Abstract
To identify new sources of effective resistance to four foliar diseases of wheat, 173 accessions of four wheat species, Triticum boeoticum, T. urartu, T. araraticum, and T. dicoccoides, from the VIR collection were tested at the juvenile and adult growth stages for resistance to leaf rust (Pt = Puccinia triticina), powdery mildew (Bgt = Blumeria graminis tritici), Septoria nodorum blotch (SNB), and dark-brown leaf spot blotch (HLB = Helminthospjrium leaf blotch). The accessions included new additions to the collection, some old samples that had never been tested before, as well as earlier tested samples noted for high levels of juvenile resistance to some fungal diseases. Natural populations of Puccinia triticina and Blumeria graminis f. sp. tritici, mixture of Parastagonospora nodorum and Bipolaris sorokiniana isolates were used to inoculate and to evaluate resistance to Pt, Bgt, SNB, and HLB, respectively. Two samples of T. boeoticum, three of T. urartu, and one of T. araraticum were resistant to leaf rust at both tested stages. Further tests (phytopathological and molecular analyses) excluded Lr9, Lr19, Lr24, Lr41, or Lr47 as single genes controlling resistance; hence, these accessions likely carry new effective leaf rust resistance genes. High level of Bgt resistance was identified in three entries of T. boeoticum, one of T. araraticum, and eleven of T. dicoccoides. All tested accessions were susceptible to HLB and SNB at both tested stages. Accessions identified as resistant are valuable plant material for introgressive hybridization in bread and durum wheat breeding. The results are discussed in the context of N.I. Vavilov’s concept of crop origin and diversity, and the laws of plant natural immunity to infectious diseases.
1. Introduction
Bread wheat (Triticum aestivum L.) is one of the most important cereal crops in the Russian Federation (RF) as well as all over the world. It is the main product of grain export for the RF. The yield of the crop and its quality are significantly reduced because of foliar fungal diseases. Leaf rust (Pt) (causal agent—Puccinia triticina Erikss.), Septoria nodorum blotch (SNB) (Parastagonospora nodorum (Berk.) Quaedvlieg, Verkley, and Crous syn. Stagonospora nodorum (Berk.) Castell. et Germano), dark-brown leaf spot blotch (indicating Helminthosporium leaf blotch (HLB)) (Bipolaris sorokiniana (Sacc.) Shoemaker, teleomorph Cochliobolus sativus (Ito et Curib.), and powdery mildew (Bgt) (Blumeria graminis (DC.) E.O. Speer f. sp. tritici Em. Marchal syn. Erysiphe graminis f. sp. tritici) are devastating diseases of common wheat in many of the crop-growing regions. Under severe epiphytotic conditions, yield losses from leaf rust development have been estimated to occur in up to 14 percent of the total wheat yield for the Great Plains of North America [1], and in more than 40% in Russia [2]. In the Southern Cone of South America Pt can cause grain yield losses higher than 50% during severe epidemics if fungicides are not applied [3]. Leaf rust infections at earlier stages of wheat growth may also cause yield losses higher than 50% [4]. Losses up to 50% due to SNB have been reported [5]. HLB can result in yield losses up to 100% in susceptible varieties under favorable conditions [6]. Powdery mildew causes yield losses as high as 48% [7]. Yield losses from Bgt under severe epiphytotic conditions in Russia can reach 50% or more [8]. In addition to yield losses, the diseases decrease the qualities of the wheat grains [5,9,10,11].
The most effective and environmentally friendly method to avoid economically significant losses of wheat yield from these, as well as other diseases, has been well proven to be the cultivation of resistant varieties. To develop such varieties, the sources and donors of effective genes and genetic systems for the resistance are needed.
The collection of the All-Russian Institute of Plant Genetic Resources (VIR, Saint Petersburg, Russia), the basis of which was established by N.I. Vavilov, represents a very valuable plant material for searching sources and donors and for breeding important traits, including resistance to diseases [12,13]. However, our previous work with bread wheat from this collection showed very narrow genetic diversity for effective resistance to modern populations of leaf rust, HLB, SNB, and powdery mildew pathogens from the Russian geographic habitat [14,15].
All samples from the Vavilov’ collection with effective seedling resistance to leaf rust are protected with genes Lr9, Lr19, Lr24, and Lr41 [14,15,16]. These genes have been highly effective in many regions of the world, but now virulence to genes Lr9, Lr19, and Lr24 has been shown in Russia [17,18,19,20]. Pathotypes virulent to Lr9 have been found in the United States [21], Canada [22], Mexico [23], the Czech Republic [24], and in Egypt [25]. Pathotypes virulent to Lr19 have been found in Mexico [26], India [27], the Czech Republic [24], and in Egypt [25]. Virulence for Lr24 has occurred in the United States, Argentina, Brazil [21], South Africa [21,28] and other southern Africa countries [29], in south Australia [30], and in Canada [22]. Clones virulent to Lr41 have been found in the United States [31]. Evidently wide commercial growing of varieties with these genes will result in rapid accumulation of virulent pathogen genotypes and, hence, loss of resistance.
Not a single bread wheat sample from the VIR collection possessed high resistance to SNB and HLB at different stages of plant growth [14,15,16].
The low genetic diversity for effective resistance to diseases in T. aestivum presents the urgent task of expanding it through various approaches, including interspecific hybridization with related species in the Triticum genus. Study of seedling resistance to the diseases in cultivated species of the genus did not reveal significant genetic diversity for effective resistance [14,15,16,32]. Thus, special attention should be paid to wild Triticum L. wheat species [12], which are represented by: wild einkorn wheat T. boeoticum Boiss., red wild einkorn T. urartu Thum. ex Gandil., Armenian wild emmer T. araraticum Jakubz., and wild emmer T. dicoccoides (Koern. ex Aschers. et Graebn.) Schweinf. Some samples of such species from the VIR collection were earlier screened for resistance to diseases [12,14,15,16,33,34,35,36,37,38]. For example, in the Vavilov study all collection samples of T. boeoticum, T. urartu, and those of T. dicoccoides from Syria were resistant to leaf rust, while samples of T. dicoccoides from Palestine were differentiated for the resistance [12]. Some samples of T. dicoccoides have been described as resistant to leaf rust and 86% of the samples as resistant to powdery mildew, almost all entries of T. boeoticum were characterized by their immunity to these diseases, and T. araraticum has been described as highly resistant to Bgt [37].
In 1990, in a study of juvenile resistance to SNB, Yamaleev et al. [34] described T. boeoticum and T. urartu to offer the highest resistance to S. nodorum among the Triticum species. However, in our previous studies, such a result was not confirmed; all entries of these species were highly susceptible to the disease [14,15,16,32]. Mikhailova et al. [36] revealed 28, 50, 44, and 47% of VIR samples to be resistant and moderately resistant to dark-brown leaf spot blotch in T. dicoccoides, T. araraticum, T. urartu, and T. boeoticum, respectively. However, our work [14,15,16,32] has indicated that the predominant number of these species’ samples were highly susceptible to the disease. Samples resistant at the juvenile stage to leaf rust were found among all wild wheats from the VIR collection [35]; however, later genotypes resistant to the disease were only found in T. boeoticum [14,32]. As for Bgt, resistant entries with high frequencies were identified in T. boeoticum; all samples of T. araraticum were resistant to the disease and among 13 T. urartu samples, two were highly resistant [35].
There are several reasons to study resistance to these diseases in wild wheat samples from the VIR collection. There are evident contradictions in earlier obtained results for frequencies of samples resistant to certain disease. Several samples were introduced into the Vavilov’ collection quite long ago, and newer entries introduced into the collection during the last 10 years had never been studied for resistance to the diseases. Additionally, possible changes in virulence and aggressiveness of the causal agents can lead to current susceptibility of wild wheat samples that had earlier been described as resistant.
The purpose of this research was to study effective resistance to four diseases in wild Triticum L. species from the VIR collection and to identify entries with high level of the trait expression.
2. Materials and Methods
2.1. Plant Material
The plant material included 173 accessions of four wild wheat species (two diploids and two tetraploids) from the Vavilov’ collection (Table 1). In this study, classification of Triticum genus adopted in Russia [37,38] was used.

Table 1.
Characteristics of wild wheat species samples studied for resistance to the diseases.
The accessions included 138 new additions to the collection, six old samples that had never been tested before, as well as 29 earlier tested samples noted for high level of juvenile resistance to some fungal diseases [32,35,36,37].
All the samples were evaluated for resistance to Pt, SNB and HLB. Ninety-seven of these, including 15 samples of T. boeoticum, 22 of T. urartu, 10 of T. araraticum, and 50 of T. dicoccoides, were tested for resistance to Bgt.
2.2. Pathogen Material
Phytopathogens were sampled in the fields of Pushkin and Pavlovsk laboratories of VIR (Russia, Northwest Region) in 2020–2022.
The leaf rust population was drawn from the inoculums from leaves of several susceptible wheat varieties. Under laboratory conditions, the population was maintained and multiplied on seedlings of susceptible cv. Leningradka in a light chamber (2500 lux, temperature–20–22 °C, 24 h daylight). Under these conditions, the population was virulent / avirulent to seedlings of near-isogenic lines of wheat cv. Thatcher and samples of wheat lines with resistance genes Lr1, Lr2а, Lr2с, Lr3bg, Lr10, Lr11, Lr12, Lr13, Lr14а, Lr14b, Lr15, Lr16, Lr17, Lr18, Lr20, Lr21, Lr22а, Lr22b, Lr23, Lr25, Lr26, Lr28, Lr29, Lr27+31, Lr32, Lr33, Lr34, Lr35, Lr36, Lr37, Lr38, Lr43, Lr44, Lr45, Lr46, Lr48, Lr49, Lr51, Lr52, Lr57, Lr60, Lr63, Lr64, Lr67 / Lr9, Lr19, Lr24, Lr39 (=Lr41), and Lr47.
B. sorokiniana was isolated from the diseased wheat and barley leaves in Petri dishes on a semi-selective medium [14,39]. The dishes were incubated at 22–25 °С in the darkness; abundant spores’ production was observed within 10–14 days. Inoculum multiplication was done on CZLU medium in Petri dishes [14,39].
Conidia from the medium surface were transferred into water using scalpel; suspension of spores of five fungus isolates in approximately equal quantities were filtered through a double layer of cheesecloth.
S. nodorum was isolated from diseased wheat leaves on Potato Glucose Agar for microbiology in Petri dishes and multiplied on pearl barley in flasks under UV light as earlier described [39]. For plant infections, water suspension consisting of a mixture of six S. nodorum isolates was prepared by combining equal numbers of pearl grains with each isolate. Grains were placed into water for 15 min.
Inoculum for powdery mildew was sampled as diseased leaves from susceptible wheat accessions in 2021 and 2022 at Pushkin and Pavlovsk laboratories of VIR. The populations of Bgt were used to inoculate seedlings of wheat near-isogenic lines and samples with genes for the resistance Pm1a, Pm2, Pm3a, Pm3b, Pm3c, Pm3d, Pm4a, Pm4b, Pm4d, Pm5a, Pm6, Pm7, Pm8, Pm9, Pm10, Pm11, Pm12, Pm16, Pm17, Pm19, Pm28, PmKu, and PmSp. All of these were heavily infected with the pathogen populations, so no one particular gene available in this study was effective.
2.3. Seedling Resistance Screening
In order to study juvenile resistance to the diseases, 15–20 seeds of each sample were placed on cotton wool rolls wetted with water. Ten to fifteen days later, seedlings at the stage of 1–2 leaves were placed horizontally into cuvettes and sprayed with water suspensions of the pathogens’ spores using a hand atomizer (the concentration of P. triticina uredospores was 3 × 104 spores ml−1, of B. sorokiniana conidia was 4 × 104 spores ml−1 and of S. nodorum spores was 5 × 106 conidia ml−1).
After inoculation, the cuvettes were wrapped with polyethylene, covered with glasses, and placed in the darkness. After 24 h, plants were transferred into the light chamber (see above). For the leaf rust experiments, polyethylene film and glass were removed, and seedlings were returned to a vertical position. When studying SNB and HLB resistance, the cuvettes with seedlings remained wrapped until disease scorings. When studying resistance to Bgt, vertical seedlings were powdered with spores of B. graminis from field collected leaves; infected plants were sprayed with water and were not covered. The types of reaction to P. triticina and B. graminis infections were scored on the 12th day after inoculation according to generally accepted scales [40,41] with modifications, where 0—no symptoms; 0;—necrotic spots without pustules formation; 1—very small pustules surrounded by necrosis; 2—medium-sized pustules surrounded by necrosis or chlorosis; 3—large pustules without necrosis; s.p.—single pustules of susceptible type without necrosis; and X—pustules of different types on one leaf. Samples with types 0, 0;, and 1 were classified as highly resistant, 2, s.p. and Х–moderately resistant, and 3—susceptible.
Disease ratings of SNB and HLB were scored on the 7th day after inoculation on a scale, where 0—no symptoms of disease; 1, 2, 3, 4—10, 20, 30, 40% of leaf surface affected; 5—50% and more of leaf surface affected, and 6—the death of the leaves. Samples with the disease scores 0, 1 and 2 after inoculation were considered highly resistant, 3 and 4—possessing moderate level of the resistance, and scores of 5 and 6 were classified as susceptible [39].
All samples which showed any level of resistance to certain diseases were reevaluated for the trait in 3–4 additional independent experiments. For all diseases, seedlings of wheat cv. Leningradka were used as susceptible controls and were sown after each five experimental samples.
In order to determine the presence of effective leaf rust resistance genes in those samples, which have been resistant to the population of leaf rust, leaf segments of seedlings 0.8–1.0 cm were placed on cotton wool wetted with solution of KCl (0.48 g L−1) + NaH2PO4 × 2H2O (0.66 g L−1) + maleic acid hydrazide (10 mg L−1) [42]. Leaf segments were sprayed with water suspensions of uredospores of single P. triticina clones, which were virulent to seedlings of near-isogenic lines of wheat cv. Thatcher and also to samples of bread wheat lines with resistance genes Lr9, Lr19, Lr24, Lr41, and Lr47 under these conditions. For each gene we used five virulent monopustule isolates that had been earlier independently isolated from complex population [42]. Infections with each clone were undertaken in separate experiments without mixing clones. The wild wheat samples, which showed a high type of reaction to inoculation with at least one clone could be considered as possibly protected with that single particular gene [14].
Molecular markers, tightly linked to effective genes for seedling and adult plant leaf rust resistance Lr9, Lr19, Lr24, Lr41, and Lr47 in bread wheat (Table 2) under conditions of the NW of the RF were used to identify the corresponding genes in six resistant samples of T. boeoticum, T. urartu, and T. araraticum. All these genes were transferred into bread wheat genome from species that differed from wild Triticum (Aegilops umbellulata, Thinopirum ponticum, Th. ponticum, Ae. tauschii, and Ae. speltoides, respectively) [43]. It is notable that several alien genes for resistance from some species were identified with the use of molecular markers in other wheat relatives or wheat lines with genetic material from those relatives [44,45,46], possibly due to the presence of orthologous genes in different genomes.
Total genomic DNA was isolated from 10-day-old seedlings (five plants) with a micro method proposed by Edwards et al. [47] with modifications by Dorokhov and Klocke [48]. A polymerase chain reaction (PCR) was performed in 25 μL reaction volume containing: 2 μL 50 ng/μL of DNA, 2.5 μL 10 × PCR buffer, 1 μL (2.5 mM) dNTPs, 12.5 pmol of each primer, 16 μL MQ H2O, and 0.5 μL (2 U/μ) Taq DNA Polymerase. Nucleotide sequences of primers listed in Table 2. The PCR was performed in MiniAmp™ Plus Thermal Cycler (Thermo Fisher Scientific, USA) according to original protocols [31,49,50,51,52]. PCR products were separated in 1.5% agarose gel in 1 × TBE buffer, stained in a solution of ethidium bromide (0.5 mg L−1) and visualized under UV light. GeneRuler™ 100 bp Plus DNA. Ladder (Fermentas) was used to estimate the size of PCR amplified fragments. Positive controls were ThLr9, Olga, Tertsia (Lr9), ThLr19, Ulia (Lr19), ThLr24, Scua (Lr24), KS90WGRC10 (Lr41), Pavon Lr47 [14].

Table 2.
DNA markers linked to effective leaf rust resistance genes in wheat.
Table 2.
DNA markers linked to effective leaf rust resistance genes in wheat.
| Gene | Marker | Primer Sequence (5’-3’) | Fragment Size (bp) | Reference |
|---|---|---|---|---|
| Lr9 | SCS5550 | F: TGC GCC CTT CAA AGG AAG R: TGC GCC CTT CTG AAC TGT AT | 550 | [49] |
| Lr19 | Gb | F: CAT CCT TGG GGA CCT C R: CCA GCT CGC ATA CAT CCA | 130 | [50] |
| Lr24 | SCS73719 | F: TCG TCC AGA TCA GAA TGT G R: CTC GTC GAT TAG CAG TGA G | 719 | [51] |
| Lr41 | GDM35 | F: CCT GCT CTG CCC TAG ATA CG R: ATG TGA ATG TGA TGC ATG CA | 190 | [31] |
| Lr47 | PS10 | F: GCT GAT GAC CCT GAC CGG T R: TCT TCA TGC CCG GTC GGG T | 282 | [52] |
2.4. Adult Plant Resistance Screening
Adult plant resistance was studied in 2021 and 2022 in the field of Pushkin and Pavlovsk VIR Laboratories. Each sample grew in one row (length 1 m). Plants in the flag-leaf stage were sprayed with pathogen spore water suspensions and infected with the use of microchambers [39]. Samples highly susceptible to all four diseases under laboratory conditions were screened for adult resistance in the field only with use of microchambers method [39].
The types of reaction to P. triticina and B. graminis infections were scored on the 12th day after inoculation according to generally accepted scales [40,41] with modifications. Diseases with ratings of SNB and HLB were scored on the 7th day after inoculation according to above-presented scale and as described in [39].
3. Results
3.1. Juvenile Resistance in Samples of Wild Wheat Species to the Diseases
According to the results of four independent experiments, the majority of test samples under study were susceptible to leaf rust, powdery mildew, Septoria nodorum blotch, and dark-brown leaf spot blotch at the seedling stage.
Only six entries of three species were highly resistant to the Northwest population of Pt (Table 3). Resistant T. boeoticum samples originated from Bulgaria and Turkey—T. urartu from Lebanon and T. araraticum from Turkey (Table 1). The reactions to leaf rust infection of three samples out these six, as well as the susceptible sample, are presented in Figure 1.

Table 3.
Samples of wild wheat species from the VIR collection that are highly resistant to leaf rust.

Figure 1.
Seedlings of wild wheat species samples from the VIR collection after infection with population of P. triticina: (a) T. boeoticum k-66369; (b) T. urartu k-64777; (c) T. araraticum k-66372; and (d) T. boeoticum k-59178 (susceptible).
Samples of T. urartu k-33870, k-33871, T. boeoticum k-27141, k-28283, k-58611, k-59159, k-59163, k-59178, T. dicoccoides k-15907, k-20403, and T. araraticum k-30258, k-30240, k-30234, k-31628 from the VIR collection have been previously described as resistant to Pt [35], as well as k-62492 of T. boeoticum [32] and were present in this study. In this work, only sample of T. boeoticum k-62492 was resistant at seedling stage to leaf rust.
Juvenile genes for resistance Lr9, Lr19, Lr24, Lr41, and Lr47 are still effective in the Northwest of Russia [39] and were highly effective in this study. Leaf segments of six leaf rust resistant samples were inoculated with the single pathogen monopustule isolates virulent to wheat samples with five effective genes for resistance. All of these were highly resistant to these Pt isolates (Table 3), thus these samples evidently cannot have the single specific genes Lr9, Lr19, Lr24, Lr41, and Lr47.
The DNA markers specific to Lr9, Lr19, Lr24, Lr41, and Lr47 genes were amplified only in wheat samples carrying the corresponding genes. None of the resistant to leaf rust sample of T. boeoticum, T. urartu, and T. araraticum had amplification products after PCR with primers to these genes’ markers. The electropherograms of the amplicons in samples with the SCS5550 marker primers and the PS10 marker primers are presented in Figure 2 and Figure 3.
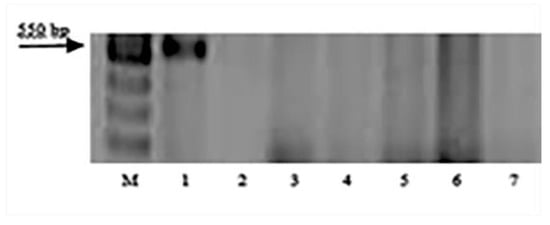
Figure 2.
Amplification products of SCAR marker SCS5550 linked to leaf rust resistance gene Lr9: M—100 bp DNA Ladder; 1—Thatcher Lr9; 2—k-62492 T. boeoticum; 3—k-66369 T. boeoticum; 4—k-64777 T. urartu; 5—k-64782 T. urartu; 6—k-64783 T. urartu; and 7—k-66372 T. araraticum.
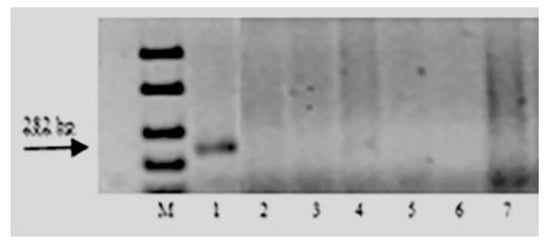
Figure 3.
Amplification products of SCAR marker PS10 linked to leaf rust resistance gene Lr47: M–100 bp DNA Ladder; 1—Pavon Lr47; 2—k-62492 T. boeoticum; 3—k-66369 T. boeoticum; 4—k-64777 T. urartu; 5—k-64782 T. urartu; 6—k-64783 T. urartu; and 7—k-66372 T. araraticum.
Fifteen samples of T. dicoccoides, T. boeoticum, and T. araraticum were resistant to the population of Bgt sampled in the Northwest region of the Russian Federation (Table 4). All samples of T. urartu, including four that were earlier identified as resistant [35], were classified as susceptible to Bgt (type of reaction 3). Among 74 evaluated samples of T. dicoccoides, 11 were highly resistant to Bgt. These samples belonged to five botanical varieties and originated from Israel, Jordan, and Syria (Table 1 and Table 4). Resistant T. boeoticum samples were from Azerbaijan, Bulgaria, and Turkey; and the resistant T. araraticum entry was collected in Turkey.

Table 4.
Samples of wild wheat species from the VIR collection that are highly resistant to powdery mildew.
Two samples of T. urartu (k-58504, k-33871), six samples of T. boeoticum (k-18424, k-28280, k-40117, k-58506, k-58489, k-59163), and five of T. araraticum (k-28132, k-28247, k-58668, k-30258, k-31628) present in this research were earlier described as resistant to powdery mildew at seedling stage [35]. Only one sample out of these—T. boeoticum k-58489, from Azerbaijan—was confirmed as resistant in this study.
All studied samples of wild wheat species from the VIR collection, including 17 earlier described as highly or moderately resistant to dark-brown leaf spot blotch (T. urartu k-33871, k-58504, k-62458, k-62464, disease score 2 for all); T. boeoticum k-18424, k-40117, k-61660, k-62492 (disease score 1 for all); T. dicoccoides, k-61720, k-61828 (2), k-26117, k-62362, k-62364 (1–2); and T. araraticum k-28244 (2), k-30240 ), k-30268 (both 1–2), k-59940 (3) [36]), were susceptible to HLB in this work (disease ratings 5–6). The effects on disease seedlings of four samples in four wheat species from [36] are shown in Figure 4.

Figure 4.
HLB development on seedlings of wild wheat species samples from the VIR collection after infection with a mixture of B. sorokiniana isolates: (a) T. urartu k-58504; (b) T. araraticum k-28244; (c) T. boeoticum k-62492; and (d) T. dicoccoides k-62364.
Similarly, all entries under study were highly susceptible to SNB (disease ratings 5–6). The disease development on four samples of T. urartu and T. boeoticum, the species most resistant to Septoria nodorum blotch according to [34] is shown in Figure 5.

Figure 5.
SNB development on seedlings of wild wheat species samples from the VIR collection after infection with a mixture of S. nodorum isolates: (a) T. urartu k-58497; (b) T. urartu k-66361; (c) T. boeoticum k-58506; and (d) T. boeoticum k-59178.
3.2. Adult Resistance of Wild Wheat Species to the Diseases
Under field conditions, two samples of T. boeoticum, three of T. urartu, and one sample of T. araraticum, classified as resistant to the rust at seedling stage, displayed resistant types of reaction to the disease with two inoculation methods (Table 3). All other accessions were susceptible.
Fifteen samples of T. dicoccoides, T. boeoticum, and T. araraticum that had been classified as resistant to the Northwest population of Bgt under laboratory conditions, were shown to be highly resistant to the disease at the adult stages of plant growth (Table 4). Therefore, we did not identify accessions with only adult resistance to Pt or Bgt.
All samples were highly susceptible to SNB and HLB after inoculation of the microchambers in the fields of Pushkin and Pavlovsk laboratories of VIR with the respective pathogens.
4. Discussion
Leaf rust (Pt), powdery mildew (Bgt), dark-brown leaf spot blotch (HLB), and Septoria nodorum blotch (SNB) are economically significant diseases of bread wheat in many regions where the crop is cultivated. Leaf rust infections at even earlier stages may cause yield losses higher than 50% [4]. HLB was usually considered a harmful disease in non-traditional wheat-growing areas with high temperatures and humidity [53,54,55,56]. However, severe development of the disease has recently been reported in two regions of Russia [57,58]. HLB can result in yield losses up to 100% [6]. SNB is widespread across the world and losses from the disease can reach up to 50% [5]. Bgt is another common worldwide disease of wheat. Yield losses from powdery mildew under severe epiphytotic conditions can reach 50% or more [8]. For losses the maximum data are cited, indicating the possibility of lower or higher losses in the field.
The economic value of these diseases has led to an intensive study of the bread wheat gene pool for resistance to them. The world collection of the N.I. Vavilov All-Russian Institute of Plant Genetic Resources is of great interest for the search of valuable breeding material, including wheat for resistance to harmful foliar diseases. Up until now, a large number of sources for resistance have been identified in the bread wheat collection of the VIR [34,36,59,60,61,62,63,64,65,66,67,68,69,70,71]. However, our earlier studies have shown that many VIR bread wheat samples previously identified in scientific literature as highly resistant are susceptible to Pt, SNB, and HLB [14,15,16,72,73]. The extremely limited number of genes is effective against Bgt in the Russian Federation [15,70,71], moreover samples earlier described as having very effective genes for resistance Pm12, PmSp, and PmKu were identified as susceptible to B. graminis populations from the Northwest region of the RF in 2022 (this study).
If the viewpoint on extremely narrow genetic diversity for effective resistance in T. aestivum to these diseases is correct, it is urgently important to use introgressive hybridization with closely related wheat species for its broadening.
According to Vavilov’s concept of the centers of crops origin and diversity on utilization of plant genetic resources, the wild species of Triticum genus can be a very useful source of new effective genes for resistance to diseases for bread wheat breeding [12,13,33]. The Vavilov’ collection of this species has already been evaluated by several scientific groups for seedling resistance to Pt [14,15,16,32,35], HLB [14,32,36], SNB [14,32,34], and Bgt [35]. However, the data obtained on resistance to leaf rust, HLB, and SNB are very contradictory. So, among 56, 13, 28, 168 screened samples of T. boeoticum, T. urartu, T. araraticum and T. dicoccoides, 14%, 15%, 14%, and 13%, respectively, were resistant to leaf rust [35]. However, in the study of 258 samples of these species only four entries of T. boeoticum were found to be resistant to Pt [32]. For HLB resistance 17 entries of T. boeoticum, nine for T. urartu, eight for T. araraticum and 18 for T. dicoccoides were studied and eight, four, four, and five resistant samples were selected, respectively [36]. However, in our previous work no samples of those species with VIR catalogue number were resistant to HLB [32]. Yamaleev et al. [34] described T. boeoticum and T. urartu as the most resistant to S. nodorum species at the juvenile stage. Unfortunately, the authors did not provide detailed information on the characteristics of selected samples, which is why it is impossible to re-evaluate the resistance of particular entries. None of the samples of these species possessed any level of resistance in our previous study [32].
Here, 32 collection entries of T. boeoticum, 33 of T. urartu, 34 of T. araraticum, and 74 of T. dicoccoides were studied for effective seedling and adult resistance to four foliar diseases. Most samples under this study were susceptible to the diseases. Only six entries—two of T. boeoticum (k-62492, k-66369), three of T. urartu (k-64777, k-64782, k-64783), and one of T. araraticum (k-66372)—were highly resistant to Pt in seedling at the flag-leaf stages of plant growth. Notably, entry k-62492 was shown to be resistant at the seedling stage to Pt more than 15 year ago [32]. All samples of T. dicoccoides were classified as susceptible to the disease, although some genotypes of this species were described as resistant to leaf rust [74,75,76,77]; it is worth noting that resistant entries were often identified only after inoculation with extremely limited number of Pt isolates. Wheat line with gene Lr64 from T. dicoccoides [77] was susceptible to the disease in this study. Resistance to the rust was earlier found in T. araraticum samples [78], though only one isolate of Pt was used for inoculation. Several wheat lines with genes for Pt resistance from this species were developed [79], but they were susceptible to at least two leaf rust races. After seedling inoculations with three Pt isolates many samples of T. boeoticum and T. urartu were found to be particularly useful for obtaining leaf rust resistance, moreover they were described as carriers of Lr25 (gene from rye), Lr28 (gene from Aegilops speltoides Tausch) and Lr47 (from Ae. speltoides, too) [46]. T. dicoccoides k-17256 was identified as unaffected by the rust [37] but was found to be susceptible in this study. Samples of T. urartu k-33870, k-33871; T. boeoticum k-27141, k-28283, k-58611, k-59159, k-59163, k-59178; T. dicoccoides k-15907, k-20403; and T. araraticum k-30258, k-30240, k-30234, k-31628 from the VIR collection were identified as possessing resistance to leaf rust [35] but were found to be susceptible in this research. Several possible reasons could explain their susceptibility. We studied disease development on intact seedlings, and, during original work, leaf segments were infected with Pt on benzimidazole solution. Significant differences in types of reaction were often observed between these two methods of evaluation [14]. The changes in Pt population structure possibly could be the most important reason for the susceptibility of earlier resistant samples. From a practical viewpoint, it is evident that these entries cannot be recommended for breeding for resistance to leaf rust. The samples identified in this study can be used in interspecific hybridization to transfer the resistance genes to bread wheat. Genes Lr9, Lr19, Lr24, Lr41, and Lr47 are still effective in the Northwest of the Russian Federation [39] (this study). The molecular markers for these genes were used to identify the corresponding genes in 6 samples of wild wheat species resistant to leaf rust. The DNA fragments specific to Lr9 (550 bp) [49], Lr19 (130 bp) [50], Lr24 (719 bp) [51], Lr41 (190 bp) [31], and Lr47 (282 bp) [52] were amplified only in bread wheat lines and varieties carrying the corresponding genes. Phytopathological test with Pt isolates marked with virulence to the genes showed that resistance of T. boeoticum, T. urartu, and T. araraticum entries is controlled with new genes or possibly combinations of known effective genes for the trait.
Fifteen entries out of 97 studied were resistant at two ontogenesis stages to the population of Bgt sampled in the Northwest region of the Russian Federation in 2021–2022. They belong to three species (Table 3). All samples of T. urartu, including two earlier identified as resistant (k-58504, k-33871) [35], were classified as susceptible. Three samples (k-58489, k-62491, k-66370) of T. boeoticum from three countries were resistant to the disease, all others, including five—k-18424, k-28280, k-40117, k-58506, k-58489, and k-59163— shown to be resistant to Bgt according to Makarova et al. [35] have now been shown to be susceptible. Species T. araraticum was considered to be absolutely resistant (immune) or highly resistant to this disease [37]; this was confirmed for 28 accessions in 1993 [35]. The earlier susceptibility of most studied samples of this species has been already described [78]. In this study only one sample—k-64846 from Turkey—was found to be resistant, whereas seedlings and adult plants of others, including k-28132, k-28247, k-58668, k-30258, and k-31628, previously shown as resistant in [35], were severely affected by the modern B. graminis populations The main reason for the susceptibility of the earlier identified entries of three species is highly likely to lie in the drastic changes in Bgt population for virulence during the last 18 years; confirmation of this could be seen in high frequencies of Bgt isolates virulent to the previously highly effective genes Pm12, PmSp, and PmKu [80]. In this study, the majority of entries resistant to the B. graminis population belong to T. dicoccoides and originated from Israel (nine samples), while two samples were entered into the collection from Iordania and Syria. In an earlier study of wild wheat species from samples other than VIR’s genetic collections, the resistance to Bgt was found, for instance, in T. araraticum [78], T. boeoticum, T. urartu [46], and T. dicoccoides [81,82], but only a limited number of fungal isolates (1–3) were used for inoculations. The samples identified in the present work as highly resistant to complex powdery mildew populations, especially those of the T. dicoccoides species, are of great interest for hybridization with bread wheat. Preliminary study of the resistance genetic control and possible allelic relationships of genes for the trait is necessary to prevent transfer of identical genes in T. aestivum genotypes. Notably, the effective resistance of identified accessions could not be controlled by 23 single genes listed in the Materials and Methods section because they are not effective against modern populations of Bgt from the Northwest region of the RF.
Earlier, samples of T. urartu k-33871, k-58504, k-62458, k-62464; T. boeoticum k-18424, k-40117, k-61660, k-62492; T. dicoccoides k-26117, k-61720, k-61828, k-62362, k-62364; and T. araraticum k-28244, k-30240, k-30268, k-59940 have all been described as resistant (eight accessions) or moderately resistant (nine accessions) to HLB [36]. All these samples were susceptible to the disease in the present work. At least three possible reasons could explain this serious inconsistency of the results. To identify the resistance, we inoculated intact seedlings with the pathogen while Mikhailova et al. [36] studied resistance of leaf segments on benzimidazole. Then we scored the ratings on the seventh day after inoculation vs. the third day after infection with B. sorokiniana conidia in [36]. Finally, we inoculated leaves with a mixture of five pathogen isolates, only one was used in Mikhailova et al. [36] and then possibly of lower aggressiveness. None of the samples from newer entries of the collection was resistant to the disease in seedlings and at adult stage. Hence, the genetic diversity for HLB resistance in wild wheat species is extremely low, as has been proposed earlier [14,15,16,32,72].
In this study we also determined that all samples were highly susceptible to SNB (disease ratings 5–6) under the laboratory and field conditions. Yamaleev et al. [34] studied seedling resistance in Triticum species and identified wild wheat species T. boeoticum and T. urartu as the most resistant to SNB. Unfortunately, information on the number of studied samples in each species and variability for SNB resistance among samples was not presented in the publication [34]. As mentioned above, no one entry of those species possessed any level of seedling resistance to S. nodorum in our study. The sources of SNB resistance were also found also in some genetic collections of wild wheat species, namely among samples of T. dicoccoides [83,84]; however, in the present work all 83 entries were shown to be highly susceptible. Similar to HLB, our current research confirmed conclusions of a very narrow genetic diversity for effective seedling and adult resistance to Septoria nodorum blotch in wild wheats from the VIR collection [14,15,16,32].
As a result, we selected entries of wild Triticum species from the world VIR collection with effective resistance to populations of leaf rust in three species and to powdery mildew also in three species. These samples are of great interest for interspecific hybridization with bread wheat in order to develop new valuable donors for resistance to two harmful foliar fungal diseases. Additionally, we here confirm the extremely narrow genetic diversity for resistance to SNB and HLB in wild wheats from the Vavilov’ collection [14,15,16,32]. Hence, our data confirm the basis position of Vavilov’s concept of the wheat’s diversity for resistance to diseases [12,13,33] at least to those caused by biotrophic pathogens, and not caused by hemibiotrophic pathogens. It should be especially mentioned that all Vavilov’s concepts concerning the genetic diversity of wheats for disease resistance was based on experimental data on resistance to obligate parasites.
Besides practical conclusions, our data could be regarded in the view of another outstanding concept from Vavilov— the “keys for finding plant immune forms” or laws of natural immunity (resistance in our modern terms) [33,85,86,87,88]. This concept is the theoretical and practical basis for the identification of cultivated plants and their relatives’ sources and donors of effective resistance to pests and diseases. Although even Vavilov outlined that these laws are not absolute [33] and later some contradictions were found with regards to the current data on plant resistance [89]. This aspect of his conclusions is supposed to be absolutely actual to this day. The general six points of this concept’s last version [33,87] are briefly discussed below in context of our experimental data.
First to begin the breeding for resistance to diseases, it is necessary to know the biology of the parasite and, above all, its specialization. The narrower the specialization of the parasite in limits of plant genera and species, the greater the chances to identify resistant forms within individual species [33,88]. For example, in the works of Vavilov, many bread wheat varieties resistant to a highly specialized pathogen of yellow rust have been identified, and, conversely, the vast majority of varieties were susceptible to stem rust, the causal agent of which has a wide range of hosts. At the same time, a relatively large number of examples can be presented that refute this conclusion for cases of intraspecific diversity for effective resistance in widely cultivated species today. For instance, in the VIR collection there are currently no barley samples that are resistant (at least in the juvenile stage) to a highly specialized pathogen of leaf rust, but there are several accessions with a fairly high level of resistance to dark-brown leaf spot blotch (a pathogen with extremely wide specialization) [14,90]. In the VIR collection there are many samples of bread wheat that are resistant to leaf rust, but they are all created based on the use of introgressive hybridization [14,16]; if one were to consider only those genotypes without alien genes, then the number of varieties resistant to the rust (narrow specialized parasite) would not be more than those resistant to dark-brown leaf spot blotch. It should be especially noted that, if replacing the term “within species” with the term “between species”, or even further with “between genera”, there is no doubt about the universality of the law. Furthermore, when discussing genetic diversity for effective resistance to disease in plant species that have never been grown widely, the correctness of this aspect of Vavilov’s natural immunity (now resistance) concept is absolutely evident. Therefore, in this study of resistance to diseases in four wild Triticum species (173 accessions) we identified six samples of three species resistant to wheat leaf rust (narrow specialized pathogen) and 15 entries resistant to powdery mildew (also a narrowly specialized pathogen), but all of these were highly susceptible to dark-brown leaf spot blotch and septoriosis (both of which are caused by parasites with low specialization).
The second point of the law claims that the most highly contrasting differences in resistance are revealed in plants that are cytogenetically sharply differentiated into different species [33,88]. As one of the examples, Vavilov regarded the exceptional resistance of einkorn wheat to leaf rust and resistance to crown rust and smut in genetically separate species of wild and cultivated oats [88]. This point of the concept is partially supported by our present data. All bread and durum wheat from the VIR collection were susceptible to Pt and Bgt (not considering samples with alien genes for the resistance) [14,15]; however, six and 15 samples of wild species cytogenetically differing from T. aestivum and T. durum Desf. were highly resistant to these diseases at the two stages of plant growth, respectively, in this study. However, in terms of reactions to weakly specialized pathogens (B. sorokiniana and P. nodorum) we did not find differences in the frequencies of resistant forms among many Triticum species [14,15,16] including those studied for this research.
The most highly contrasting differences in resistance are detected in the most highly contrasting environmental conditions; there is a certain relationship between ecological differentiation of species and differences in the response of varieties to various parasitic diseases [88]. Therefore, according to Vavilov, most varieties and ecological–geographical groups of barley are very susceptible to barley leaf rust; however, a large number of immune forms have been identified among the material from the Mediterranean region [33,88]. Similarly, resistance to yellow rust, powdery mildew and other diseases was found in the Abyssinian subspecies of barley. Vavilov also noted that the accessions of bread wheat from Central and Southern China regions, but not from Western China, included a number of forms with drastically pronounced resistance to leaf and yellow rusts [88]. Vavilov explained this fact by creating resistant forms under conditions of humid and warm climate, favorable for the development of these diseases. Without questioning the correctness of this law’s point at the time of its development, it should be noted that all VIR wheat samples from China are susceptible to modern populations of the leaf rust pathogen from the RF [14]. Additionally, barley samples (including local forms from different regions) lack high level of resistance to leaf rust [14,90]. One of the most plausible explanations of this contradiction is a change in virulence structures of phytopathogen populations because of breeding achievements in the creation of resistant varieties, as well as changes in the virulence of pathogens under the effects of global climate change. The data obtained in our work are difficult to consider regarding this point of Vavilov’s concept because of the relatively small number of identified resistant samples. However, it is necessary to emphasize that accessions of T. boeoticum resistant to Pt originated from two different regions, resistance to Bgt from three regions (Table 1) and samples of T. dicoccoides resistant to Bgt were also from three different regions (Table 1). Vavilov [33] described samples of T. dicoccoides from Syria as resistant to leaf rust and that those from Palestine could be differentiated for their resistance; however, in this study all accessions of this species were susceptible. Thus, we were not able to find any close relationship between resistance of samples and their origin possibly due to limited quantity of plant material from certain regions.
The next point declared that group, or complex, immunity (i.e., resistance, in view of modern terminology) is a quite real fact that is widespread in nature and can therefore be used in practical breeding [33,88]. Species resistant to a single disease, are often resistant to many other diseases. Therefore, T. timopheevii is highly resistant both to leaf and yellow rusts, loose smut, powdery mildew, frit, and Hessian flies [88]. Complex resistance is also characteristic for T. monococcum [33]. There should be no doubt that, for this point of Vavilov’s concept, it is necessary to underline the key phrase “resistant species” i.e., species for which all accessions are resistant to certain diseases. When there is intraspecific differentiation for resistance this point of law of natural immunity should be considered as doubtful. For instance, in this study of 173 accessions of Triticum species, we found six samples resistant to Pt and 15 to Bgt, but none were resistant to more than one disease. At the same time resistance to several diseases is not completely rare. For instance, bread wheat genotypes, created as a result of interspecific hybridization by transferring into their genomes relatively large segments of alien chromosomes, are often resistant to several diseases. We have also left behind the discussion of resistance (immunity) to parasites that are non-pathogenic to a given host species (in this case any sample possesses complex immunity to thousands of diseases).
Distribution of immune and susceptible species and varieties is not an accident. Knowing the evolution of a certain cultivated plant, its differentiation into certain genetic and ecological–geographical groups, it is possible to foresee to a large extent the location of immune forms interesting for breeding [33,88]. This conclusion, as Vavilov indicated himself [88], is a logical consequence from the first four concept points, so its correctness does not require additional proof beyond the consideration of our remarks given above (the first four statements).
Additionally, as a final point of Vavilov’s law we will notice only his brilliant foresight. That, in the near future, we will be witnesses to changes in our varieties, in the sense of radical increases in their immunity (resistance), by means of distant hybridization [33,88]. Indeed, in many cases, there have been successes in the modern breeding of cultivated plants for effective resistance to fungal diseases (including complex diseases), the causal agents of which are able to spread over considerable distances (and therefore, theoretically can overcome existing resistance due to the mutational process and selection) and are associated with the success of introgressive hybridization. The samples of wild Triticum species identified in this study as resistant to Pt and Bgt could serve as very useful initial material in this direction of wheat breeding.
Author Contributions
Conceptualization, methodology, L.G.T., formal analysis, L.G.T. and M.A.K.; investigation, L.G.T. and M.A.K., resources, L.G.T., M.A.K. and N.S.L.; data curation, L.G.T., M.A.K. and N.S.L.; original draft preparation, L.G.T.; writing—review and editing, L.G.T. and M.A.K..; visualization, L.G.T. and M.A.K.; project administration, N.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation under agreement No 075-15-2021-1050 at 28.09.2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wegulo, S.N. Rust Diseases of Wheat. University of Nebraska–Lincoln Extension Neb Guide G2180, 2012. Available online: https://extensionpublications.unl.edu/assets/pdf/g2180.pdf (accessed on 6 November 2022).
- Krupnov, V.A. Strategy for genetic protection of wheat from leaf rust in the Volga region. Vestn. Russ. Agric. Sci. 1997, 6, 12–15. (In Russian) [Google Scholar]
- Germán, S.; Barcellos, A.; Chaves, M.; Kohli, M.; Campos, P.; Viedma, L. The situation of common wheat rusts in the Southern Cone of America and perspectives for control. Austr. J. Agric. Res. 2007, 58, 620–630. [Google Scholar] [CrossRef]
- Huerta-Espino, J.; Singh, R.P.; Germán, S.; McCallum, B.D.; Park, R.F.; Chen, W.Q.; Bhardwa, S.C.; Goyeau, H. Global status of wheat leaf rust caused by Puccinia triticina. Euphytica 2011, 179, 143–160. [Google Scholar] [CrossRef]
- Mehra, L.; Adhikari, U.; Cowger, C.; Ojiambo, P.S. Septoria nodorum blotch of wheat. PeerJ Prepr. 2018, 6, e27039v2. [Google Scholar]
- Chowdhury, A.K.; Singh, G.; Tyagi, B.S.; Ojha, A.; Dhar, T.; Bhattacharya, P.M. Spot blotch disease of wheat—A new thrust area for sustaining productivity. J. Wheat Res. 2013, 5, 1–11. [Google Scholar]
- Maxwell, J.J.; Lyerly, J.H.; Cowger, C.; Marshall, D.; Brown-Guedira, G.; Murphy, J.P. MlAG12: A Triticum timopheevii-derived powdery mildew resistance gene in common wheat on chromosome 7AL. Theor. Appl. Genet. 2009, 119, 1489–1495. [Google Scholar] [CrossRef]
- Vedeneeva, M.L.; Markelova, T.S.; Kirillova, T.V.; Anikeeva, N.V. Strategy of wheat disease-resistant varieties breeding in Volga region 1. Leaf rust, powdery mildew, loose smut and bunt. AgroХХI 2002, 2, 12–13. (In Russian) [Google Scholar]
- Spanic, V.; Matthew, N.R.; Kolmer, J.A.; Anderson, J.A. Leaf and stem seedling rust resistance in wheat cultivars grown in Croatia. Euphytica 2015, 203, 437–448. [Google Scholar] [CrossRef]
- Gessese, M.K. Description of wheat rusts and their virulence variations determined through annual pathotype surveys and controlled multi-pathotype tests. Adv. Agric. 2019, 2019, 2673706. [Google Scholar] [CrossRef]
- Singh, D.P.; Kumar, P. Role of spot blotch (Bipolaris sorokiniana) in deteriorating seed quality, its management in different wheat genotypes using fungicidal seed treatment. Indian Phytopathol. 2008, 61, 49–54. [Google Scholar]
- Vavilov, N.I. Selected Works in Two Volumes; Nauka: Leningrad, Russia, 1967; Volume 1, pp. 1–424. (In Russian) [Google Scholar]
- Vavilov, N.I. Origin and Geography of Cultivated Plants; Nauka: Leningrad, Russia, 1987; pp. 1–440. (In Russian) [Google Scholar]
- Tyryshkin, L.G. Genetic Diversity of Wheat and Barley for Effective Diseases Resistance and the Possibility of Its Broadening. Doctoral Dissertation, N.I. Vavilov All-Russian Institute of Plant Industry, Saint Petersburg, Russia, 2007. (In Russian). [Google Scholar]
- Tyryshkin, L.G.; Kolesova, M.A.; Kovaleva, M.M.; Lebedeva, T.V.; Zuev, E.V.; Brykova, A.N.; Gashimov, M.E. Current status of bread wheat and its relatives from VIR collection study for effective resistance to fungal diseases. In Proceedings of the 8th International Wheat Conference, Saint Petersburg, Russia, 1–4 June 2010; Dzyubenko, N.I., Vavilov, N.I., Eds.; Research Institute of Plant Industry (VIR): Saint Petersburg, Russia, 2010; pp. 134–135. (In Russian). [Google Scholar]
- Tyryshkin, L.G.; Syukov, V.V.; Zaharov, V.G.; Zuev, E.V.; Gashimov, M.E.; Kolesova, M.A.; Chikida, N.N.; Ershova, M.A.; Belousova, M.H. Sources of effective resistance to fungal diseases in wheat and its relatives—Search, creation and use in breeding. Proc. Appl. Bot. Genet. Breed. 2012, 170, 187–201. (In Russian) [Google Scholar]
- Meshkova, L.V.; Rosseeva, L.P.; Korenyuk, E.A.; Belan, I.A. Dynamics of distribution of the wheat leaf rust pathotypes virulent to the cultivars with Lr9 gene in Omsk region. Mikol. Fitopatol. 2012, 46, 397–400. (In Russian) [Google Scholar]
- Leonova, I.N.; Skolotneva, E.S.; Salina, E.A. Genome-wide association study of leaf rust resistance in Russian spring wheat varieties. BMC Plant Biol. 2020, 20, 135. [Google Scholar] [CrossRef] [PubMed]
- Skolotneva, E.S.; Leonova, I.N.; Bukatich, E.Y.; Boiko, N.I.; Piskarev, V.V.; Salina, E.A. Effectiveness of leaf rust resistance genes against Puccinia triticina populations in Western Siberia during 2008–2017. J. Plant Dis. Prot. 2018, 125, 549–555. [Google Scholar] [CrossRef]
- Markelova, T.S. Study of the structure and variability of the wheat brown rust population in the Volga region. AgroХХI 2007, 4–6, 37–40. (In Russian) [Google Scholar]
- Roelfs, A.P.; Singh, R.P.; Saari, E.E. Rust Diseases of Wheat: Concepts and Methods of Disease Management; CIMMYT: Mexico City, Mexico, 1992; pp. 1–81. [Google Scholar]
- McCallum, B.D.; Seto-Goh, P.; Xue, A. Physiologic specialization of Puccinia triticina, the causal agent of wheat leaf rust, in Canada in 2008. Can. J. Plant. Pathol. 2011, 33, 541–549. [Google Scholar] [CrossRef]
- Huerta-Espino, J.; Singh, R.P.; Reyna-Martinez, J. First detection of virulence to genes Lr9 and Lr25 conferring resistance to leaf rust of wheat caused by Puccinia triticina in Mexico. Plant Dis. 2008, 92, 311. [Google Scholar] [CrossRef]
- Hanzalová, A.; Bartoš, P. The virulence spectrum of the wheat leaf rust population analyzed in the Czech Republic from 2002 to 2011. Czech J. Genet. Plant Breed. 2014, 50, 288–292. [Google Scholar] [CrossRef]
- El-Orabey, W.M. Virulence of some Puccinia triticina races to the effective wheat leaf rust resistant genes Lr9 and Lr19 under Egyptian field conditions. Physiol. Mol. Plant Pathol. 2018, 102, 163–172. [Google Scholar] [CrossRef]
- Huerta-Espino, J.; Singh, R.P. First report of virulence to wheat with leaf rust resistance gene Lr19 in Mexico. Plant Dis. 1994, 78, 640. [Google Scholar] [CrossRef]
- Bhardwaj, S.C.; Prashar, M.; Kumar, S.; Jain, S.K.; Datta, D. Lr19 resistance in wheat becomes susceptible to Puccinia triticina in India. Plant Dis. 2005, 89, 1360. [Google Scholar] [CrossRef] [PubMed]
- Boshoff, W.H.P.; Labuschagne, R.; Terefe, T.; Pretorius, Z.A.; Visser, B. New Puccinia triticina races on wheat in South Africa. Australas. Plant Pathol. 2018, 47, 325–334. [Google Scholar] [CrossRef]
- Figlan, S.; Ntushelo, K.; Mwadzingeni, L.; Terefe, T.; Tsilo, T.J.; Shimelis, H. Breeding wheat for durable leaf rust resistance in southern Africa: Variability, distribution, current control strategies, challenges, and future prospects. Front. Plant Sci. 2020, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Park, R.F. Breeding cereals for rust resistance in Australia. Plant Pathol. 2008, 57, 591–602. [Google Scholar] [CrossRef]
- Singh, S.; Franks, C.D.; Huang, L.; Brown-Guedira, G.L.; Marshall, D.S.; Gill, B.S.; Fritz, A. Lr41, Lr39, and a leaf rust resistance gene from Aegilops cylindrica may be allelic and are located on wheat chromosome 2DS. Theor. Appl. Genet. 2004, 108, 586–591. [Google Scholar] [CrossRef]
- Tyryshkin, L.G.; Gashimov, M.E.; Kolesova, M.A.; Anphilova, N.A. Juvenile resistance to diseases in samples of Triticum L. species from VIR World Collection. Cereal Res. Comm. 2006, 34, 1073–1079. [Google Scholar] [CrossRef]
- Vavilov, N.I. Selected Works in Two Volumes; Nauka: Leningrad, Russia, 1967; Volume 2, pp. 1–480. (In Russian) [Google Scholar]
- Yamaleev, A.M.; Dolotovskij, I.M.; Nuriakhmetov, D.F.; Zolotov, A.L. Resistance in various species of wheats and Aegilops to Septoria nodorum Berk. Proc. Appl. Bot. Genet. Breed. 1990, 132, 44–50. (In Russian) [Google Scholar]
- Makarova, N.A.; Lebedeva, T.V.; Radchenko, E.E. Wheat. (Immunological characteristics of rare species). In Catalogue of the VIR World Collection; N.I. Vavilov All-Russian Institute of Plant Industry: Saint Petersburg, Russia, 1993; Volume 640, pp. 1–59. (In Russian) [Google Scholar]
- Mikhailova, L.A.; Kovalenko, N.M.; Smurova, S.G.; Ternyuk, I.G.; Mitrofanova, O.P.; Lyapunova, O.A.; Zuev, E.V.; Chikida, N.N.; Loskutova, N.P.; Pyukkenen, V.P. Resistance of Triticum L. and Aegilops L. species from the VIR collection to tan and dark-brown leaf spots (catalogue). In Catalogue; All-Russian Institute of Plant Protection: Saint Petersburg, Russia, 2007; pp. 1–60. (In Russian) [Google Scholar]
- Dorofeev, V.F.; Filatenko, A.A.; Migushova, E.F.; Udachin, R.A.; Jacubziner, M.M. Cultural Flora of USSR. Wheat; Kolos: Leningrad, Russia, 1979; pp. 1–348. (In Russian) [Google Scholar]
- Dorofeev, V.F.; Udachin, R.A.; Semenova, L.V.; Novikova, M.V.; Gradchaninova, O.D.; Shitova, I.P.; Merezhko, A.F.; Filatenko, A.A. Wheats of the World, 2nd ed.; Agropromisdat: Leningrad, Russia, 1987; pp. 1–560. (In Russian) [Google Scholar]
- Kolesova, M.M.; Lysenko, N.S.; Tyryshkin, L.G. Resistance to diseases in samples of rare wheat species from the N.I. Vavilov All-Russian Institute of Plant Genetic Resources. Cereal Res. Commun. 2022, 50, 287–296. [Google Scholar] [CrossRef]
- Mains, E.В.; Jасkson, H.S. Physiological specialization in leaf rust of wheat Puccinia triticina Erikss. Phytopathology 1926, 16, 89–120. [Google Scholar]
- Mains, E.B.; Dietz, S.M. Physiologic forms of barley mildew, Erysiphe graminis hordei Marchal. Phytopathology 1930, 20, 229–239. [Google Scholar]
- Tyryshkin, L.G. Modification Variability of Virulence and Aggressiveness in Phytopathogens of Cereal Crops: Conclusions, Consequences, Possibilities of Practical Application; Saint Petersburg State Agrarian University: Saint Petersburg, Russia, 2016; pp. 1–137. (In Russian) [Google Scholar]
- McIntosh, R.A.; Yamazaki, Y.; Dubcovsky, J.; Rogers, J.; Morris, C.; Appels, R.; Xia, X.C. Catalogue of Gene Symbols for Wheat. 2013. Available online: https://shigen.nig.ac.jp/wheat/komugi/genes/macgene/2013/GeneSymbol.pdf (accessed on 3 September 2022).
- Dzhenin, S.V.; Lapochkina, I.F.; Zhemchuzhina, A.I.; Kovalenko, E.D. Donors of spring wheat resistant to the leaf rust with the genetic material of Aegilops speltoides L., Aegilops triuncialis L., Triticum kiharae Dorof. et Migusсh. Russ. Agric. Sci. 2009, 5, 3–7. (In Russian) [Google Scholar]
- Gajnullin, N.R.; Lapochkina, I.F.; Zhemchuzhina, A.I.; Kiseleva, M.I.; Kolomiets, T.M.; Kovalenko, E.D. Phytopathological and molecular genetic identification of leaf rust resistance genes in common wheat accessions with alien genetic material. Russ. J. Genet. 2007, 43, 875–881. (In Russian) [Google Scholar] [CrossRef]
- Hovhannisyan, N.A.; Dulloo, M.E.; Yesayan, A.H.; Knüpffer, H.; Amri, A. Tracking of powdery mildew and leaf rust resistance genes in Triticum boeoticum and T. urartu, wild relatives of common wheat. Czech J. Genet. Plant Breed. 2011, 47, 45–57. [Google Scholar] [CrossRef]
- Edwards, K.; Johnstone, C.; Thompson, C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucl. Acids Res. 1991, 19, 1349. [Google Scholar] [CrossRef]
- Dorokhov, D.B.; Klocke, E. A rapid and economic technique for RAPD analysis of plant genomes. Russ. J. Genet. 1997, 33, 358–365. [Google Scholar]
- Gupta, S.K.; Charpe, A.; Koul, S.; Prabhu, K.V.; Haq, Q.M.R. Development and validation of molecular markers linked to an Aegilops umbellulate-derived leaf-rust-resistance gene, Lr9, for marker-assisted selection in bread wheat. Genome 2005, 48, 823–830. [Google Scholar] [CrossRef]
- Prins, R.; Groenewald, J.Z.; Marais, G.F.; Snape, J.W.; Koebner, R.M.D. AFLP and STS tagging of Lr19, a gene conferring resistance to leaf rust in wheat. Theor. Appl. Genet. 2001, 103, 618–624. [Google Scholar] [CrossRef]
- Prabhu, K.V.; Gupta, S.K.; Charpe, A.; Koul, S. SCAR marker tagged to the alien leaf rust resistance gene Lr19 uniquely marking the Agropyron elongatum-derived gene Lr24 in wheat: A revision. Plant Breed. 2004, 123, 417–420. [Google Scholar] [CrossRef]
- Helguera, M.; Khan, I.A.; Dubcovsky, J. Development of PCR markers for the wheat leaf rust resistance gene Lr47. Theor. Appl. Genet. 2000, 100, 1137–1143. [Google Scholar] [CrossRef]
- Hetzler, J. Host-Pathogen Interactions in Populations of Bipolaris sorokiniana in the Warm Non-Traditional Areas. Ph.D. Thesis, Georg August University Göttingen, Göttingen, Germany, 1992. [Google Scholar]
- Kumar, J.; Schäfer, P.; Hückelhoven, R.; Langen, G.; Baltruschat, H.; Stein, E.; Nagarajan, S.; Kogel, K.H. Bipolaris sorokiniana, a cereal pathogen of global concern: Cytological and molecular approaches towards better control. Mol. Plant Pathol. 2002, 3, 185–195. [Google Scholar] [CrossRef]
- Duveiller, E.M.; Sharma, R.C. Genetic improvement, and crop management strategies to minimize yield losses in warm non-traditional wheat growing areas due to spot blotch pathogen Cochliobolus sativus. J. Phytopathol. 2009, 157, 521–534. [Google Scholar] [CrossRef]
- Joshi, A.K.; Chand, R.; Arun, B. Relationship of plant height and days to maturity with resistance to spot blotch in wheat. Euphytica 2002, 123, 221–228. [Google Scholar] [CrossRef]
- Smurova, S.G. New Sources and Donors of Wheat Resistance to Cochliobolus sativus Drechs. ex Dastur. Candidate Dissertation (Ph.D.), All-Russian Institute of Plant Protection, Saint Petersburg, Russia, 2008. (In Russian). [Google Scholar]
- Askhadullin, D.F.; Askhadullin, D.F.; Vasilova, N.Z.; Khusainova, I.I.; Bagavieva, E.Z.; Tazutdinova, M.R. Dark-brown leaf spot on spring bread wheat in Tatarstan. Plant Prot. Quar. 2018, 9, 17–19. (In Russian) [Google Scholar]
- Alexandrov, A.E. Sources of Spring Bread Wheat Resistance to Powdery Mildew in the Lower Volga Region. Candidate Dissertation (Ph.D.), NIISH of the South-East, Saratov, Russia, 2000. (In Russian). [Google Scholar]
- Smurova, S.G.; Mikhailova, L.A. Sources of resistance to wheat spot blotch. Russ. Agric. Sci. 2007, 33, 378–380. [Google Scholar] [CrossRef]
- Kolesnikov, L.E.; Vlasova, E.A.; Vinogradov, A.A. On wheat leaf blotch agent’s development in plants of collection samples of soft wheat. Agric. Biol. 2009, 5, 90–93. (In Russian) [Google Scholar]
- Lebedeva, T.V.; Zuev, E.V. Studies of powdery mildew resistance (Blumeria graminis f. sp. tritici Golov.) in varieties of common wheat (Triticum aestivum L.). Ach. Sci. Technol. AIC 2015, 29, 17–20. (In Russian) [Google Scholar]
- Sadovaya, A.S.; Gultyaeva, E.I.; Mitrofanova, O.P.; Shaidayuk, E.L.; Hakimova, A.G.; Zuev, E.V. Leaf rust resistance in common wheat varieties and lines from the collection of the Vavilov Plant Industry Institute carrying alien genetic material. Russ. J. Genet. Appl. Res. 2015, 5, 233–241. [Google Scholar] [CrossRef]
- Kolomiets, T.M.; Pankratova, L.F.; Skatenok, O.O.; Pakholkova, E.V. Creation of genebank of wheat resistance sources to Septoriosis. Plant Prot. Quar. 2015, 7, 44–46. (In Russian) [Google Scholar]
- Podgorny, S.V.; Samofalov, A.P.; Skripka, O.V. Collection Samples of Soft Winter Wheat Tolerant to Leaf Rust and Powdery Mildew. Available online: http://ej.kubagro.ru/2015/09/pdf/105.pdf (accessed on 12 June 2022).
- Riaz, A.; Athiyannan, N.; Periyannan, S.; Afanasenko, O.; Mitrofanova, O.; Aitken, E.A.B.; Lagudah, E.; Hickey, L.T. Mining Vavilov’s treasure chest of wheat diversity for adult plant resistance to Puccinia triticina. Plant Dis. 2017, 101, 317–323. [Google Scholar] [CrossRef]
- Riaz, A.; Athiyannan, N.; Periyannan, S.K.; Afanasenko, O.; Mitrofanova, O.P.; Platz, G.J.; Aitken, E.A.B.; Snowdon, R.J.; Lagudah, E.S.; Hickey, L.T.; et al. Unlocking new alleles for leaf rust resistance in the Vavilov wheat collection. Theor. Appl. Genet. 2018, 131, 127–144. [Google Scholar] [CrossRef]
- Phan, H.T.T.; Rybak, K.; Bertazzoni, S.; Furuki, E.; Dinglasan, E.; Hickey, L.T.; Oliver, R.P.; Tan, K.C. Novel sources of resistance to Septoria nodorum blotch in the Vavilov wheat collection identified by genome-wide association studies. Theor. Appl. Genet. 2018, 131, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Tyryshkin, L.G.; Volkova, G.V.; Kolomiets, T.M.; Brykova, A.N.; Zuev, E.V. Effective resistance to leaf rust in spring bread wheat accessions among recent additions to the VIR collection. Vavilovia 2019, 2, 35–43. (In Russian) [Google Scholar] [CrossRef]
- Lebedeva, T.V.; Zuev, E.V.; Brykova, A.N. Prospects of employing modern European cultivars of spring bread wheat in the breeding for powdery mildew resistance in the Northwestern region of Russia. Proc. Appl. Bot. Genet. Breed. 2019, 180, 170–176. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Lebedeva, T.V.; Brykova, A.N.; Zuev, E.V. Powdery mildew resistance of Nordic spring bread wheat accessions from the Collection of the Vavilov Institute (VIR). Proc. Appl. Bot. Genet. Breed. 2020, 181, 146–154. (In Russian) [Google Scholar] [CrossRef]
- Tyryshkin, L.G.; Tyryshkina, N.A. Resistance to diseases in wheat collection samples and somaclonal variants. Czech. J. Genet. Plant Breed. 2003, 39, 21–23. [Google Scholar] [CrossRef]
- Tyryshkin, L.G.; Ershova, M.A. Inheritance of resistance to Septoria blotch in common wheat sample MN81330. Russ. J. Genet. 2004, 40, 454–457. [Google Scholar] [CrossRef]
- Anikster, Y.; Manisterski, J.; Long, D.L.; Leonard, K.J. Leaf rust and stem rust resistance in Triticum dicoccoides populations in Israel. Plant Dis. 2005, 89, 55–62. [Google Scholar] [CrossRef]
- Elkot, A.F.; Singh, R.; Kaur, S.; Kaur, J.; Chhuneja, P. Mapping novel sources of leaf rust and stripe rust resistance introgressed from Triticum dicoccoides in cultivated tetraploid wheat background. J. Plant Biochem. Biotechnol. 2021, 30, 336–342. [Google Scholar] [CrossRef]
- Marais, G.F.; Pretorius, Z.A.; Wellings, C.R.; McCallum, B.; Marais, A.S. Leaf rust and stripe rust resistance genes transferred to common wheat from Triticum dicoccoides. Euphytica 2005, 143, 115–123. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Bernardo, A.; Bai, G.; Hayden, M.J.; Anderson, J.A. Thatcher wheat line RL6149 carries Lr64 and a second leaf rust resistance gene on chromosome 1DS. Theor. Appl. Genet. 2019, 132, 2809–2814. [Google Scholar] [CrossRef]
- Brown-Guedira, G.L.; Gill, B.S.; Cox, T.S.; Leath, S. Transfer of disease resistance genes from Triticum araraticum to common wheat. Plant Breed. 1997, 116, 105–112. [Google Scholar] [CrossRef]
- Brown-Guedira, G.L.; Singh, S.; Fritz, A.K. Performance and mapping of leaf rust resistance transferred to wheat from Triticum timopheevii subsp. armeniacum. Phytopathology 2003, 93, 784–789. [Google Scholar] [CrossRef]
- Lebedeva, T.V.; Zuev, E.V. Inheritance of powdery mildew resistance in selected spring bread wheat accessions from the VIR collection. Vavilovia 2018, 1, 18–24. (In Russian) [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, K.; Dong, L.; Liang, Y.; Li, G.; Fang, T.; Guo, G.; Wu, Q.; Xie, J.; Chen, Y.; et al. Wheat powdery mildew resistance gene Pm64 derived from wild emmer (Triticum turgidum var. dicoccoides) is tightly linked in repulsion with stripe rust resistance gene Yr5. Crop J. 2019, 7, 761–770. [Google Scholar] [CrossRef]
- Xie, C.; Sun, Q.; Ni, Z.; Yang, T.; Nevo, E.; Fahima, T. Chromosomal location of a Triticum dicoccoides-derived powdery mildew resistance gene in common wheat by using microsatellite markers. Theor. Appl. Genet. 2003, 106, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Mergoum, M.; Ali, S.; Adhikari, T.B.; Elias, E.M.; Hughes, G.R. Identification of new sources of resistance to tan spot, Stagonospora nodorum blotch, and Septoria tritici blotch of wheat. Crop Sci. 2006, 46, 2047–2053. [Google Scholar] [CrossRef]
- Chu, C.G.; Xu, S.S.; Faris, J.D.; Nevo, E.; Friesen, T.L. Seedling resistance to tan spot and Stagonospora nodorum leaf blotch in wild emmer wheat (Triticum dicoccoides). Plant Dis. 2008, 92, 1229–1236. [Google Scholar] [CrossRef]
- Vavilov, N.I. Materials on the question of the resistance of cereals against parasitic fungi. Proc. Breed. Stn. Mosc. Agric. Inst. 1913, 1, 1–118. (In Russian) [Google Scholar]
- Vavilov, N.I. Plant immunity to infectious diseases. Proc. Petrovsk. Agric. Acad. 1918, 1–4, 1–244. (In Russian) [Google Scholar]
- Vavilov, N.I. The theory of plant immunity to infectious diseases. In Theoretical Bases of Plant Breeding; State Publishing House of State and Collective Farms Literature: Moscow/Leningrad, Russia, 1935; Volume 1, pp. 893–990. (In Russian) [Google Scholar]
- Vavilov, N.I. Laws of natural immunity of plants to infectious diseases (keys to finding immune forms). Izv. Akad. Nauk. SSSR. Ser. Biol. 1961, 1, 117–157. (In Russian) [Google Scholar]
- Tyryshkin, L.G. Vavilov’s laws of natural immunity to diseases: A modern view. In Proceedings of the Conference Scientific Support for the Development of Agriculture Industrial Complex under Conditions of Import Substitution, Saint Petersburg, Russia, 25–27 January 2018; Zhgulev, E.V., Ed.; Ministry of Agriculture of the Russian Federation, Saint Petersburg State Agrarian University: Saint Petersburg, Russia, 2018. Part I. pp. 41–45. (In Russian). [Google Scholar]
- Tyryshkin, L.G.; Gashimov, M.E.; Petrova, N.S.; Zveinek, I.A.; Kovaleva, O.N.; Chernov, V.E. Effective barley resistance to foliar fungal diseases (leaf rust, powdery mildew, dark-brown leaf spot blotch). Proc. Appl. Bot. Genet. Breed. 2013, 171, 57–60. (In Russian) [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).