Rootstocks with Different Tolerance Grade to Citrus Tristeza Virus Induce Dissimilar Volatile Profile in Citrus sinensis and Avoidance Response in the Vector Aphis gossypii Glover
Abstract
1. Introduction
2. Results
2.1. Behavioral Bioassays
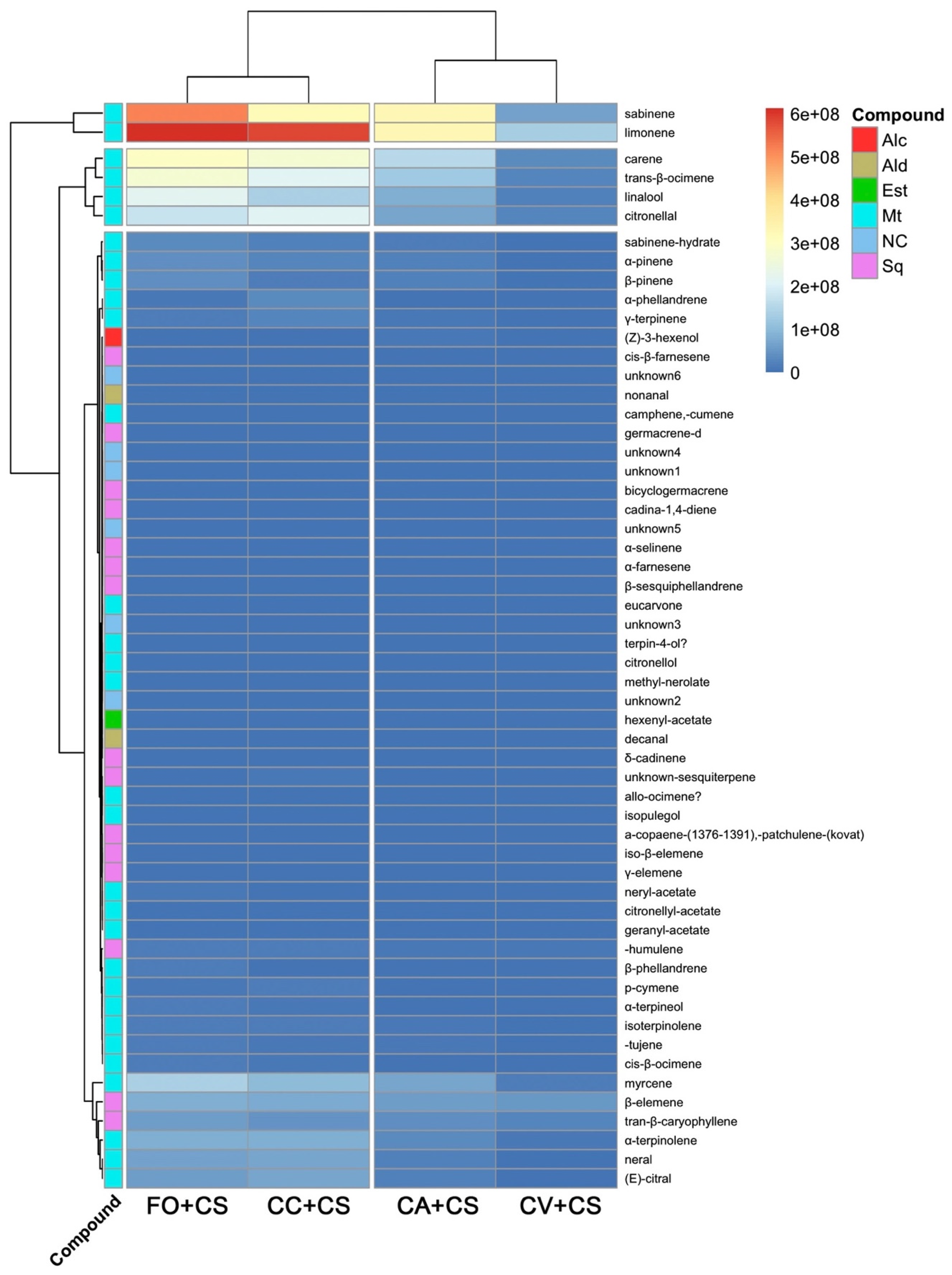
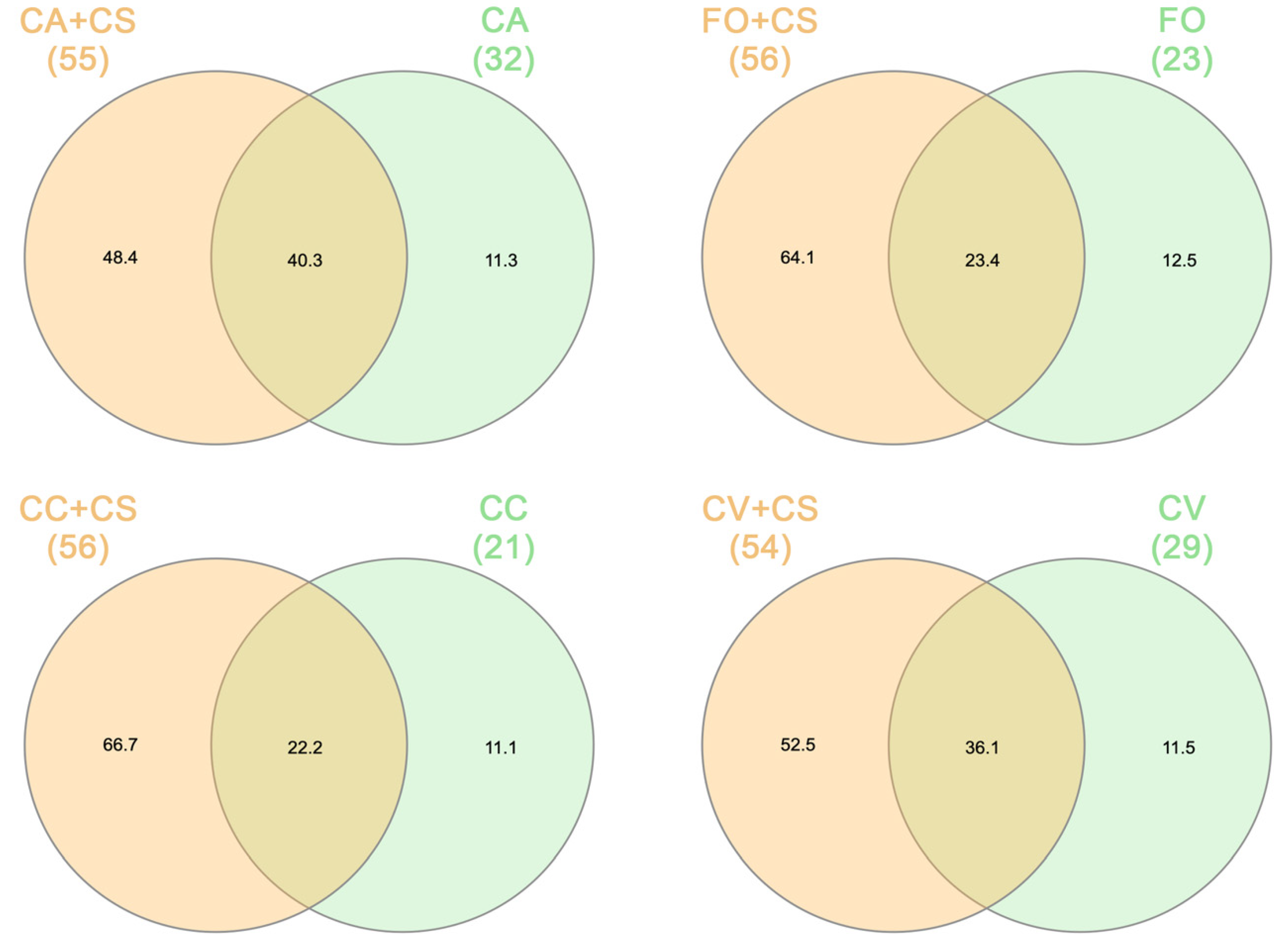
2.2. VOC Chemical Analysis
3. Discussion
4. Materials and Methods
4.1. Plant and Insects
4.2. VOC Chemical Analysis
4.3. Behavioral Bioassays
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreno, P.; Ambrós, S.; Albiach-Martí, M.R.; Guerri, J.; Pena, L. Citrus tristeza virus: A pathogen that changed the course of the citrus industry. Mol. Plant Pathol. 2008, 9, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ze’ev, I.S.; Bar-Joseph, M.; Nitzan, Y.; Marcus, R. A severe citrus tristeza virus isolate causing the collapse of trees of sour orange before virus is detectable throughout the canopy. Ann. Appl. Biol. 1989, 114, 293–300. [Google Scholar] [CrossRef]
- Castle, W.S.; Nunnallee, J.; Manthey, J.A. Screening citrus rootstocks and related selections in soil and solution culture for tolerance to low-iron stress. Hort. Sci. 2009, 44, 638–645. [Google Scholar] [CrossRef]
- Laino, P.; Russo, M.P.; Guardo, M.; Reforgiato-Recupero, G.; Valè, G.; Cattivelli, L.; Moliterni, V.M. Rootstock–scion interaction affecting citrus response to CTV infection: A proteomic view. Physiol. Plant. 2016, 156, 444–467. [Google Scholar] [CrossRef]
- Folimonova, S.Y. Developing an understanding of cross-protection by Citrus tristeza virus. Front. Microbiol. 2013, 4, 76–85. [Google Scholar] [CrossRef]
- Abbate, L.; Panno, S.; Mercati, F.; Davino, S.; Fatta Del Bosco, S. Citrus rootstock breeding: Response of four allotetraploid somatic hybrids to Citrus tristeza virus induced infections. Eur. J. Plant Pathol. 2019, 153, 837–847. [Google Scholar] [CrossRef]
- Roose, M.L. Choosing a Rootstock. In Citrus Production Manual; Ferguson, L., Grafton-Carwell, E.E., Eds.; University of California: California, CA, USA, 2014; Volume 3539, p. 95. [Google Scholar]
- Dambier, D.; Benyahia, H.; Pensabene-Bellavia, G. Somatic hybridization for citrus rootstock breeding: An effective tool to solve some important issues of the Mediterranean citrus industry. Plant Cell Rep. 2011, 30, 883–900. [Google Scholar] [CrossRef]
- Forner-Giner, M.A.; Primo-Millo, E.; Forner, J.B. Performance of Forner-Alcaide 5 and Forner-Alcaide 13, hybrids of Cleopatra mandarin × Poncirus trifoliate, as salinity-tolerant citrus rootstocks. J. Am. Pomol. Soc. 2009, 63, 72–80. [Google Scholar]
- Salibe, A.A.; Cereda, E. Limitations on the use of Volkamer Lemon as rootstock for Citrus. In Proceedings of the International Organization of Citrus Virologists Conference Proceedings, (1957–2010); University of California; California, CA, USA, 1984; Volume 9. [Google Scholar]
- Emmanouilidou, M.G.; Kyriacou, M.C. Rootstock-modulated yield performance, fruit maturation and phytochemical quality of ‘Lane Late’ and ‘Delta’ sweet orange. Sci. Hortic. 2017, 225, 112–121. [Google Scholar] [CrossRef]
- Castle, W.S. Rootstock as a fruit quality factor in citrus and deciduous tree crops. N. Z. J. Crop Hort. Sci. 1995, 23, 383–394. [Google Scholar] [CrossRef]
- Castle, W.S. A career perspective on citrus rootstocks, their development, and commercialization. Hort. Sci. 2010, 45, 11–15. [Google Scholar] [CrossRef]
- Forner, J.B.; Forner-Giner, M.A.; Alcaide, A. Forner-Alcaide 5 and Forner-Alcaide 13: Two new citrus rootstocks released in Spain. Hort. Sci. 2003, 38, 629–630. [Google Scholar] [CrossRef]
- Forner-Giner, M.A.; Continella, A.; Grosser, J.W. Citrus Rootstock Breeding and Selection. In The Citrus Genome. Compendium of Plant Genomes; Gentile, A., La Malfa, S., Deng, Z., Eds.; Springer: Cham, Switzerland, 2020; pp. 49–74. [Google Scholar]
- Guarino, S.; Abbate, L.; Mercati, F.; Fatta Del Bosco, S.; Motisi, A.; Arif, M.A.; Cencetti, G.; Palagano, E.; Michelozzi, M. Citrus varieties with different tolerance grades to Tristeza virus show dissimilar volatile terpene profiles. Agronomy 2021, 11, 1120. [Google Scholar] [CrossRef]
- Roistacher, C.N.; Bar-Joseph, M. Aphid transmission of citrus tristeza virus: A review. Phytophylactica 1987, 19, 163–168. [Google Scholar] [CrossRef]
- Roy, A.; Brlansky, R.H. Population dynamics of a Florida Citrus tristeza virus isolate and aphid-transmitted subisolates: Identification of three genotypic groups and recombinants after aphid transmission. Phytopathology 2009, 99, 1297–1306. [Google Scholar] [CrossRef]
- Brlansky, R.H.; Damsteegt, V.D.; Howd, D.S.; Roy, A. Molecular analyses of Citrus tristeza virus subisolates separated by aphid transmission. Plant Dis. 2003, 87, 397–401. [Google Scholar] [CrossRef]
- Halbert, S.E.; Genc, H.; Cevik, B.; Brown, L.G.; Rosales, I.M.; Manjunath, K.L.; Pomerinke, M.; Davidson, D.A.; Lee, R.F.; Niblett, C.L. Distribution and characterization of Citrus tristeza virus in South Florida following establishment of Toxoptera citricida. Plant Dis. 2004, 88, 935–941. [Google Scholar] [CrossRef]
- Rocha-Peña, M.A.; Lee, R.F.; Lastra, R.; Niblett, C.L.; Ochoa-Corona, F.M.; Garnsey, S.M.; Yokomi, R.K. Citrus tristeza virus and its aphid vector Toxoptera citricida: Threats to citrus production in the Caribbean and Central and North America. Plant Dis. 1995, 79, 437–445. [Google Scholar] [CrossRef]
- Yokomi, R.K.; Lastra, R.; Stoetzel, M.B.; Damsteegt, V.D.; Lee, R.F.; Garnsey, S.M.; Gottwald, T.R.; Rocha-Pena, M.A.; Niblett, C.L. Establishment of the brown citrus aphid (Homoptera: Aphididae) in Central America and the Caribbean Basin and transmission of citrus tristeza virus. J. Econ. Entomol. 1994, 87, 1078–1085. [Google Scholar] [CrossRef]
- Ilharco, F.A.; Sousa-Silva, C.R.; Álvarez Álvarez, A. First report on Toxoptera citricidus (Kirkaldy) in Spain and Continental Portugal (Homoptera, Aphidoidea). Agron. Lusit. 2005, 51, 19–21. [Google Scholar]
- Cambra, M.; Gorris, M.T.; Marroquın, C.; Román, M.P.; Olmos, A.; Martınez, M.C.; Hermoso De Mendoza, A.; Lopez, A.; Navarro, L. Incidence and epidemiology of Citrus tristeza virus in the Valencian Community of Spain. Virus Res. 2000, 71, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Marroquín, C.; Olmos, A.; Gorris, M.T.; Bertolini, E.; Martınez, M.C.; Carbonell, E.A.; Hermoso de Menzoza, A.; Cambra, M. Estimation of the number of aphids carrying Citrus tristeza virus that visit adult citrus trees. Virus Res. 2004, 100, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Satar, S.; Kersting, U.; Uygun, N. Transmission of Turkish citrus tristeza virus isolates by Aphis gossypii Glover (Homoptera: Aphididae) in laboratory conditions. IOBC/wprs Bull. 2008, 38, 328–335. [Google Scholar]
- Yokomi, R.K.; Garnsey, S.M. Transmission of citrus tristeza virus by Aphis gossypii and Aphis citricola in Florida. Phytophylactica 1987, 19, 169–172. [Google Scholar] [CrossRef]
- Carletto, J.; Martin, T.; Vanlerberghe-Masutti, F.; Brévault, T. Insecticide resistance traits differ among and within host races in Aphis gossypii. Pest Manag. Sci. Former. Pestic. Sci. 2010, 66, 301–307. [Google Scholar] [CrossRef]
- Silver, A.R.; Van Emden, H.F.; Battersby, M. A biochemical mechanism of resistance to pirimicarb in two glasshouse clones of Aphis gossypii. Pestic. Sci. 1995, 43, 21–29. [Google Scholar] [CrossRef]
- Li, F.; Han, Z.; Tang, B. Insensitivity of acetylcholinesterase and increased activity of esterase in the resistant cotton aphid, Aphis gossypii Glover. Acta Entomol. Sin. 2003, 46, 578–583. [Google Scholar]
- Storer, J.R.; Van Emden, H.F. Antibiosis and antixenosis of chrysanthemum cultivars to the aphid Aphis gossypii. Entomol. Exp. Appl. 1995, 77, 307–314. [Google Scholar] [CrossRef]
- Hernández-Suárez, E.; Suárez-Méndez, L.; Parrilla, M.; Arjona-López, J.M.; Hervalejo, A.; Arenas-Arenas, F.J. Feeding and Oviposition Behaviour of Trioza erytreae (Hemiptera: Triozidae) on Different Citrus Rootstock Material Available in Europe. Insects 2021, 12, 623. [Google Scholar] [CrossRef]
- Urbaneja-Bernat, P.; Carrillo, D.; Jaques, J.A. Behavior of Diaphorina citri: An investigation of the potential risk to the most commonly used citrus rootstock in Europe. Entomol. Gen. 2020, 40, 79–86. [Google Scholar] [CrossRef]
- Züst, T.; Agrawal, A.A. Mechanisms and evolution of plant resistance to aphids. Nat. Plants 2016, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Guarino, S.; Peri, E.; Colazza, S.; Luchi, N.; Michelozzi, M.; Loreto, F. Impact of the invasive painted bug Bagrada hilaris on physiological traits of its host Brassica oleracea var botrytis. Arthropod-Plant Interact. 2017, 11, 649–658. [Google Scholar] [CrossRef]
- Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M.; Hardie, J. The chemical ecology of aphids. Ann. Rev. Entomol. 1992, 37, 67–90. [Google Scholar] [CrossRef]
- Döring, T.F. How aphids find their host plants, and how they don’t. Ann. Appl. Biol. 2014, 165, 3–26. [Google Scholar] [CrossRef]
- Chapman, R.F.; Bernays, E.A.; Simpson, S.J. Attraction and repulsion of the aphid, Cavariella aegopodii, by plant odors. J. Chem. Ecol. 1981, 7, 881–888. [Google Scholar] [CrossRef]
- Hardie, J.; Visser, J.H.; Piron, P.G.M. Perception of volatiles associated with sex and food by different adult forms of the black-bean aphid, Aphis fabae. Physiol. Entomol. 1994, 19, 278–284. [Google Scholar] [CrossRef]
- Park, K.C.; Hardie, J. Electrophysiological characterization of olfactory sensilla in the black bean aphid, Aphis fabae. J. Insect Physiol. 2004, 50, 647–655. [Google Scholar] [CrossRef]
- Smith, M.T.; Severson, R.F. Host recognition by the blackmargined aphid (Homoptera: Aphididae) on pecan. J. Entomol. Sci. 1992, 27, 93–112. [Google Scholar] [CrossRef]
- Powell, G.; Tosh, C.R.; Hardie, J. Host plant selection by aphids: Behavioral, evolutionary, and applied perspectives. Annu. Rev. Entomol. 2006, 51, 309–330. [Google Scholar] [CrossRef]
- Kennedy, J.S.; Booth, C.O. Host alternation in Aphis fabae Scop. I. Feeding preferences and fecundity in relation to the age and kind of leaves. Ann. Appl. Biol. 1951, 38, 25–64. [Google Scholar] [CrossRef]
- Kennedy, J.S.; Booth, C.O.; Kershaw, W.J.S. Host finding by aphids in the field. II. Aphis fabae Scop. (gynoparae) and Brevicoryne brassicae L. with a reappraisal of the role of host-finding behaviour in virus spread. . Ann. Appl. Biol. 1959, 47, 424–444. [Google Scholar] [CrossRef]
- Webster, B.E.N. The role of olfaction in aphid host location. Physiol. Entomol. 2012, 37, 10–18. [Google Scholar] [CrossRef]
- Verheggen, F.J.; Haubruge, E.; De Moraes, C.M.; Mescher, M.C. Aphid responses to volatile cues from turnip plants (Brassica rapa) infested with phloem-feeding and chewing herbivores. Arthropod-Plant Interact. 2013, 7, 567–577. [Google Scholar] [CrossRef]
- Bruce, T.J. Glucosinolates in oilseed rape: Secondary metabolites that influence interactions with herbivores and their natural enemies. Ann. Appl. Biol. 2014, 164, 348–353. [Google Scholar] [CrossRef]
- Bruce, T.J.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Bruce, T.J.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects–finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef]
- Webster, B.; Bruce, T.; Pickett, J.; Hardie, J. Volatiles functioning as host cues in a blend become nonhost cues when presented alone to the black bean aphid. Anim. Behav. 2010, 79, 451–457. [Google Scholar] [CrossRef]
- Jones, S.E.; Killiny, N. Influence of rootstock on the leaf volatile organic compounds of citrus scion is more pronounced after the infestation with Diaphorina citri. Plants 2021, 10, 2422. [Google Scholar] [CrossRef]
- Trapero Muñoz, S.; Hervalejo García, Á.; Jiménez Pérez, M.; Boyero, J.R.; Vela, J.M.; Martínez-Ferri, E. Effects of rootstock and flushing on the incidence of three insects on ‘clementine de nules’ citrus trees. Environ. Entomol. 2008, 37, 1531–1537. [Google Scholar] [CrossRef]
- Che-Castaldo, C.; Crisafulli, C.M.; Bishop, J.G.; Zipkin, E.F.; Fagan, W.F. Disentangling herbivore impacts in primary succession by refocusing the plant stress and vigor hypotheses on phenology. Ecol. Mongraphs 2019, 89, e01389. [Google Scholar] [CrossRef]
- Saska, P.; Skuhrovec, J.; Platková, H.; Kosová, K.; Tylová, E.; Tuan, S.-J.; Vítámvás, P. Response of the spring wheat–cereal aphid system to drought: Support for the plant vigour hypothesis. J. Pest Sci. 2022. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Davari, M.; Razmjou, J.; Naseri, B. Separate and combined effects of Mentha piperata and Mentha pulegium essential oils and a pathogenic fungus Lecanicillium muscarium against Aphis gossypii (Hemiptera: Aphididae). J. Econ. Entomol. 2017, 110, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Behi, F.; Bachrouch, O.; Boukhris-Bouhachem, S. Insecticidal activities of Mentha pulegium L., and Pistacia lentiscus L., essential oils against two citrus aphids Aphis spiraecola Patch and Aphis gossypii Glover. J. Essent. Oil Bear. Plants 2019, 22, 516–525. [Google Scholar] [CrossRef]
- Hegde, M.; Oliveira, J.N.; da Costa, J.G.; Loza-Reyes, E.; Bleicher, E.; Santana, A.E.; Caulfield, J.C.; Mayon, P.; Dewhrist, S.Y.; Bruce, T.J.; et al. Aphid antixenosis in cotton is activated by the natural plant defence elicitor cis-jasmone. Phytochemistry 2012, 78, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Solid Phase Microextraction: Theory and Practice; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]


| df | Sum Sq | Mean Sq | F Value | Pr(>F) | |
|---|---|---|---|---|---|
| Scion/rootstock combinations | 3 | 167.1 | 55.69 | 4.114 | 0.0131 * |
| Times | 2 | 100.5 | 50.27 | 3.714 | 0.0342 * |
| Combinations:times | 6 | 29.1 | 4.85 | 0.359 | |
| Residuals | 36 | 487.3 | 13.53 |
| Peak | RT | LRI | Chemicals | Group | CA + CS | FO + CS | CV + CS | CC + CS |
|---|---|---|---|---|---|---|---|---|
| 1 | 7.124 | 860 | (Z)-3-hexenol | Alc. GLV | 798.02 ± 460.62 | 19.46 ± 7.70 | 8.33 ± 5.89 | 220.77 ± 109.31 |
| 2 | 9.104 | 914 | tujene | Mt. hd. | 851.84 ± 380.29 | 1357.68 ± 385.62 | 111.53 ± 38.80 | 988.69 ± 337.15 |
| 3 | 9.277 | 925 | α-pinene * | Mt. hd. | 2394.37 ± 760.63 | 3980.35 ± 1049.00 | 366.93 ± 123.02 | 2503.18 ± 742.30 |
| 4 | 9.72 | 942 | camphene, cumene | Mt. hd. | 71.86 ± 23.41 | 82.92 ± 37.30 | 91.99 ± 51.96 | 122.24 ± 54.47 |
| 5 | 10.259 | 968 | sabinene | Mt. hd. | 32,748.92 ± 8259.47 | 51,545.36 ± 11,420.14 | 6550.30 ± 2123.65 | 31,993.75 ± 8245.60 |
| 6 | 10.356 | 973 | β-pinene * | Mt. hd. | 2293.41 ± 716.19 | 4070.04 ± 1037.37 | 357.42 ± 114.70 | 1669.62 ± 871.07 |
| 7 | 10.618 | 985 | myrcene * | Mt. hd. | 6812.364 ± 2417.51 | 13,669.03 ± 3534.04 | 1269.10 ± 371.40 | 10,333.08 ± 2930.60 |
| 8 | 10.95 | 1001 | hexenyl acetate | Est. GLV | 183.64 ± 58.58 | 66.22 ± 33.11 | 206.27 ± 55.27 | 165.64 ± 69.95 |
| 9 | 11.032 | 1003 | carene | Mt. hd. | 15,815.20 ± 5681.08 | 30,026.01 ± 7916.07 | 3527.89 ± 916.41 | 27,053.19 ± 7171.47 |
| 10 | 11.208 | 1010 | α-phellandrene | Mt. hd. | 490.42 ± 350.66 | 1224.15 ± 387.80 | 59.52 ± 26.10 | 3092.84 ± 2440.88 |
| 11 | 11.371 | 1019 | p cymene | Mt. hd. | 391.90 ± 216.34 | 1141.53 ± 334.52 | 64.87 ± 30.64 | 1242.60 ± 410.88 |
| 12 | 11.499 | 1026 | limonene * | Mt. hd. | 32,943.90 ± 13,842.73 | 61,289.52 ± 13,772.38 | 13,122.13 ± 4495.85 | 58,836.01 ± 14,022.15 |
| 13 | 11.569 | 1032 | cis β-ocimene | Mt. hd. | 600.04 ± 158.46 | 1307.78 ± 292.60 | 69.51 ± 35.51 | 1095.56 ± 226.63 |
| 14 | 11.789 | 1044 | trans β-ocimene | Mt. hd. | 12,706.48 ± 2560.20 | 27,137.86 ± 5385.58 | 2672.65 ± 735.35 | 21,118.00 ± 5553.26 |
| 15 | 11.935 | 1050 | β-phellandrene | Mt. hd. | 271.54 ± 183.16 | 1687.85 ± 999.24 | 42.87 ± 18.71 | 598.01 ± 228.26 |
| 16 | 12.024 | 1055 | γ-terpinene * | Mt. hd. | 647.56 ± 304.32 | 1550.49 ± 511.75 | 72.35 ± 27.17 | 3026.11 ± 2282.45 |
| 17 | 12.255 | 1069 | sabinene hydrate | Mt. est. | 1289.81 ± 450.91 | 3572.42 ± 1270.21 | 195.13 ± 22.15 | 1872.28 ± 734.53 |
| 18 | 12.447 | 1078 | isoterpinolene | Mt. hd. | 702.78 ± 344.82 | 1659.58 ± 485.62 | 132.92 ± 37.86 | 1373.23 ± 451.32 |
| 19 | 12.524 | 1082 | α-terpinolene | Mt. hd. | 3630.55 ± 1735.17 | 8233.42 ± 2348.61 | 746.35 ± 214.08 | 7997.21 ± 2320.90 |
| 20 | 12.779 | 1097 | linalool * | Mt. alc. | 8026.00 ± 2193.94 | 21,911.55 ± 6873.58 | 2222.82 ± 975.31 | 13,765.83 ± 4454.73 |
| 21 | 12.854 | 1099 | nonanal * | Ald. | 39.11 ± 23.29 | 87.98 ± 56.99 | 55.49 ± 22.70 | 121.32 ± 24.69 |
| 22 | 13.239 | 1127 | allo ocimene | Mt. hd. | 114.23 ± 21.13 | 310.39 ± 76.35 | 13.28 ± 9.39 | 537.47 ± 224.51 |
| 23 | 13.676 | 1151 | citronellal | Mt. ald. | 7293.26 ± 2811.10 | 17,251.92 ± 5625.10 | 2627.51 ± 226.28 | 21,392.37 ± 4304.05 |
| 24 | 13.825 | 1162 | isopulegol | Mt. alc. | 63.35 ± 30.39 | 116.99 ± 58.49 | 4.24 ± 2.99 | 383.29 ± 244.25 |
| 25 | 14.003 | 1171 | unknown | 28.44 ± 11.95 | 74.35 ± 37.17 | 9.26 ± 3.89 | 155.20 ± 79.58 | |
| 26 | 14.096 | 1178 | unknown | 46.08 ± 33.98 | 189.40 ± 118.52 | 14.45 ± 5.65 | 278.03 ± 77.08 | |
| 27 | 14.168 | 1181 | terpin 4-ol | Mt. alc. | 80.28 ± 24.17 | 337.22 ± 135.53 | 33.74 ± 13.55 | 163.17 ± 39.90 |
| 28 | 14.276 | 1186 | unknown | 0,00 | 233.99 ± 116.99 | 0 | 28.12 ± 20.14 | |
| 29 | 14.393 | 1195 | α-terpineol * | Mt. alc. | 276.33 ± 133.51 | 1247.17 ± 486.20 | 33.46 ± 16.47 | 823.98 ± 379.95 |
| 30 | 14.468 | 1199 | unknown | 32.82 ± 32.82 | 125.63 ± 50.95 | 13.37 ± 9.46 | 123.35 ± 51.03 | |
| 31 | 14.509 | 1197 | decanal * | Ald. | 162.49 ± 26.79 | 235.78 ± 46.99 | 154.31 ± 33.48 | 299.19 ± 59.71 |
| 32 | 14.807 | 1224 | citronellol * | Mt. alc. | 143.21 ± 90.79 | 293.54 ± 146.77 | 52.40 ± 33.07 | 212.47 ± 53.36 |
| 33 | 15.025 | 1239 | neral | Mt. ald. | 2156.31 ± 900.69 | 6235.03 ± 2596.47 | 338.38 ± 98.55 | 7071.17 ± 2316.41 |
| 34 | 15.205 | 1249 | eucarvone | Mt. ket. | 19.21 ± 15.21 | 269.63 ± 134.81 | 212.81 ± 97.08 | 41.04 ± 34.14 |
| 35 | 15.457 | 1268 | (E)-citral * | Mt. ald. | 2277.47 ± 1070.30 | 6024.86 ± 2599.98 | 103.01 ± 72.83 | 6853.64 ± 22.662 |
| 36 | 16.187 | 1317 | methyl nerolate | Mt. est. | 91.62 ± 43.20 | 194.71 ± 88.41 | 6.56 ± 2.33 | 181.06 ± 66.81 |
| 37 | 16.560 | 1344 | citronellyl acetate | Mt. est. | 74.58 ± 41.79 | 538.38 ± 269.19 | 301.74 ± 201.44 | 170.19 ± 58.38 |
| 38 | 16.688 | 1355 | neryl acetate | Mt. est. | 113.17 ± 54.55 | 830.55 ± 415.27 | 189.25 ± 121.52 | 354.29 ± 145.93 |
| 39 | 16.955 | 1375 | geranyl acetate | Mt. est. | 81.39 ± 69.73 | 496.57 ± 248.28 | 7.75 ± 3.84 | 228.30 ± 132.06 |
| 40 | 17.063 | 1382 | α-copaene | Sq. hd. | 348.76 ± 101.38 | 546.47 ± 141.59 | 257.51 ± 131.36 | 562.71 ± 147.59 |
| 41 | 17.122 | 1385 | iso β-elemene | Sq. hd. | 336.67 ± 131.31 | 457.75 ± 154.70 | 328.42 ± 221.38 | 460.94 ± 154.69 |
| 42 | 17.162 | 1388 | unknown | 10.78 ± 8.42 | 34.51 ± 17.25 | 0 | 75.33 ± 32.54 | |
| 43 | 17.232 | 1393 | β-elemene | Sq. hd. | 5769.96 ± 1890.71 | 8054.76 ± 2419.72 | 4925.58 ± 3196.47 | 7561.09 ± 3158.02 |
| 44 | 17.343 | 1400 | unknown sesquiterpene | Sq. hd. | 61.42 ± 20.12 | 83.13 ± 30.69 | 57.29 ± 36.82 | 823.55 ± 753.85 |
| 45 | 17.693 | 1429 | tran β-caryophyllene * | Sq. hd. | 3886.93 ± 1060.34 | 6016.15 ± 1780.72 | 2616.40 ± 1554.05 | 4416.97 ± 1462.25 |
| 46 | 17.787 | 1436 | γ-elemene | Sq. hd. | 330.60 ± 79.69 | 476.03 ± 181.18 | 271.92 ± 171.84 | 427.06 ± 163.10 |
| 47 | 17.964 | 1450 | cis β-farnesene | Sq. hd. | 376.12 ± 138.09 | 257.78 ± 110.69 | 342.80 ± 234.45 | 49.98 ± 28.85 |
| 48 | 18.026 | 1453 | unknown | 105.76 ± 30.95 | 120.34 ± 40.25 | 120.85 ± 71.58 | 125.56 ± 56.39 | |
| 49 | 18.154 | 1464 | humulene | Sq. hd. | 1030.95 ± 295.69 | 1600.16 ± 463.95 | 750.07 ± 462.95 | 1489.90 ± 555.65 |
| 50 | 18.382 | 1481 | α-selinene | Sq. hd. | 23.60 ± 9.80 | 39.28 ± 19.64 | 7.20 ± 50.09 | 51.68 ± 20.39 |
| 51 | 18.464 | 1488 | germacrene d | Sq. hd. | 73.40 32.67 | 117.00 ± 32.61 | 77.29 ± 51.73 | 97.67 ± 63.23 |
| 52 | 18.617 | 1500 | α-farnesene | Sq. hd. | 66.42 ± 31.89 | 100.42 ± 48.89 | 92.41 ± 62.08 | 53.85 ± 47.60 |
| 53 | 18.644 | 1502 | bicyclogermacrene | Sq. hd. | 34.06 ± 19.24 | 45.69 ± 21.45 | 1.82 ± 1.28 | 110.71 ± 60.73 |
| 54 | 18.876 | 1521 | δ-cadinene | Sq. hd. | 160.88 ± 57.19 | 219.47 ± 86.81 | 200.84 ± 138.41 | 220.76 ± 108.37 |
| 55 | 18.926 | 1525 | β-sesquiphellandrene | Sq. hd. | 29.64 ± 21.64 | 45.365 ± 28.18 | 102.08 ± 72.181 | 45.12 ± 33.37 |
| 56 | 19.087 | 1538 | cadina-1,4-diene | Sq. hd. | 13.06 ± 5.55 | 26.80 ± 14.72 | 32.76 ± 23.16 | 34.44 ± 11.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarino, S.; Mercati, F.; Fatta Del Bosco, S.; Motisi, A.; Abbate, L. Rootstocks with Different Tolerance Grade to Citrus Tristeza Virus Induce Dissimilar Volatile Profile in Citrus sinensis and Avoidance Response in the Vector Aphis gossypii Glover. Plants 2022, 11, 3426. https://doi.org/10.3390/plants11243426
Guarino S, Mercati F, Fatta Del Bosco S, Motisi A, Abbate L. Rootstocks with Different Tolerance Grade to Citrus Tristeza Virus Induce Dissimilar Volatile Profile in Citrus sinensis and Avoidance Response in the Vector Aphis gossypii Glover. Plants. 2022; 11(24):3426. https://doi.org/10.3390/plants11243426
Chicago/Turabian StyleGuarino, Salvatore, Francesco Mercati, Sergio Fatta Del Bosco, Antonio Motisi, and Loredana Abbate. 2022. "Rootstocks with Different Tolerance Grade to Citrus Tristeza Virus Induce Dissimilar Volatile Profile in Citrus sinensis and Avoidance Response in the Vector Aphis gossypii Glover" Plants 11, no. 24: 3426. https://doi.org/10.3390/plants11243426
APA StyleGuarino, S., Mercati, F., Fatta Del Bosco, S., Motisi, A., & Abbate, L. (2022). Rootstocks with Different Tolerance Grade to Citrus Tristeza Virus Induce Dissimilar Volatile Profile in Citrus sinensis and Avoidance Response in the Vector Aphis gossypii Glover. Plants, 11(24), 3426. https://doi.org/10.3390/plants11243426








