu, D.; Shen, C. Genome-wide identification and expression analysis of the 14-3-3 family genes in Medicago truncatula. Front. Plant Sci. 2016, 7, 320. [Google Scholar] [CrossRef]
- Dubrow, Z.; Sunitha, S.; Kim, J.G.; Aakre, C.D.; Girija, A.M.; Sobol, G.; Teper, D.; Chen, Y.C.; Ozbaki-Yagan, N.; Vance, H.; et al. Tomato 14-3-3 proteins are required for Xv3 disease resistance and interact with a subset of Xanthomonas euvesicatoria effectors. Mol. Plant Microbe Interact. 2018, 31, 1301–1311. [Google Scholar] [CrossRef]
- Aitken, A. Post-translational modification of 14-3-3 isoforms and regulation of cellular function. Semin. Cell Dev. Biol. 2011, 22, 673–680. [Google Scholar] [CrossRef]
- Obšlova, V.; Šilhan, J.; BouŔa, E.; Teisinger, J.; Obšil, T. 14-3-3 proteins: A family of versatile molecular regulators Physiol. Res. 2008, 57, S11–S21. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Thilmony, R.; You, F.M.; Gu, Y.Q.; Coleman-Derr, D. PIECE 2.0: An update for the plant gene structure comparison and evolution database. Nucleic Acids Res. 2017, 45, 1015–1020. [Google Scholar] [CrossRef]
- Mukherjee, S.; Stamatis, D.; Bertsch, J.; Ovchinnikova, G.; Sundaramurthi, J.C.; Lee, J.; Kandimalla, M.; Chen, I.M.A.; Kyrpides, N.C.; Reddy, T.B.K. Genomes online database (GOLD) v.8: Overview and updates. Nucleic Acids Res. 2021, 49, D723–D733. [Google Scholar] [CrossRef]
- Testerink, C.; van Zeijl, M.J.; Drumm, K.; Palmgren, M.G.; Collinge, D.B.; Kijne, J.W.; Wang, M. Post-translational modification of barley 14-3-3A is isoform-specific and involves removal of the hypervariable C-terminus. Plant Mol. Biol. 2002, 50, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Börnke, F. The variable C-terminus of 14-3-3 proteins mediates isoform-specific interaction with sucrose-phosphate synthase in the yeast two-hybrid system. J. Plant Physiol. 2005, 162, 161–168. [Google Scholar] [CrossRef]
- Aducci, P.; Camoni, L.; Marra, M.; Visconti, S. From cytosol to organelles: 14-3-3 proteins as multifunctional regulators of plant cell. IUBMB Life 2002, 53, 49–55. [Google Scholar] [CrossRef]
- Waese, J.; Fan, J.; Pasha, A.; Yu, H.; Fucile, G.; Shi, R.; Cumming, M.; Kelley, L.A.; Sternberg, M.J.; Krishnakumar, V.; et al. ePlant: Visualizing and exploring multiple levels of data for hypothesis generation in plant biology. Plant Cell 2017, 29, 1806–1821. [Google Scholar] [CrossRef]
- Paul, A.-L.; Denison, F.C.; Schultz, E.R.; Zupanska, A.K.; Ferl, R.J. 14-3-3 phosphoprotein interaction networks—Does isoform diversity present functional interaction specification? Front. Plant Sci. 2012, 3, 190. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Landherr, L.L.; Frohlich, M.W.; Leebens-Mack, J.; Ma, H.; dePamphilis, C.W. Patterns of gene duplication in the plant SKP1 gene family in angiosperms: Evidence for multiple mechanisms of rapid gene birth. Plant J. 2007, 50, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Flagel, L.E.; Wendel, J.F. Gene duplication and evolutionary novelty in plants. New Phytol. 2009, 183, 557–564. [Google Scholar] [CrossRef]
- Zhang, Z. KaKs_calculator 3.0: Calculating selective pressure on coding and non-coding sequences. Genom. Proteom. Bioinf. 2022; in press. [Google Scholar] [CrossRef]
- Blanc, G.; Wolfe, K.H. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell 2004, 16, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.M.; Vision, T.; Liu, J.; Tanksley, S.D. Comparing sequenced segments of the tomato and Arabidopsis genomes: Large-scale duplicationfollowed by selective gene loss creates anetwork of synteny. Proc. Natl. Acad. Sci. USA 2000, 97, 9121–9126. [Google Scholar] [CrossRef]
- Folta, K.M.; Paul, A.-L.; Mayfield, J.D.; Ferl, R.J. 14-3-3 isoforms participate in red light signaling and photoperiodic flowering. Plant Signal Behav. 2014, 3, 304–306. [Google Scholar] [CrossRef]
- Sullivan, S.; Thomson, C.E.; Kaiserli, E.; Christie, J.M. Interaction specificity of Arabidopsis14-3-3 proteins with phototropin receptor kinases. FEBS Lett. 2009, 583, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Keicher, J.; Jaspert, N.; Weckermann, K.; Möller, C.; Throm, C.; Kintzi, A.; Oecking, C. Arabidopsis 14-3-3 epsilon members contribute to polarity of PIN auxin carrier and auxin transport-related development. eLife 2017, 6, e24336. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.M.; Kieber, J.J. 14-3-3 regulates 1-aminocyclopropane-1-carboxylate synthase protein turnover in Arabidopsis. Plant Cell 2013, 25, 1016–1028. [Google Scholar] [CrossRef]
- Ishida, S.; Fukazawa, J.; Yuasa, T.; Takahashi, Y. Involvement of 14-3-3 signaling protein binding in the functional regulation of the transcriptional activator REPRESSION OF SHOOT GROWTH by gibberellins. Plant Cell 2004, 16, 2641–2651. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Sethi, S.; Zutshi, I.; Bhalothia, P.; Mehrotra, S. Patterns and evolution of ACGT repeat cis-element landscape across four plant genomes. BMC Genom. 2013, 14, 203. [Google Scholar] [CrossRef]
- Coblitz, B.; Shikano, S.; Wu, M.; Gabelli, S.B.; Cockrell, L.M.; Spieker, M.; Hanyu, Y.; Fu, H.; Amzel, L.M.; Li, M. C-terminal Recognition by 14-3-3 proteins for surface expression of membrane receptors. J. Biol. Chem. 2005, 280, 36263–36272. [Google Scholar] [CrossRef] [PubMed]
- de Boer, A.H.; van Kleeff, P.J.M.; Gao, J. Plant 14-3-3 proteins as spiders in a web of phosphorylation. Protoplasma 2012, 250, 425–440. [Google Scholar] [CrossRef]
- Liu, J.; Sun, X.; Liao, W.; Zhang, J.; Liang, J.; Xu, W. Involvement of OsGF14b adaptation in the drought resistance of rice plants. Rice 2019, 12, 82. [Google Scholar] [CrossRef]
- Purwestri, Y.A.; Ogaki, Y.; Tamaki, S.; Tsuji, H.; Shimamoto, K. The 14-3-3 protein GF14c acts as a negative regulator of flowering in rice by interacting with the florigen Hd3a. Plant Cell Physiol. 2009, 50, 429–438. [Google Scholar] [CrossRef]
- Manosalva, P.M.; Bruce, M.; Leach, J.E. Rice 14-3-3 protein (GF14e) negatively affects cell death and disease resistance. Plant J. 2011, 68, 777–787. [Google Scholar] [CrossRef]
- Dou, Y.; Liu, X.; Yin, Y.; Han, S.; Lu, Y.; Liu, Y.; Hao, D. Affinity chromatography revealed insights into unique functionality of two 14-3-3 protein species in developing maize kernels. J. Proteom. 2015, 114, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| Group | Name | ID | Length (AA) | MW (Da) | pI | GRAVY | Instability Index | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|
| non-ε | SlTFT1 | Solyc11g010470.2.1 | 249 | 28,201.16 | 4.76 | −0.0261 | 37.97 (Stable) | Cytoplasmic (2.968) |
| non-ε | SlTFT2 | Solyc12g057120.2.1 | 401 | 45,149.05 | 4.67 | −0.689 | 54.17 (Unstable) | Cytoplasmic (2.695) |
| non-ε | SlTFT3 | Solyc04g074510.3.1 | 268 | 30,439.91 | 5.4 | −0.353 | 42.24 (Unstable) | Cytoplasmic (2.492) |
| non-ε | SlTFT4 | Solyc02g063070.3.1 | 260 | 29,338.81 | 4.66 | −0.517 | 43.74 (Unstable) | Cytoplasmic (3.355) |
| non-ε | SlTFT5 | Solyc04g012120.3.1 | 255 | 28,795.31 | 4.74 | −0.525 | 41.08 (Unstable) | Cytoplasmic (3.528) |
| non-ε | SlTFT6 | Solyc11g010200.2.1 | 259 | 29,107.68 | 4.76 | −0.494 | 39.24 (Stable) | Cytoplasmic (3.666) |
| ε | SlTFT7 | Solyc04g074230.3.1 | 243 | 27,757.46 | 5.31 | −0.344 | 52.12 (Unstable) | Nuclear (1.160) |
| ε | SlTFT8 | Solyc12g010860.2.1 | 261 | 29,478.94 | 4.61 | −0.531 | 43.40 (Unstable) | Cytoplasmic (2.519) |
| ε | SlTFT9 | Solyc07g053260.3.1 | 261 | 29,431.93 | 4.74 | −0.538 | 47.55 (Unstable) | Cytoplasmic (2.867) |
| non-ε | SlTFT10 | Solyc04g076060.3.1 | 295 | 33,701.28 | 5.14 | −0.397 | 40.59 (Unstable) | Cytoplasmic (2.356) |
| non-ε | SlTFT11 | Solyc03g034180.3.1 | 258 | 29,122.59 | 4.69 | −0.492 | 44.61 (Unstable) | Cytoplasmic (3.792) |
| ε | SlTFT12 | Solyc05g012420.3.1 | 367 | 42,073.87 | 5.33 | −0.387 | 50.02 (Unstable) | Cytoplasmic (1.076) |
| ε | SlTFT13 | Solyc01g010360.3.1 | 249 | 28,193.67 | 4.95 | −0.718 | 51.66 (Unstable) | Cytoplasmic (2.979) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, C.; Guo, B.; Wang, B.; Li, X.; Yang, T.; Li, N.; Wang, J.; Yu, Q. Genome-Wide Identification and Expression Analysis of the 14-3-3 (TFT) Gene Family in Tomato, and the Role of SlTFT4 in Salt Stress. Plants 2022, 11, 3491. https://doi.org/10.3390/plants11243491
Jia C, Guo B, Wang B, Li X, Yang T, Li N, Wang J, Yu Q. Genome-Wide Identification and Expression Analysis of the 14-3-3 (TFT) Gene Family in Tomato, and the Role of SlTFT4 in Salt Stress. Plants. 2022; 11(24):3491. https://doi.org/10.3390/plants11243491
Chicago/Turabian StyleJia, Chunping, Bin Guo, Baike Wang, Xin Li, Tao Yang, Ning Li, Juan Wang, and Qinghui Yu. 2022. "Genome-Wide Identification and Expression Analysis of the 14-3-3 (TFT) Gene Family in Tomato, and the Role of SlTFT4 in Salt Stress" Plants 11, no. 24: 3491. https://doi.org/10.3390/plants11243491
APA StyleJia, C., Guo, B., Wang, B., Li, X., Yang, T., Li, N., Wang, J., & Yu, Q. (2022). Genome-Wide Identification and Expression Analysis of the 14-3-3 (TFT) Gene Family in Tomato, and the Role of SlTFT4 in Salt Stress. Plants, 11(24), 3491. https://doi.org/10.3390/plants11243491






