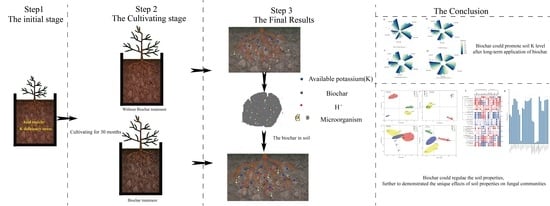
30-Month Pot Experiment: Biochar Alters Soil Potassium Forms, Soil Properties and Soil Fungal Diversity and Composition in Acidic Soil of Southern China
Abstract
1. Introduction
2. Results
2.1. The Changes in Soil Chemical Properties over Time under Biochar and Chemical Fertilizer Treatments
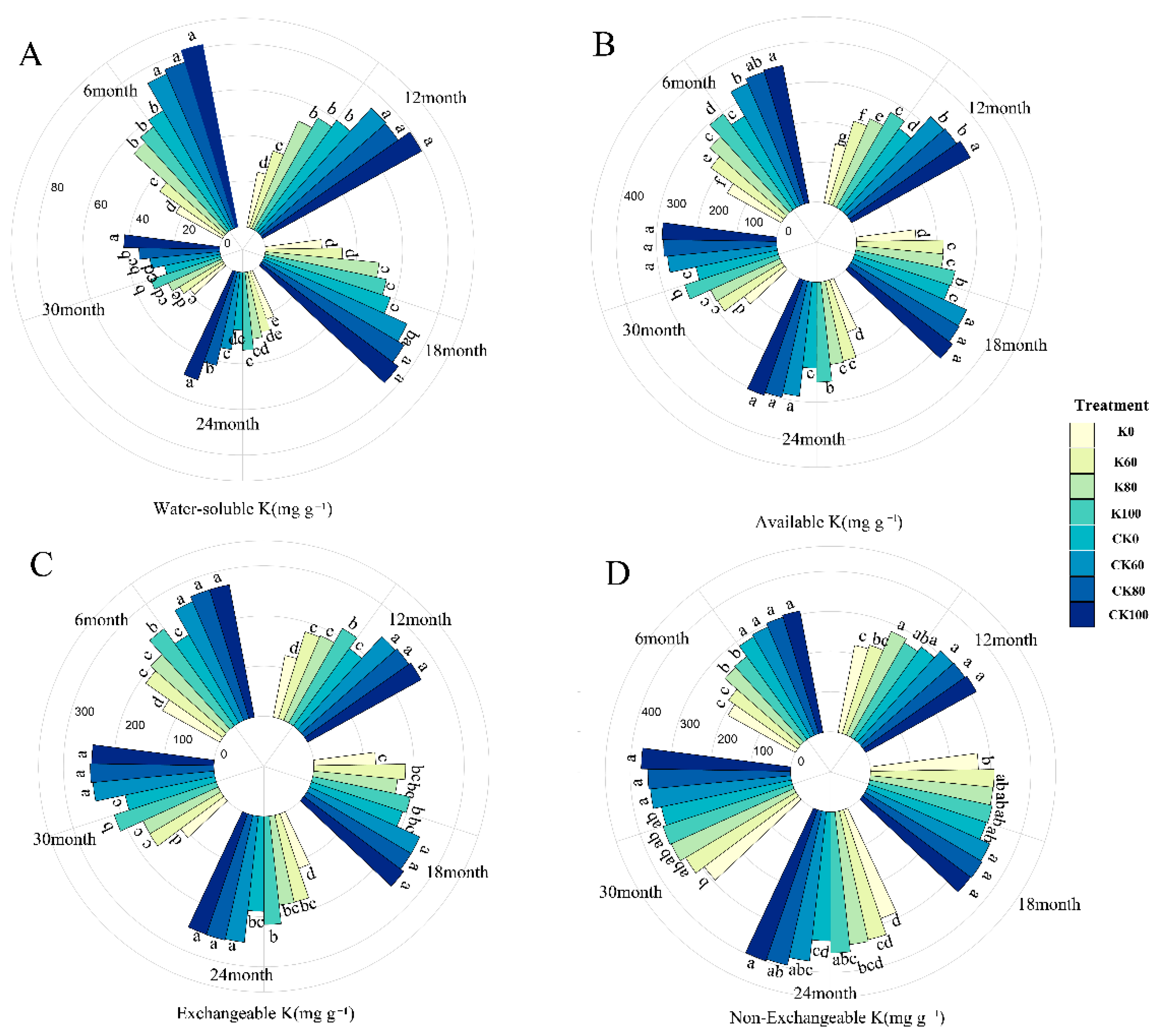
2.2. The Changes in K Forms under Biochar and Chemical Fertilizer Treatments
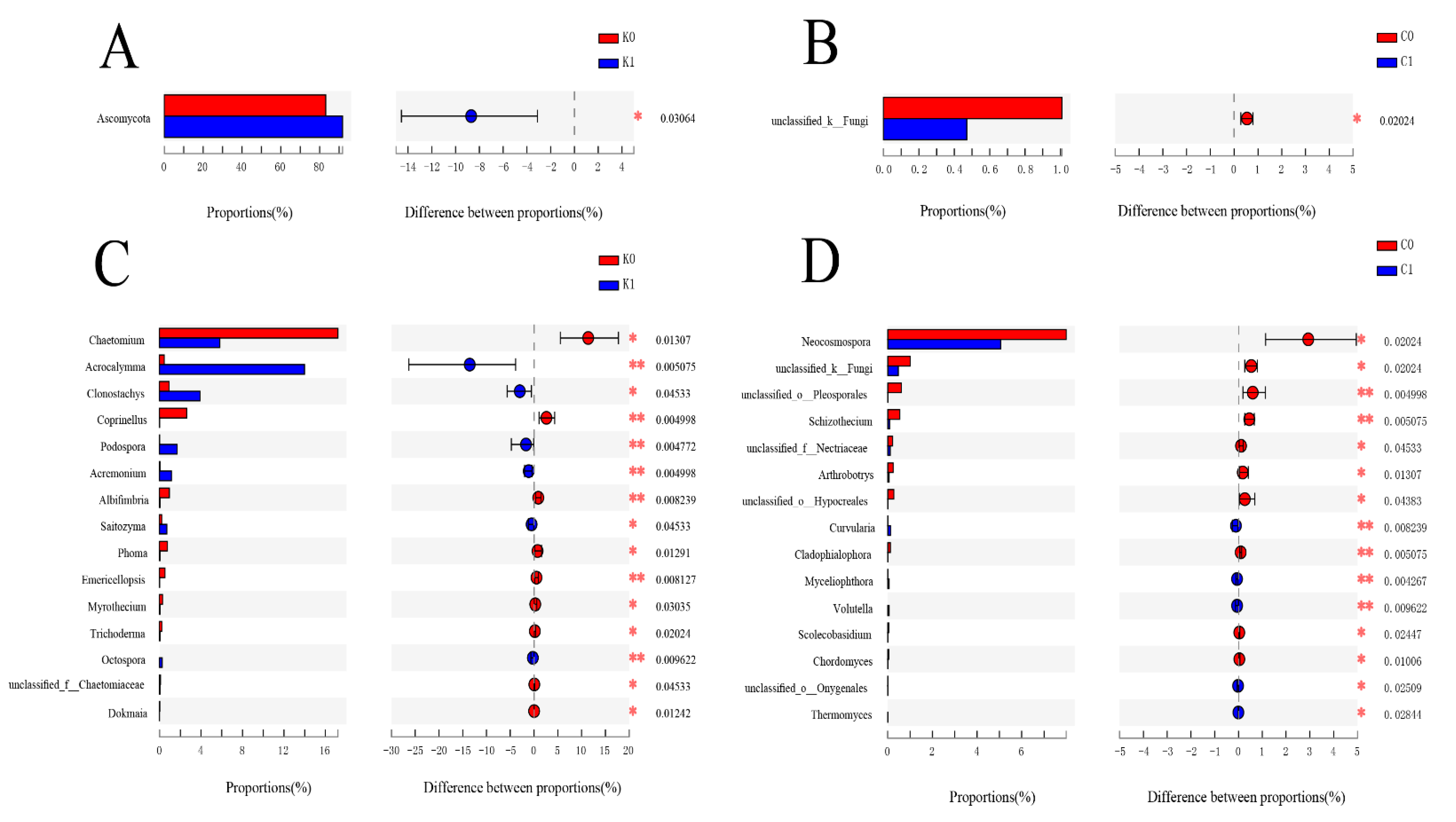
2.3. The Changes in Fungi over Time under Biochar and Chemical Fertilizer Treatment
2.3.1. The Response of Alpha Diversity to Different Treatments
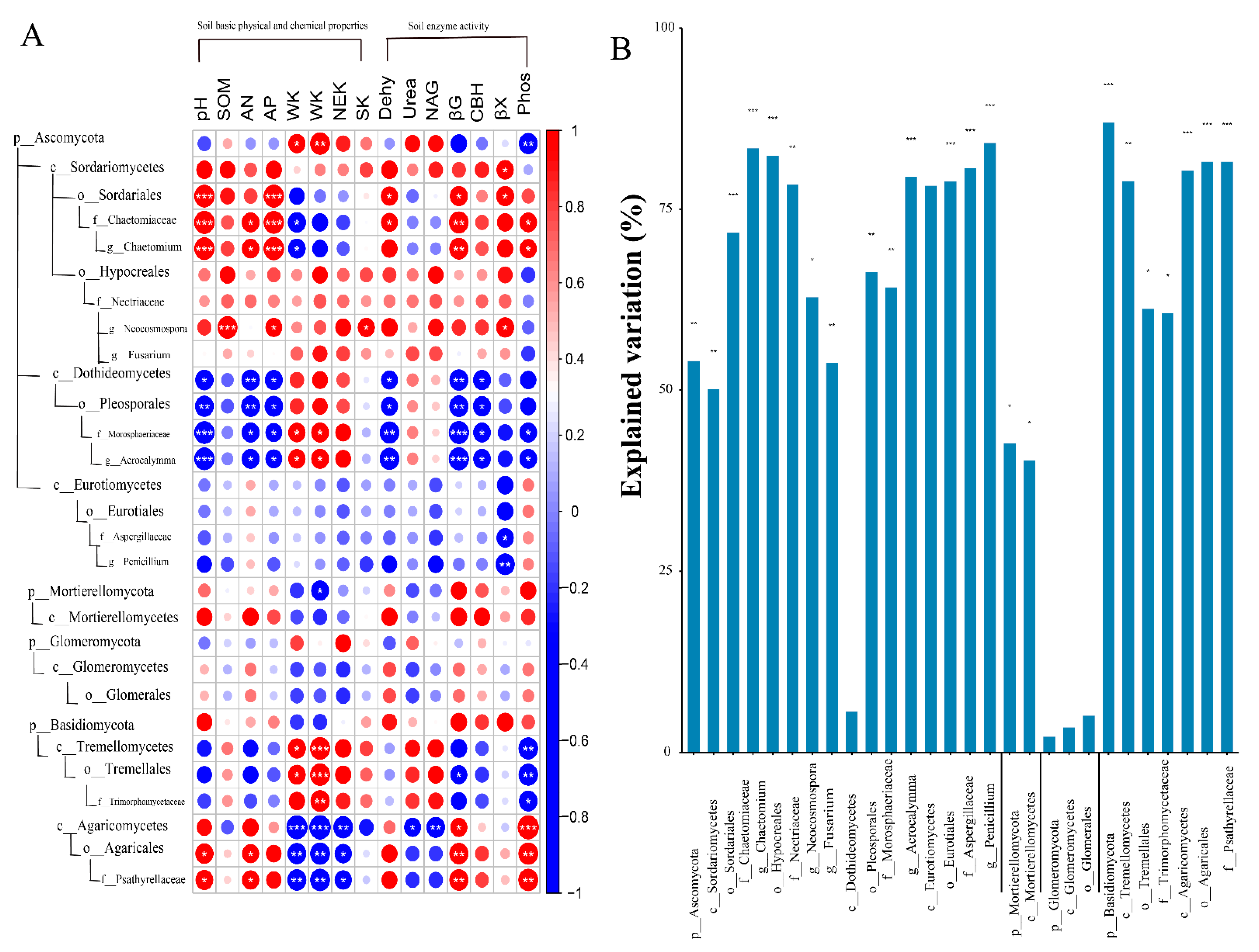
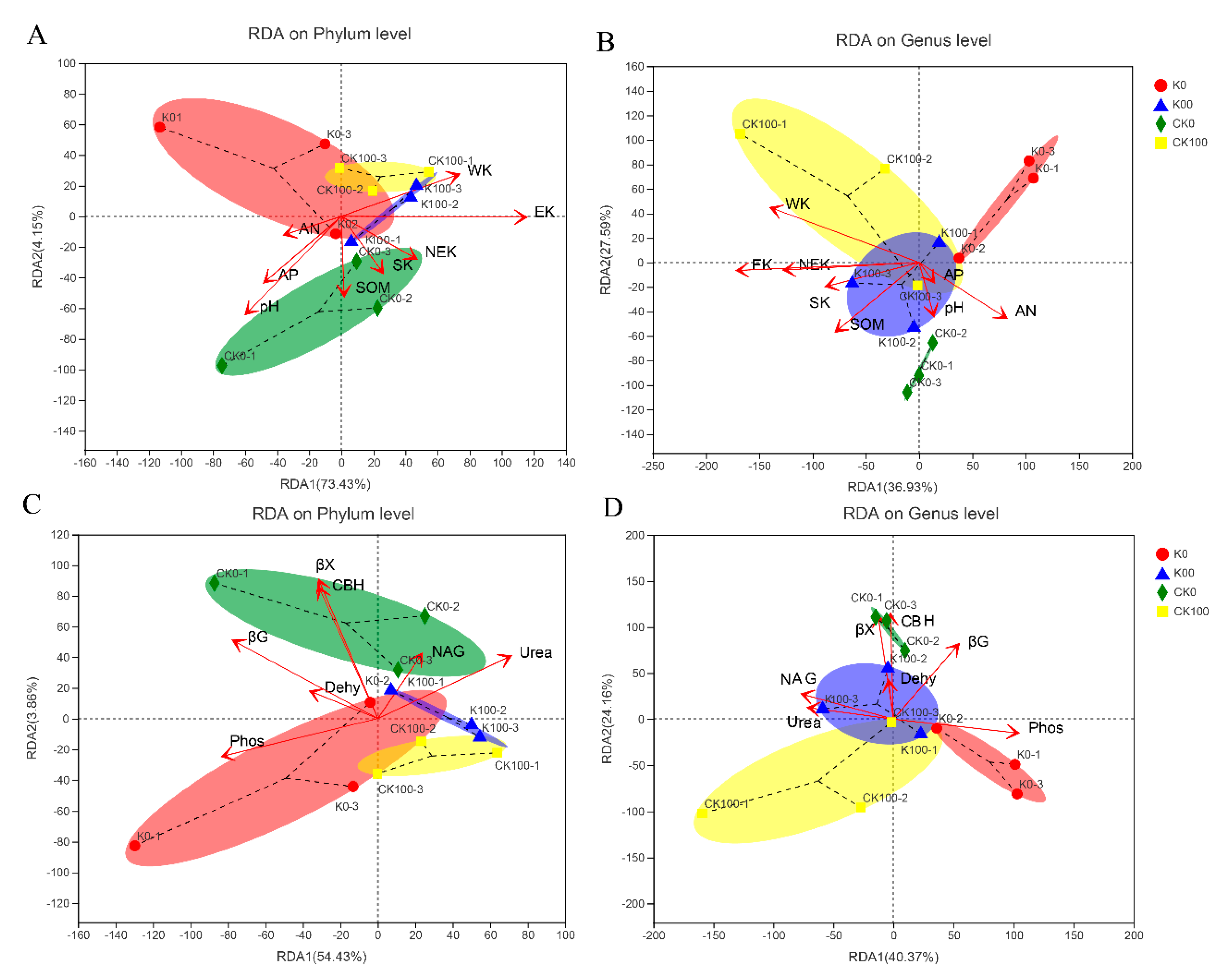
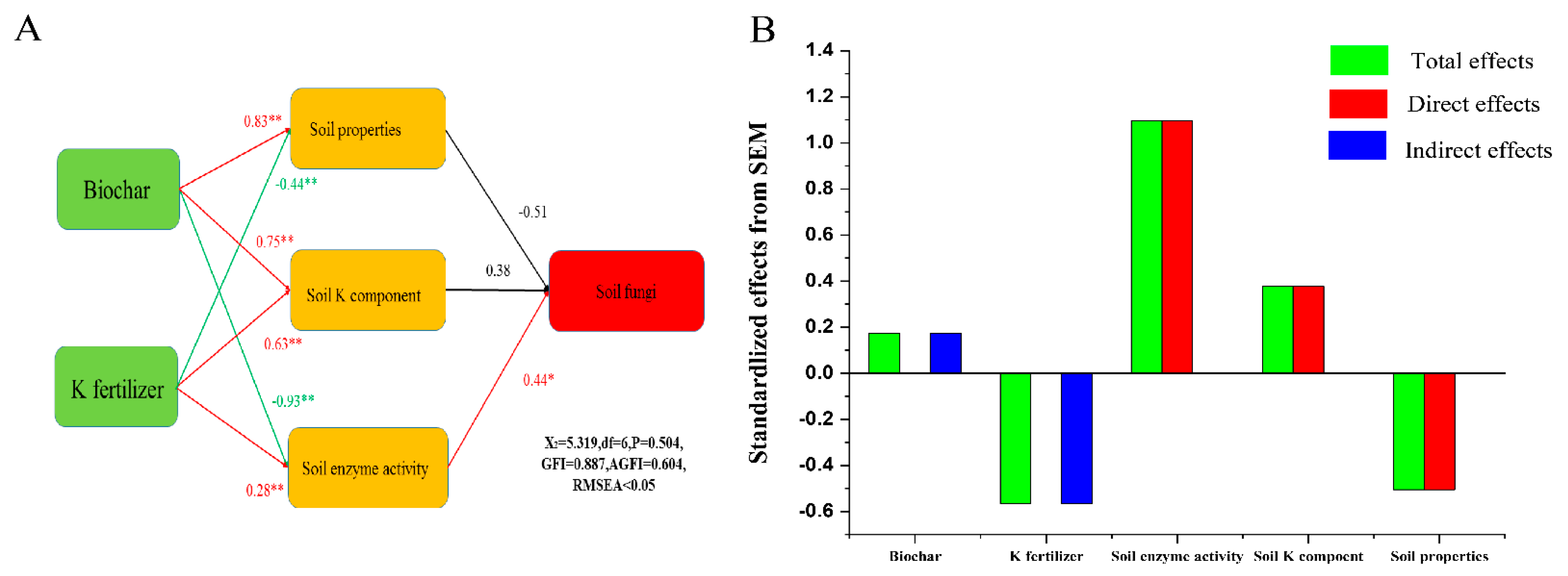
2.3.2. The Associations between Individual Taxa of Fungi and Selected Soil Properties
3. Discussion
3.1. Changes in Chemical Properties over Time
3.2. Associations between Fungal and Soil Properties
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Design and Management
4.3. Soil Sample and Analyses
4.4. Soil DNA Extraction, PCR, and Sequencing
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Qi, C.J. Empircal study on dynamic evolution and its influencing factors of international competiveness of China’s citrus industry. J. Huazhong Agric. Univ. 2013, 6, 33–38. [Google Scholar]
- Yang, S.Q. Studies on the Influences of Soil and Leaf Nutrient on Citrus Fruit’s Output and Quality. Master’s Dissertation, Southwest University, Chongqing, China, 2008. [Google Scholar]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Imran, M.; ShoaibRanaa, M.; Jianga, C. Boron reduces aluminum-induced growth inhibition, oxidative damage and alterations in the cell wall components in the roots of trifoliate orange. Ecotoxicol. Environ. Saf. 2018, 153, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Riaz, M.; Liu, Y.; Zeng, Y.; Jiang, C.C. Aluminum toxicity could be mitigated with boron by altering the metabolic patterns of amino acids and carbohydrates rather than organic acids in trifoliate orange. Tree Physiol. 2019, 39, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Quaggio, J.A.; Mattos, D.; Cantarella, H. Fruit yield and quality of sweet oranges affected by nitrogen, phosphorus and potassium fertilization in tropical soils. Fruits 2006, 61, 293–302. [Google Scholar] [CrossRef][Green Version]
- Li, C.J.; Xiong, Y.W.; Qu, Z.Y.; Xu, X.; Huang, Q.Z.; Huang, G.H. Impact of biochar addition on soil properties and water-fertilizer productivity of tomato in semi-arid region of Inner Mongolia, China. Geoderma 2018, 331, 100–108. [Google Scholar] [CrossRef]
- Embleton, T.; Jones, W.W. Effects of fertilization of citrus on fruit quality and groundwater nitrate-pollution potential. Proc. Int. Soc. Citric. 1980, 1, 280–285. [Google Scholar]
- Simonsson, M.; Hillier, S.; Oborn, I. Changes in clay minerals and potassium fixation capacity as a result of release and fixation of potassium in long-term field experiments. Geoderma 2009, 151, 109–120. [Google Scholar] [CrossRef]
- Wang, L.; Xue, C.; Nie, X.X.; Liu, Y.; Chen, F. Effects of biochar application on soil potassium dynamics and crop uptake. J. Plant Nutr. Soil Sci. 2018, 181, 635–643. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Muhammad, R.; Zhang, L.; Xia, H.; El-desouki, Z.; Jiang, C.C. Response of fungal communities in different soils to biochar and chemical fertilizers under simulated rainfall conditions. Sci. Total Environ. 2019, 691, 654–663. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Zhang, M.Y.; Zhang, L.; Jiang, C.C. Investigating the effects of biochar on soil properties and alleviating aluminum toxicity for improving cabbage (Brassica oleracea var. Capitata) productivity while reducing potash fertilizer. Int. J. Agric. Biol. 2019, 22, 1415–1424. [Google Scholar]
- Yuan, J.C.; Xu, R.K. Progress of the research on the properties of biochars and their influence on soil environmental functions. Ecol. Environ. Sci. 2011, 20, 779–785. [Google Scholar]
- Zhang, J.; Liu, J.; Liu, R. Effects of pyrolysis temperature and heating time on biochar obtained from the pyrolysis of straw and lignosulfonate. Bioresour. Technol. 2015, 176, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Kookana, R.S.; Sarmah, A.K.; Van Zwieten, L.; Krull, E.; Singh, B. Biochar application to soil: Agronomic and environmental benefits and unintended consequences. Adv. Agron. 2011, 112, 103–143. [Google Scholar]
- Zhang, Y.; Jeyakumar, P.; Xia, C.L.; Lam, S.S.; Jiang, J.; Sun, H.J.; Shi, W.M. Being applied at rice or wheat season impacts biochar’s effect on gaseous nitrogen pollutants from the wheat growth cycle. Environ. Pollut. 2022, 306, 119409. [Google Scholar] [CrossRef]
- Smider, B.; Singh, B. Agronomic performance of a high ash biochar in two contrasting soils. Agric. Ecosyst. Environ. 2014, 191, 99–107. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Yang, S. Impacts of biochar addition on rice yield and soil properties in a cold waterlogged paddy for two crop seasons. Field Crops Res. 2016, 191, 161–167. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Xu, G.; Wei, L.L.; Sun, J.N. What is more important for enhancing nutrient bioavailability with biochar application into a sandy soil: Direct or indirect mechanism? Ecol. Eng. 2013, 52, 119–124. [Google Scholar] [CrossRef]
- Chen, W.F.; Meng, J.; Han, X.R.; Lan, Y.; Zhang, W.M. Past, present, and future of biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef]
- Sun, H.J.; Yi, Z.H.; Jeyakumar, P.; Xia, C.L.; Feng, Y.F.; Lam, S.S.; Sonne, C.; Wang, H.L.; Shi, W.M. Citric acid modified biochar application at a low dosage can synchronically mitigate the nitrogenous gas pollutants emission from rice paddy soils. Environ. Pollut. 2022, 312, 120068. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Matsufuru, H.; Takaoka, M.; Tanida, H.; Sato, T. Impacts of chemical amendment and plant growth on lead speciation and enzyme activities in a shooting range soil: An X-ray absorption fine structure investigation. J. Environ. Qual. 2009, 38, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Varma, A. Role of Enzymes in Maintaining Soil Health, Soil Enzymology; Springer: New York, NY, USA, 2010; pp. 25–42. [Google Scholar]
- Bhaduri, D.; Saha, A.; Desai, D.; Meena, H.N. Restoration of carbon and microbial activity in salt induced soil by application of peanut shell biochar during short-term incubation study. Chemosphere 2016, 148, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Mierzwa-Hersztek, M.; Gondek, K.; Baran, A. Effect of poultry litter biochar on soil enzymatic activity, ecotoxicity and plant growth. Appl. Soil Ecol. 2016, 105, 144–150. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Laghari, M.; Laghari, M.; Naidu, R.; Xiao, B.; Hu, Z.; Mirjat, M.S.; Hu, M.; Kandhro, M.N.; Chen, Z.; Guo, D.; et al. Recent developments in biochar as an effective tool for agricultural soil management: A review. J. Sci. Food Agric. 2016, 96, 4840–4849. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Wang, X.; Song, D.; Liang, G.; Zhang, Q.; Ai, C.; Zhou, W. Maize biochar addition rate influences soil enzyme activity and microbial community composition in a fluvo-aquic soil. Appl. Soil Ecol. 2015, 96, 265–272. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, L.; Riaz, M.; Zhang, M.Y.; Xia, H.; Lv, B.; Jiang, C.C. Assessing the potential of biochar and aged biochar to alleviate aluminum toxicity in an acid soil for achieving cabbage productivity. Ecotox. Environ. Safe. 2018, 161, 290–295. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Zhang, L.; Muhammad, R.; Xia, H.; Jiang, C.C. Biochar amendment improved fruit quality and soil properties and microbial communities at different depths in citrus production. J. Clean. Prod. 2021, 292, 126062. [Google Scholar] [CrossRef]
- Chen, J.H.; Liu, X.Y.; Zheng, J.W.; Zhang, B.; Lu, H.; Chi, Z. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Guo, X.W.; Chen, J.; Lu, X.Y.; Li, Y.; Tao, Y.F.; Min, W. Effects of Biochar and Straw Returning on Soil Fungal Community Structure Diversity in Cotton Field with Long-term Brackish Water Irrigation. Environ. Sci. 2022, 43, 4625–4635. [Google Scholar]
- Wang, X.; Cui, H.; Shi, J.; Zhao, X.; Zhao, Y.; Wei, Z. Relationship between bacterial diversity and environmental parameters during composting of different raw materials. Bioresour. Technol. 2015, 198, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wang, S.; Tian, L.; Li, S.; Li, X.; Shen, Y.; Tian, C. Long-term biochar application influences soil microbial community and its potential roles in semiarid farmland. Appl. Soil Ecol. 2017, 117, 10–15. [Google Scholar] [CrossRef]
- Hu, L.; Cao, L.X.; Zhang, R.D. Bacterial and fungal taxon changes in soil microbial community composition induced by short-term biochar amendment in red oxidized loam soil. World J. Microb. Biot. 2014, 30, 1085–1092. [Google Scholar] [CrossRef]
- Wu, X.; Wang, D.; Riaz, M.; Zhang, L.; Jiang, C.C. Investigating the effect of biochar on the potential of increasing cotton yield, potassium efficiency and soil environment. Ecotoxicol. Environ. Saf. 2019, 182, 109451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Muhammad, R.; Liu, B.; Xia, H.; El-desouki, Z.; Jiang, C.C. Two-year study of biochar: Achieving excellent capability of potassium supply via alter clay mineral composition and potassium-dissolving bacteria activity. Sci. Total Environ. 2020, 717, 137286. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Venterea, R.T. Biochar’s role as an alternative N-fertilizer: Ammonia capture. Plant Soil 2012, 350, 35–42. [Google Scholar] [CrossRef]
- El Sharkawi, H.M.; Tojo, S.; Chosa, T.; Malhat, F.M.; Youssef, A.M. Biochar-ammonium phosphate as an uncoated-slow release fertilizer in sandy soil. Biomass Bioenerg. 2018, 117, 154–160. [Google Scholar] [CrossRef]
- Sun, H.; Jeyakumar, P.; Xiao, H.; Li, X.; Liu, J.; Yu, M.; Rana, P.B.J.; Shi, W. Biochar can Increase Chinese Cabbage (Brassica oleracea L.) Yield, Decrease Nitrogen and Phosphorus Leaching Losses in Intensive Vegetable Soil. Phyton-Int. J. Exp. Bot. 2022, 91, 197. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Liu, B.; Li, Y.X.; El-desouki, Z.; Jiang, C.C. Over two years study: Peanut biochar promoted potassium availability by mediating the relationship between bacterial community and soil properties. Appl. Soil Ecol. 2022, 176, 104485. [Google Scholar] [CrossRef]
- Feng, X.J.; Nielsen, L.L.; Simpson, M.J. Responses of soil organic matter and microorganisms to freeze–thaw cycles. Soil Biol. Biochem. 2007, 39, 2027–2037. [Google Scholar] [CrossRef]
- Yao, F.X.; Arbestain, M.C.; Virgel, S.; Blanco, F.; Arostegui, J.; Macia-Agullo, J.A.; Macias, F. Simulated geochemical weathering of a mineral ash-rich biochar in amodified Soxhlet reactor. Chemosphere 2010, 80, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Rufty, T.; Shi, W. Soil microbial diversity and composition: Links to soil texture and associated properties. Soil Biol. Biochem. 2020, 149, 107953. [Google Scholar] [CrossRef]
- Nevarez, L.; Vasseur, V.; Le Madec, A.; Le Bras, M.A.; Coroller, L.; Leguérinel, I.; Barbier, G. Physiological traits of Penicillium glabrum strain LCP 08.5568, a filamentous fungus isolated from bottled aromatised mineral water. Int. J. Food Microbiol. 2009, 130, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Mandal, S.; Pu, S.; Adhikari, S.; Ma, H.; Kim, D.H.; Bai, Y.; Hou, D. Progress and future prospects in biochar composites: Application and reflection in the soil environment. Crit. Rev. Environ. Sci. Technol. 2020, 51, 219–271. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Zhang, M.Y.; Liu, B.; El-Desouki, Z.; Jiang, C.C. Biochar increases nitrogen use efficiency of maize by relieving aluminum toxicity and improving soil quality in acidic soil. Ecotoxicol. Environ. Saf. 2020, 196, 110531. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Bloem, J.; Vries, F.T.D.; Kalbita, K.; Wagg, C. Linking soil biodiversity and agricultural soil management. Curr. Opin. Environ. Sust. 2012, 4, 523–528. [Google Scholar] [CrossRef]
- Zhang, S.S.; Gao, L.Y.; Xu, G.F.; Wang, H.L.; Chen, H.K. Present Situation and Prospect Analysis of Comprehensive Utilization of Insoluble Potassium Resources. Guangdong Chem. Ind. 2019, 46, 98–99. [Google Scholar]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]
- Egidi, E.; Delgadobaquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D. A few ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef]
- Zhang, S.J.; Wang, L.; Ma, F. Application of arbuscular mycorrhiza on promoting the growth of rice and reducing the usage of chemical fertilizer. J. Harbin Inst. Technol. 2010, 42, 958–962. [Google Scholar]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef]
- Arines, J.; Quintela, M.; Vilari, O.A. Protein patterns and superoxide dismutase activity in nonmycorrhizal and arbuscular mycorrhizal Pisum sativum L. plants. Plant Soil 1994, 166, 37–45. [Google Scholar] [CrossRef]
- Gast, V.D.; Christopher, J. Microbial biogeography: The end of the ubiquitous dispersal hypothesis? Environ. Microbiol. 2015, 17, 544–546. [Google Scholar] [CrossRef]
- Hu, H.W.; Zhang, L.M.; Yuan, C.L.; Yong, Z.; Wang, J.T.; Chen, D. The large-scale distribution of ammonia oxidizers in paddy soils is driven by soil pH, geographic distance, and climatic factors. Front. Microbiol. 2015, 6, 938. [Google Scholar] [CrossRef]
- Schadt, C.W.; Martin, A.P.; Lipson, D.A.; Schmidt, S.K. Seasonal dynamics of previously unknown fungal lineages in tundra soils. Science 2003, 301, 1359–1361. [Google Scholar] [CrossRef]
- Johnson, D.; Vandenkoornhuyse, P.J.; Leake, J.R.; Gilbert, L.; Booth, R.E.; Grime, J.P. Plant communities affect arbuscular mycorrhizal fungal diversity and community composition in grassland microcosms. New Phytol. 2004, 161, 503–515. [Google Scholar] [CrossRef]
- Paul, G.D.; Steven, P.R.; Kevin, K.N.; Vito, A.L.; Victoria, J.O.; Timothy, J.D.; David, W.H. Soil fungal community composition does not alter along a latitudinal gradient through the maritime and sub-antarctic. Fungal. Ecol. 2012, 5, 403–408. [Google Scholar]
- Liu, P.; Tian, Y.Z.; Zhong, Y.J.; Liu, H. Isolation and application of effective rhizobium strainsin peanut on acidic soils. Sci. Agric. Sin. 2019, 52, 3393–3403. [Google Scholar]
- Bao, S.D. Soil and Agriculture Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 39–61. [Google Scholar]
- Grandy, A.S.; Sinsabaugh, R.L.; Neff, J.C. Nitrogen deposition effects on soil organic matter chemistry are linked to variation in enzymes, ecosystems and size fractions. Biogeochemistry 2008, 91, 37–49. [Google Scholar] [CrossRef]
- Deforest, J. The influence of time, storage temperature, and substrate age onpotential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA. Soil Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in microbes: Fungi in indoor air are dominated by outdoor air and show dispersal limitation at short distances. ISME J. 2013, 7, 1262. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rrna sequences into the new bacterial taxonomy. Appl. Environ. Microb. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Muhammad, R.; Xia, H.; Li, Y.X.; Wang, X.L.; Jiang, C.C. Four-year biochar study: Positive response of acidic soil microenvironment and citrus growth to biochar under potassium deficiency conditions. Sci. Total Environ. 2022, 813, 152515. [Google Scholar] [CrossRef]

| pH | 6 Month | 12 Month | 18 Month | 24 Month | 30 Month | SOM | 6 Month | 12 Month | 18 Month | 24 Month | 30 Month |
|---|---|---|---|---|---|---|---|---|---|---|---|
| K0 | 4.73 ± 0.06 b | 4.76 ± 0.73 b | 5.07 ± 0.06 a | 6.26 ± 0.10 ab | 6.75 ± 0.02 bc | K0 | 17.27 ± 2.44 c | 16.50 ± 0.80 bc | 11.10 ± 1.28 c | 9.79 ± 0.62 c | 8.06 ± 0.86 b |
| K60 | 4.65 ± 0.04 bc | 4.74 ± 0.02 b | 5.07 ± 0.07 a | 6.15 ± 0.04 abc | 6.70 ± 0.01 cd | K60 | 15.83 ± 1.13 c | 15.41 ± 0.70 c | 11.33 ± 1.00 c | 9.45 ± 0.82 c | 7.92 ± 0.82 b |
| K80 | 4.56 ± 0.06 c | 4.72 ± 0.02 b | 5.02 ± 0.12 a | 6.13 ± 0.06 bc | 6.64 ± 0.04 d | K80 | 15.07 ± 1.25 c | 14.43 ± 1.41 c | 11.69 ± 1.39 c | 9.29 ± 0.89 c | 7.40 ± 0.71 b |
| K100 | 4.55 ± 0.03 c | 4.70 ± 0.01 b | 5.03 ± 0.09 a | 5.97 ± 0.23 c | 6.53 ± 0.09 e | K100 | 14.47 ± 0.51 c | 10.42 ± 0.63 d | 10.73 ± 0.48 c | 7.97 ± 0.29 d | 7.22 ± 0.96 b |
| CK0 | 5.11 ± 0.05 a | 5.18 ± 0.29 a | 6.00 ± 0.07 b | 6.37 ± 0.04 a | 6.87 ± 0.04 a | CK0 | 29.00 ± 4.89 b | 20.58 ± 1.57 b | 15.42 ± 1.44 b | 14.71 ± 0.91 b | 15.16 ± 1.47 a |
| CK60 | 5.06 ± 0.12 a | 5.14 ± 0.03 a | 6.00 ± 0.05 b | 6.31 ± 0.20 ab | 6.79 ± 0.01 b | CK60 | 32.18 ± 5.56 ab | 33.63 ± 3.47 a | 18.60 ± 1.79 a | 16.81 ± 0.28 a | 16.40 ± 0.95 a |
| CK80 | 5.07 ± 0.03 a | 5.14 ± 0.07 a | 5.95 ± 0.09 b | 6.31 ± 0.03 ab | 6.76 ± 0.04 bc | CK80 | 35.06 ± 2.27 a | 30.93 ± 2.47 a | 17.41 ± 1.86 ab | 16.55 ± 0.95 a | 15.62 ± 0.60 a |
| CK100 | 5.02 ± 0.04 a | 5.08 ± 0.03 a | 5.90 ± 0.12 b | 6.22 ± 0.05 ab | 6.73 ± 0.02 bc | CK100 | 34.78 ± 2.88 a | 29.81 ± 1.54 a | 17.27 ± 2.52 ab | 14.31 ± 1.71 b | 15.25 ± 0.69 a |
| AN | 6 month | 12 month | 18 month | 24 month | 30 month | AP | 6 month | 12 month | 18 month | 24 month | 30 month |
| K0 | 78.17 ± 8.08 a | 56.29 ± 5.83 a | 68.54 ± 2.53 ab | 60.78 ± 3.54 ab | 66.50 ± 6.06 ab | K0 | 5.77 ± 0.66 c | 5.91 ± 0.62 c | 15.49 ± 2.12 a | 12.30 ± 2.73 a | 33.01 ± 2.80 abc |
| K60 | 77.00 ± 3.50 a | 56.88 ± 8.35 a | 64.17 ± 2.02 bc | 55.24 ± 4.04 bc | 63.29 ± 0.51 ab | K60 | 6.24 ± 1.01 c | 6.20 ± 0.54 c | 16.37 ± 1.01 a | 14.29 ± 1.01 a | 29.27 ± 4.49 bcd |
| K80 | 78.17 ± 5.35 a | 53.67 ± 2.03 a | 64.17 ± 4.04 bc | 55.24 ± 2.02 bc | 61.25 ± 1.75 ab | K80 | 8.75 ± 0.65 b | 7.12 ± 0.77 bc | 16.86 ± 2.27 a | 15.43 ± 2.18 a | 27.49 ± 1.19 cd |
| K100 | 77.00 ± 3.50 a | 53.67 ± 2.03 a | 58.33 ± 2.02 d | 53.20 ± 2.32 c | 58.92 ± 7.07 b | K100 | 9.39 ± 1.22 b | 7.05 ± 1.17 bc | 17.14 ± 3.01 a | 14.75 ± 1.44 a | 26.12 ± 1.79 d |
| CK0 | 80.50 ± 3.50 a | 56.00 ± 3.5 a | 71.17 ± 5.35 a | 66.33 ± 3.03 a | 67.38 ± 2.32 a | CK0 | 9.92 ± 1.10 b | 8.06 ± 1.00 ab | 17.74 ± 1.85 a | 13.67 ± 2.18 a | 35.94 ± 3.85 a |
| CK60 | 85.17 ± 4.04 a | 53.67 ± 2.02 a | 65.04 ± 1.82 bc | 63.41 ± 5.35 a | 64.17 ± 4.04 ab | CK60 | 10.46 ± 1.46 ab | 9.64 ± 1.41 a | 18.08 ± 0.72 a | 14.55 ± 2.10 a | 34.51 ± 4.18 ab |
| CK80 | 84.00 ± 3.50 a | 52.50 ± 3.50 a | 63.88 ± 2.32 bc | 55.24 ± 1.01 bc | 61.54 ± 2.53 ab | CK80 | 12.03 ± 0.84 a | 9.77 ± 0.85 a | 18.02 ± 2.01 a | 15.59 ± 1.77 a | 31.56 ± 0.79 abcd |
| CK100 | 83.13 ± 6.95 a | 52.21 ± 3.07 a | 60.67 ± 2.02 cd | 54.66 ± 3.64 bc | 60.38 ± 3.81 ab | CK100 | 12.40 ± 1.83 a | 9.75 ± 0.65 a | 18.09 ± 1.83 a | 15.55 ± 0.82 a | 32.23 ± 3.81 abc |
| Treatment | Abbreviation | |
|---|---|---|
| Biochar treatment | 0% K | CK0 |
| KNO3 0 mg·kg−1 | ||
| 60% K | CK60 | |
| KNO3 364 mg·kg−1 | ||
| 2% | 80% K | CK80 |
| KNO3 485 mg·kg−1 | ||
| 100% K | CK100 | |
| KNO3 606 mg·kg−1 | ||
| Non-biochar treatment | 0% K | K0 |
| KNO3 0 mg·kg−1 | ||
| 60% K | K60 | |
| KNO3 364 mg·kg−1 | ||
| 0% | 80% K | K80 |
| KNO3 485 mg·kg−1 | ||
| 100% K | K100 | |
| KNO3 606 mg·kg−1 | ||
| Other fertilizer management | CO(NH2)2 402 mg·kg−1, Na2HPO4·12H2O 356.3 mg·kg−1, CaCO3 719 mg·kg−1, MgSO4·7H2O 492 mg·kg−1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, H.; Liu, B.; Riaz, M.; Li, Y.; Wang, X.; Wang, J.; Jiang, C. 30-Month Pot Experiment: Biochar Alters Soil Potassium Forms, Soil Properties and Soil Fungal Diversity and Composition in Acidic Soil of Southern China. Plants 2022, 11, 3442. https://doi.org/10.3390/plants11243442
Xia H, Liu B, Riaz M, Li Y, Wang X, Wang J, Jiang C. 30-Month Pot Experiment: Biochar Alters Soil Potassium Forms, Soil Properties and Soil Fungal Diversity and Composition in Acidic Soil of Southern China. Plants. 2022; 11(24):3442. https://doi.org/10.3390/plants11243442
Chicago/Turabian StyleXia, Hao, Bo Liu, Muhammad Riaz, Yuxuan Li, Xiangling Wang, Jiyuan Wang, and Cuncang Jiang. 2022. "30-Month Pot Experiment: Biochar Alters Soil Potassium Forms, Soil Properties and Soil Fungal Diversity and Composition in Acidic Soil of Southern China" Plants 11, no. 24: 3442. https://doi.org/10.3390/plants11243442
APA StyleXia, H., Liu, B., Riaz, M., Li, Y., Wang, X., Wang, J., & Jiang, C. (2022). 30-Month Pot Experiment: Biochar Alters Soil Potassium Forms, Soil Properties and Soil Fungal Diversity and Composition in Acidic Soil of Southern China. Plants, 11(24), 3442. https://doi.org/10.3390/plants11243442