Differentiation and Propagation Potential of Arnica montana L. Achenes as a Consequence of the Morphological Diversity of Flowers and the Position of Flower Heads on the Plant
Abstract
1. Introduction
2. Results
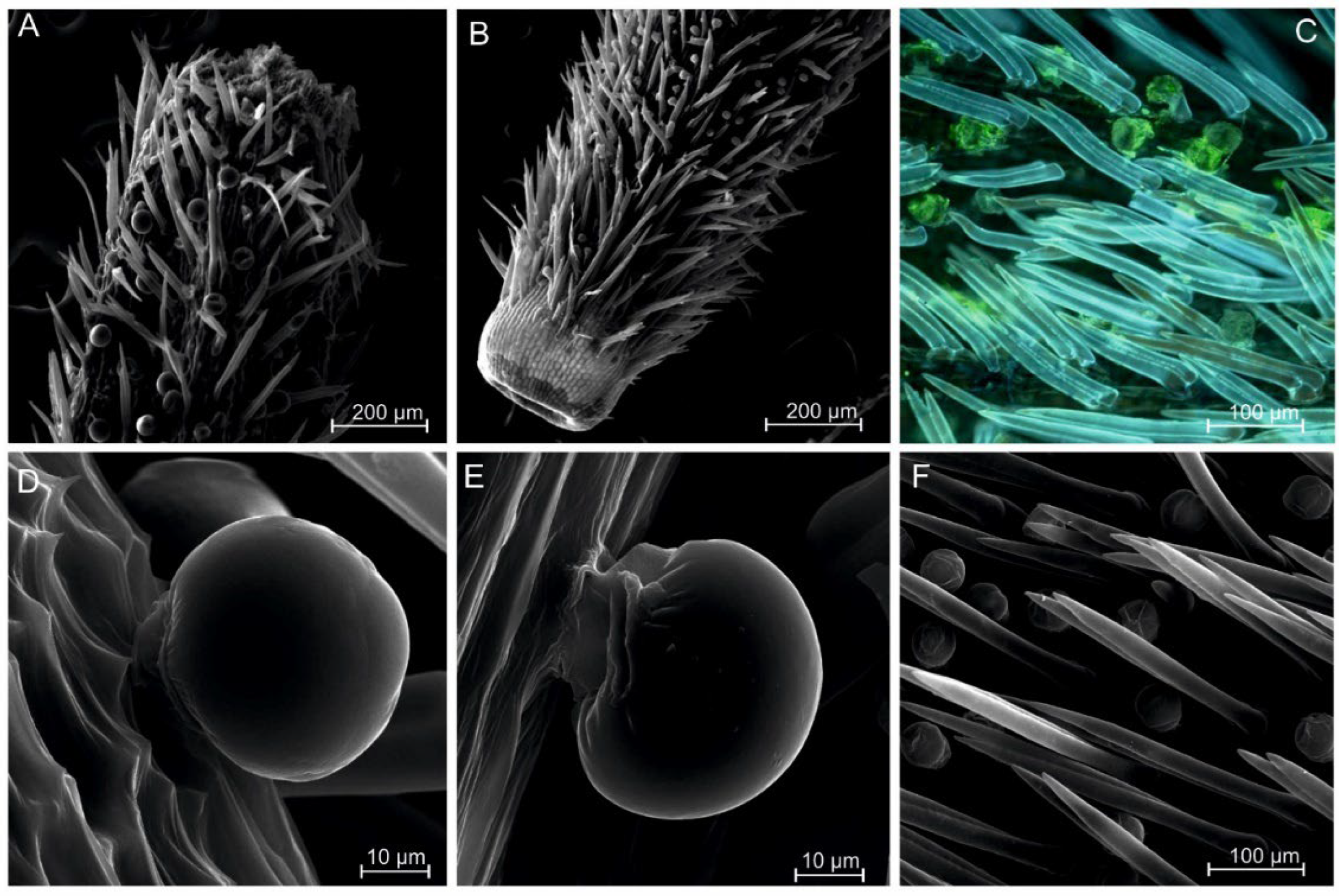
2.1. Morphological Observations of A. montana Achenes
2.2. Infructescence on the Main Stem vs. Infructescence on the Branch Stem
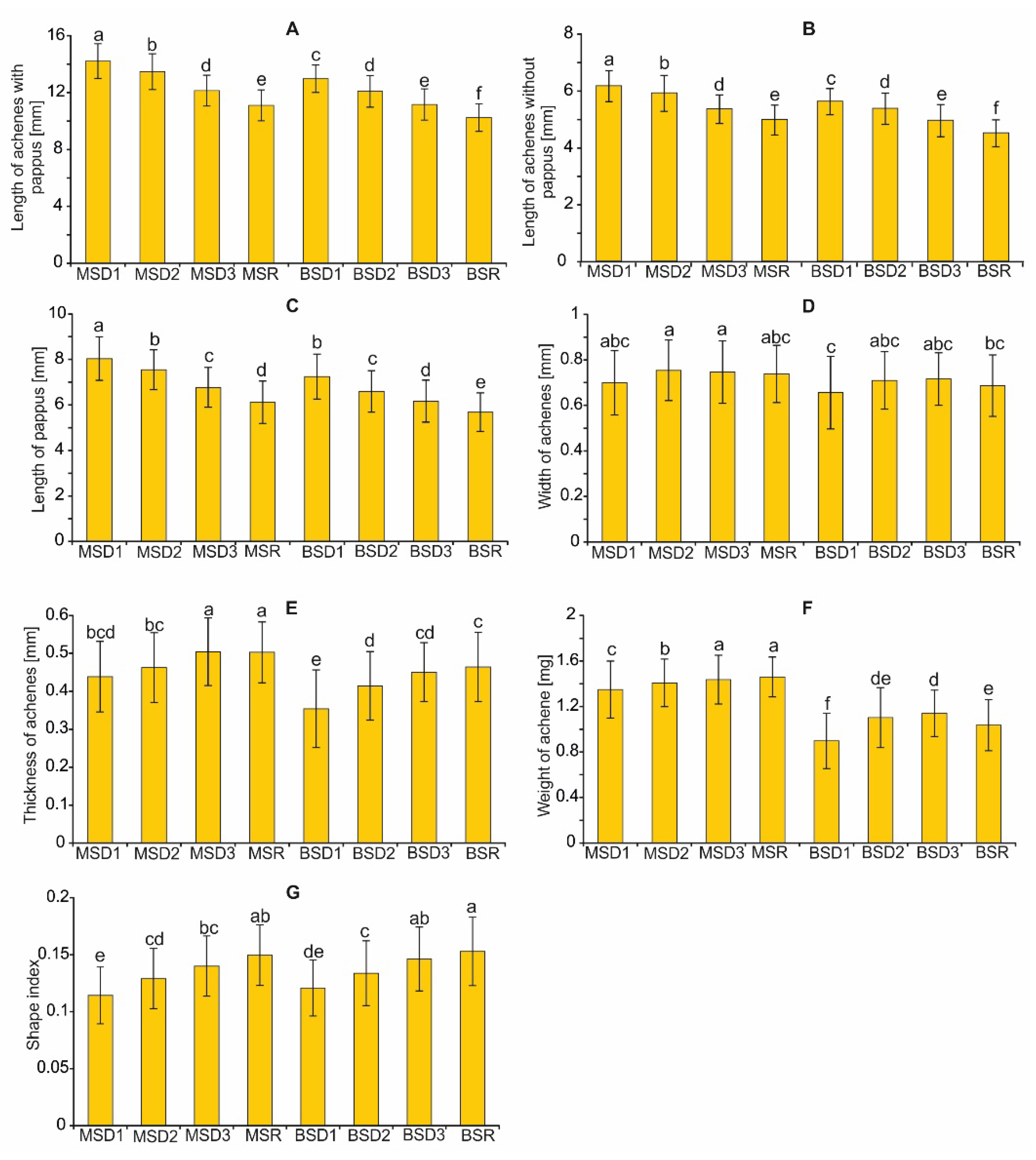
2.3. Morphometric Characteristics of Achenes
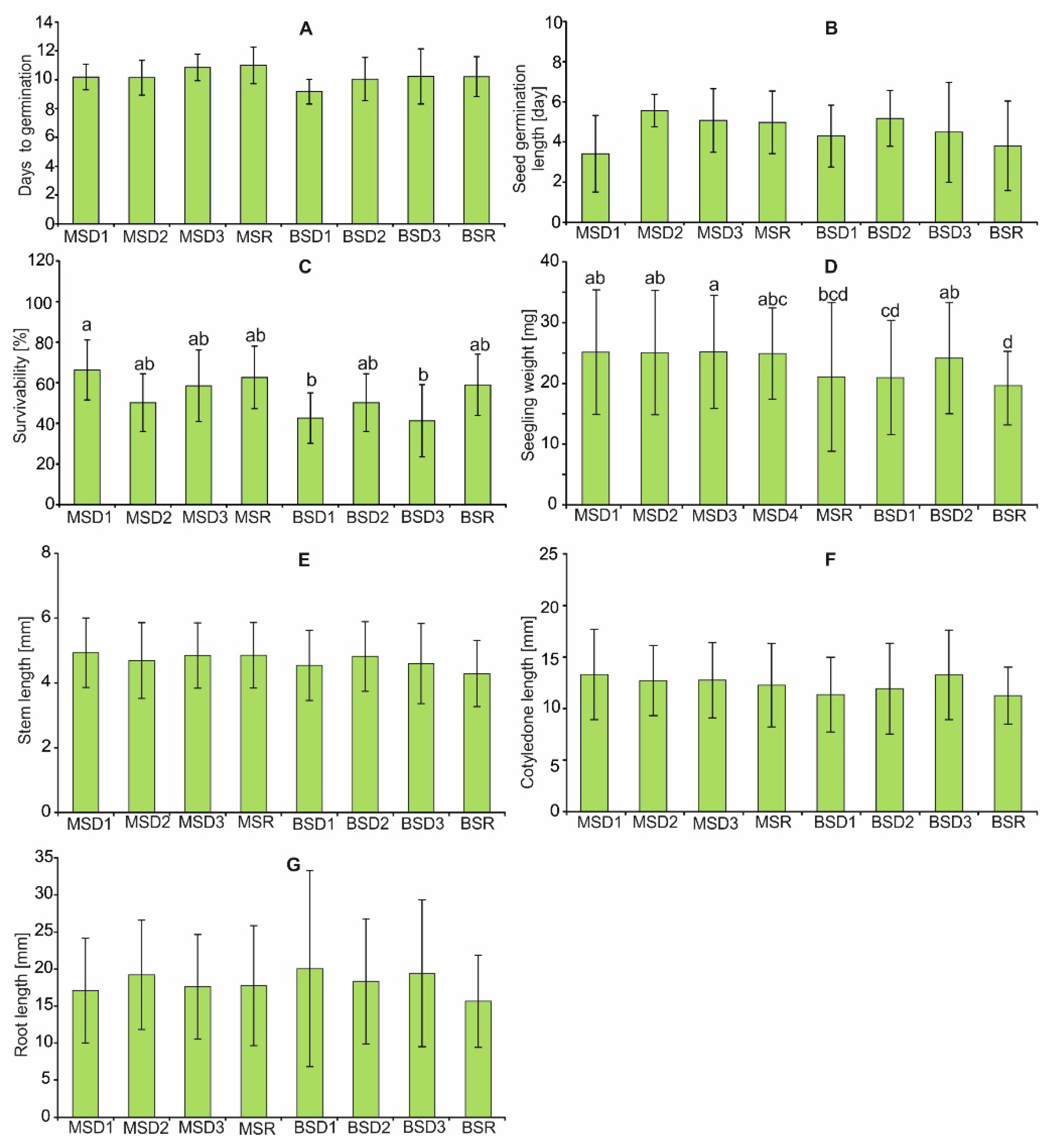
2.4. Germination, Survival and Seedling Characteristic
3. Discussion
4. Materials and Methods
4.1. Research Material
4.2. Scanning Electron Microscopy (SEM)
4.3. Fluorescence Microscopy (FM)
4.4. Light Microscopy
4.5. Morphometric Measurements
4.6. Germination Experiment
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wolfe, L.M. Why does the size of reproductive structures decline through time in Hydrophyllum appendiculatum (Hydrophyllaceae)? developmental constraints vs. resource limitation. Am. J. Bot. 1992, 79, 1286–1290. [Google Scholar] [CrossRef]
- Diggle, P.K. Architectural effects on floral form and function: A review. In Deep Morphology: Towards a Renaissance of Morphology in Plant Systematics; Stuessy, T., Hsrandl, E., Mayer, V., Eds.; Ganter Verlag: Koenigstein, Germany, 2003; pp. 63–80. [Google Scholar]
- Torices, R.; Méndez, M. Fruit size decline from the margin to the center of capitula is the result of resource competition and architectural constraints. Oecologia 2010, 164, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Kliber, A.; Eckert, C.G. Sequential decline in allocation among flowers within inflorescences: Proximate mechanism and adaptive significance. Ecology 2004, 85, 1675–1687. [Google Scholar] [CrossRef]
- Medrano, M.; Guitián, P.; Guitián, J. Patterns of fruit and seed set within inflorescences of Pacratium maritimum Amaryllidaceae: Nonuniform pollination, resource limitation, or architectural effects? Am. J. Bot. 2000, 87, 493–501. [Google Scholar] [CrossRef] [PubMed]
- El-Keblawy, A. Effects of achene dimorphism on dormancy and progeny traits in the two ephemerals Hedypnois cretica and Crepis aspera Asteraceae. Can. J. Bot. 2003, 81, 550–559. [Google Scholar] [CrossRef]
- Imbert, E.; Escarre, J.; Lepart, J. Seed heteromorphism in Crepis sancta Asteraceae: Performance of two morphologies in different environments. Oikos 1997, 79, 325–332. [Google Scholar] [CrossRef]
- Picó, F.X.; Koubek, T. Inbreeding effects on fitness traits in the heterocarpic herb Leontodon autumnalis L. Asteraceae. Acta Oecol. 2003, 24, 289–294. [Google Scholar] [CrossRef]
- Ruíz de Clavijo, E. The ecological significance of fruit heteromorphism in the amphicarphic species Catananche lutea Asteraceae. Int. J. Plant Sci. 1995, 156, 824–833. [Google Scholar] [CrossRef]
- Castillo-Sánchez, I.L.; Figueroa-Castro, D.M. Intra-inflorescence variation in reproductive traits of Conopholis alpina Orobanchaceae: Effect of flower maturation pattern and resource competition. Plant Ecol. 2019, 220, 721–729. [Google Scholar] [CrossRef]
- Lane, M.A. Pollination biology of Compositae. In Compositae: Biology and Utilization; Caligari, P.D.S., Hind, D.J.N., Eds.; Proceedings of the International Compositae Conference, Kew 1996, vol. 2; Kew: Royal Botanical Gardens, UK, 1996; pp. 61–80. [Google Scholar]
- Jeffrey, C. Evolution of Compositae flowers. In Systematics, Evolution, and Biogeography of Compositae; Funk, V., Susanna, A., Stuessy, T.F., Bayer, R.J., Eds.; International Association for Plant Taxonomy: Vienna, Austria, 2009; pp. 131–138. [Google Scholar]
- Bremer, K. Asteraceae, Cladistics and Classification; Timber Press Inc.: Portland, OR, USA, 1994; p. 752. [Google Scholar]
- Andersson, S. Pollinator and nonpollinator selection on ray morphology in Leucanthemum vulgare (oxeye daisy, Asteraceae). Am. J. Bot. 2008, 95, 1072–1078. [Google Scholar] [CrossRef]
- Bello, M.A.; Álvarez, I.; Torices, R.; Fuertes-Aguilar, J. Floral development and evolution of capitulum structure in Anacyclus (Anthemideae, Asteraceae). Ann. Bot. 2013, 112, 1597–1612. [Google Scholar] [CrossRef]
- Chapman, M.A.; Abbott, R.J. Introgression of fitness genes across a ploidy barrier. New Phytol. 2009, 186, 63–71. [Google Scholar] [CrossRef]
- Sun, M.; Ganders, F.R. Outcrossing rates and allozyme variation in rayed and rayless morphs of Bidens pilosa. Heredity 1990, 64, 139–143. [Google Scholar] [CrossRef]
- Torices, R.; Méndez, M.; Gómez, J.M. Where do monomorphic sexual systems fit in the evolution of dioecy? Insights from the largest family of Angiosperms. New Phytol. 2011, 190, 238–248. [Google Scholar] [CrossRef]
- Herrera, C.M. Multiplicity in Unity. Plant Subindividual Variation and Interactions with Animals; University of Chicago Press: Chicago, IL, USA, 2009; p. 448. [Google Scholar]
- Strykstra, R.J.; Pegtel, D.M.; Bergsma, A. Dispersal distance and achene quality of the rare anemochorous species Arnica montana L. Implication for conservation. Acta Bot. Neerl. 1998, 47, 45–56. [Google Scholar]
- Kahmen, S.; Poschlod, P. Population size, plant performance, and genetic variation in the rare plant Arnica montana L. in the Rhön, Germany. Basic Appl. Ecol. 2000, 1, 43–51. [Google Scholar] [CrossRef]
- Maurice, T.; Colling, G.; Muller, S.; Matthies, D. Habitat characteristics, stage structure and reproduction of colline and montane populations of the threatened species Arnica montana. Plant Ecol. 2012, 213, 831–842. [Google Scholar] [CrossRef]
- Sugier, P.; Kołos, A.; Wołkowycki, D.; Sugier, D.; Plak, A.; Sozinov, O. Evaluation of species inter-relations and soil conditions in Arnica montana L. habitats. A step towards active protection of endangered and high-valued medicinal plant species in NE Poland. Acta Soc. Bot. Pol. 2018, 87, 3592. [Google Scholar] [CrossRef]
- Sugier, P.; Sugier, D.; Sozinov, O.; Kołos, A.; Wołkowycki, D.; Plak, A.; Budnyk, O. Characteristics of plant communities, population features, and edaphic conditions of Arnica montana L. populations in pine forests of mid-eastern Europe. Acta Soc. Bot. Pol. 2019, 88, 3640. [Google Scholar] [CrossRef]
- Kowalski, R.; Sugier, D.; Sugier, P.; Kołodziej, B. Evaluation of the chemical composition of essential oils with respect to the maturity of flower heads of Arnica montana L. and Arnica chamissonis Less. cultivated for industry. Ind. Crops Prod. 2015, 76, 857–865. [Google Scholar] [CrossRef]
- Merfort, I.; Wendisch, D. Flavonoid glucuronides from the flowers of Arnica montana. Planta Med. 1988, 54, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.B.; Burgess, E.J.; Rodriguez, M.A.; Romero Franco, R.; López Morquero, E.; Smallfield, B.M.; Joyce, N.I.; Litrtlejohn, R.P. Sesquiterpene lactones in Arnica montana helenalin and dihydrohelenalin chemotypes in Spain. Planta Med. 2009, 75, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Sugier, P.; Jakubowicz-Gil, J.; Sugier, D.; Kowalski, R.; Gawlik-Dziki, U.; Kołodziej, B.; Dziki, D. Chemical characteristics and anticancer activity of essential oil from Arnica montana L. rhizomes and roots. Molecules 2020, 25, 1284. [Google Scholar] [CrossRef] [PubMed]
- Sugier, D.; Sugier, P.; Jakubowicz-Gil, J.; Winiarczyk, K.; Kowalski, R. Essential oil from Arnica montana L. achenes: Chemical characteristics and anticancer activity. Molecules 2019, 24, 4158. [Google Scholar] [CrossRef] [PubMed]
- Sugier, D.; Sugier, P.; Kowalski, R.; Kołodziej, B.; Olesińska, K. Foliar boron fertilization as factor affecting the essential oil content and yield of oil components from flower heads of Arnica montana L. and Arnica chamissonis Less. cultivated for industry. Ind. Crops Prod. 2017, 109, 587–597. [Google Scholar] [CrossRef]
- Gaspar, A.; Cracinescu, O.; Trif, M.; Moisei, M.; Moldovan, L. Antioxidant and anti-inflammatory properties of active compounds from Arnica montana L. Rom. Biotech. Lett. 2014, 19, 9353–9365. [Google Scholar]
- Gawlik-Dziki, U.; Świeca, M.; Sugier, D.; Cichocka, J. Comparison of in vitro lipoxygenase, xanthine oxidase inhibitory and antioxidant activity of Arnica montana and Arnica chamissonis tinctures. Acta Sci. Pol. Hortorum Cultus 2011, 10, 15–27. [Google Scholar]
- Jäger, C.; Hrenn, A.; Zwingmann, J.; Sute, A.; Merfort, I. Phytomedicines prepared from Arnica flowers inhibit the transcription factors AP-1 and NF-kappaB and modulate the activity of MMP1 and MMP13 in human and bovine chondrocytes. Planta Med. 2009, 75, 1319–1325. [Google Scholar] [CrossRef]
- Kriplani, P.; Guarve, K.; Baghael, U.S. Arnica montana L.—A plant of healing. Rev. J. Pharm. Pharmacol. 2017, 69, 925–945. [Google Scholar] [CrossRef]
- Macêdo, S.B.; Ferreira, L.R.; Perazzo, F.F.; Tavares Carvalho, J.C. Anti-inflammatory activity of Arnica montana 6cH. Preclinical study in animals. Homeopathy 2004, 93, 84–87. [Google Scholar] [CrossRef]
- Nieto-Trujillo, A.; Cruz-Sosa, F.; Luria-Pérez, R.; Gutiérrez-Rebolledo, G.A.; Román-Guerrero, A.; Burrola-Aguilar, C.; Zepeda-Gómez, C.; Estrada-Zúñiga, M.E. Arnica montana cell culture establishment, and assessment of its cytotoxic, antibacterial, α-amylase inhibitor, and antioxidant in vitro bioactivities. Plants 2021, 10, 2300. [Google Scholar] [CrossRef]
- Žitek, T.; Postružnik, V.; Knez, Ž.; Golle, A.; Dariš, B.; Marevci, M.K. Arnica montana L. supercritical extraction optimization for antibiotic and anticancer activity. Front. Bioeng. Biotechnol. 2022, 10, 897185. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Sugier, D.; Cichocka, J. Seeds of Arnica montana and Arnica chamissonis as a potential source of natural antioxidants. Herba Pol. 2009, 55, 60–71. [Google Scholar]
- Aiello, N.; Scartezzini, F.; Vender, C. Cultivation trial of Arnica montana wild accessions results of the second year. Acta Hortic. 2012, 955, 253–257. [Google Scholar] [CrossRef]
- Balabanova, V.I.; Vitkova, A.; Zheleva-Dimitrova, D. Flower yield of Arnica sp. cultivated in two floristic regions in Bulgaria. J. Agric. Ecol. Res. Int. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Pljevljakušić, D.; Janković, T.; Jelačić, S.; Novaković, M.; Menković, N.; Beatović, D.; Dajić-Stevanović, Z. Morphological and chemical characterization of Arnica montana L. under different cultivation models. Ind. Crops Prod. 2014, 52, 233–244. [Google Scholar] [CrossRef]
- Sugier, D.; Sugier, P.; Gawlik-Dziki, U. Propagation and introduction of Arnica montana L. into cultivation. A step to reduce the pressure on endangered and high-valued medicinal plant species. Sci. World J. 2013, 2013, 414363. [Google Scholar] [CrossRef]
- Leoni, V.; Borgonovo, G.; Giupponi, L.; Bassoli, A.; Pedrali, D.; Zuccolo, M.; Rodari, A.; Giorgi, A. Comparing wild and cultivated Arnica montana L. from the Italian Alps to explore the possibility of sustainable production using local seeds. Sustainability 2021, 13, 3382. [Google Scholar] [CrossRef]
- Hollmann, V.; Donath, T.W.; Grammel, F.; Himmighofen, T.; Zerahn, U.; Leyer, I. From nutrients to competition processes. Habitat specific threats to Arnica montana L. populations in Hesse, Germany. PLoS ONE 2020, 15, e0233709. [Google Scholar] [CrossRef]
- Blachnik, T.; Saller, R. In situ-Vermehrung von Arnica montana—Ergebnisse und Handlungsempfehlungen für die Artenschutz-Praxis. Anliegen Natur 2015, 37, 31–41. [Google Scholar]
- Balabanova, V.; Vitkova, A.; Zheleva-Dimitrova, D. Comparative study of germination and seed antioxidant activity of Arnica montana L. and Arnica chamissonis Less. Asteraceae. Comptes Rendus L’académie Bulg. Sci. Sci. Mathématiques Nat. 2013, 66, 1261–1268. [Google Scholar]
- Vitkova, A.; Balabanova, V. Trials on introduction and cultivation of Arnica montana L. in Bulgaria. Biol. Nyssana 2018, 9, 21–29. [Google Scholar]
- Fahn, A. Structure and function of secretory cells. In Plant Trichomes; Hallahan, D.L., Gray, J.C., Eds.; Academic Press: London, UK, 2000; pp. 37–76. [Google Scholar]
- Werker, E. Trichome diversity and development. In Plant Trichomes; Hallahan, D.L., Gray, J.C., Eds.; Academic Press: London, UK, 2000; pp. 1–36. [Google Scholar]
- Wagner, G.J.; Wang, E.; Shepherd, R. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann. Bot. 2004, 931, 3. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Tooker, J.; Peiffer, M.; Chung, S.H.; Felton, G.W. Role of trichomes in defense against herbivores: Comparison of herbivore response to woolly and hairless trichome mutants in tomato Solanum lycopersicum. Planta 2012, 236, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. Glandular trichomes—A focal point of chemical and structural interactions. Int. J. Plant Sci. 1994, 155, 617–620. [Google Scholar] [CrossRef]
- Peiffer, M.; Tooker, J.F.; Luthe, D.S.; Felton, G.W. Plants on early alert: Glandular trichomes as sensors for insect herbivores. New Phytol. 2009, 184, 644–656. [Google Scholar] [CrossRef]
- Dalin, P.; Bjökman, C. Adult beetle grazing induces willow trichome defence against subsequent larval feeding. Oecologia 2002, 134, 112–118. [Google Scholar] [CrossRef]
- Sletvold, N.; Huttunen, P.; Handley, R.; Kärkkäinen, K.; Ågren, J. Cost of trichome production and resistance to a specialist insect herbivore in Arabidopsis lyrata. Evol. Ecol. 2010, 24, 1307–1319. [Google Scholar] [CrossRef]
- Pierce, S.; Maxwell, K.; Griffiths, H.; Winter, K. Hydrophobic trichome layers and epicuticular wax powders in Bromeliaceae. Am. J. Bot. 2001, 88, 1371–1389. [Google Scholar] [CrossRef]
- Levizou, E.; Drilias, P.; Psaras, G.K.; Manetas, Y. Nondestructive assessment of leaf chemistry and physiology through spectral reflectance measurements may be misleading when changes in trichome density co-occur. New Phytol. 2005, 165, 463–472. [Google Scholar] [CrossRef]
- Savé, R.; Biel, C.; de Herralde, F. Leaf pubescence, water relations and chlorophyll fluorescence in two subspecies of Lotus creticus L. Biol. Plant. 2000, 43, 239–244. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Correia, C.M.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Lopes, J.I.; Torres-Pereira, J.M.G. Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiol. 2004, 24, 233–239. [Google Scholar] [CrossRef]
- Tan, J.; Walford, S.A.; Dennis, E.S.; Llewellyn, D. Trichomes control flower bud shape by linking together young petals. Nat. Plants 2016, 27, 1–5. [Google Scholar] [CrossRef]
- Tiwari, S.C.; Wilkins, T.A. Cotton Gossypium hirsutum seed trichomes expand via diffuse growing mechanism. Can. J. Bot. 1995, 735, 746–757. [Google Scholar] [CrossRef]
- Balabanova, V.; Vitkova, A.; Tashev, A. A study of seed propagation of Arnica montana L. (Asteraceae). Rast. Nauk. 2009, 46, 398–401. [Google Scholar]
- Aiello, N.; Carlini, A.; Fusani, P.; Scartezzini, F. Seed yield and germination characteristics of wild accessions of Arnica montana L. from Trentino (Italy). J. Appl. Res. Med. Aromat. Plants 2014, 1, e30–e33. [Google Scholar] [CrossRef]
- Yankova-Tsvetkova, E.; Yurukova-Grancharova, P.; Baldjiev, G.; Vitkova, A. Embryological features, pollen and seed viability of Arnica montana (Asteraceae)—A threatened endemic species in Europe. Acta Bot. Croat. 2016, 75, 39–44. [Google Scholar] [CrossRef]
- Luijten, S.H.; Oostermeijer, J.G.B.; van Leeuwen, N.C.; den Nijs, J.C.M. Reproductive success and clonal genetic structure of the rare Arnica montana (Compositae) in The Netherlands. Plant Syst. Evol. 1996, 210, 15–30. [Google Scholar] [CrossRef]
- Luijten, S.H. Reproduction and Genetics of Fragmented Plant Populations. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 2001. [Google Scholar]
- Torices, R.; Agudo, A.; Álvarez, I. Not only size matters: Achene morphology affects time of seedling emergence in three heterocarpic species of Anacyclus (Anthemideae, Asteraceae). An. Jard. Bot. Madr. 2013, 70, 48–55. [Google Scholar] [CrossRef]
- Diggle, P.K. Architectural effects and the interpretation of patterns of fruit and seed development. Annu. Rev. Ecol. Syst. 1995, 26, 531–532. [Google Scholar] [CrossRef]
- Heywood, V.H.; Harborne, J.B.; Turner, B.L. (Eds.) The Biology and Chemistry of the Compositae; Academic Press: London, UK, 1977. [Google Scholar]
- Stephenson, A.G. An evolutionary examination of the floral display of Catalpa speciosa (Bignoniaceae). Evolution 1979, 33, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Bawa, K.S.; Webb, C.J. Flower, fruit and seed abortion in tropical forest trees: Implications for the evolution of paternal and maternal reproductive patterns. Am. J. Bot. 1984, 71, 736–751. [Google Scholar] [CrossRef]
- Nakamura, R.R. Maternal investment and fruit abortion in Phaseolus vulgaris. Am. J. Bot. 1986, 73, 1049–1057. [Google Scholar] [CrossRef]
- Lee, T.D. Patterns of fruit and seed production. In Plant Reproductive Ecology: Patterns and Strategies; Lovett Doust, J., Lovett Doust, L., Eds.; Oxford University Press: New York, NY, USA, 1988; pp. 179–202. [Google Scholar]
- Thomson, J.D. Deployment of ovules and pollen among flowers within inflorescences. Evol. Trends Plants 1989, 3, 65–68. [Google Scholar]
- Guittian, N.J. Why Prunus mahaleb (Rosaceae) produces more flowers than fruits. Am. J. Bot. 1993, 80, 1305–1309. [Google Scholar] [CrossRef]
- Obeso, J.R. Seed mass variation in the perennial herb Asphodelus albus: Sources of variation and position effect. Oecologia 1993, 93, 571–575. [Google Scholar] [CrossRef]
- Brunet, J. Male reproductive success and variation in fruit and seed set in Aquilegia caerulea (Ranunculaceae). Ecology 1996, 77, 2458–2471. [Google Scholar] [CrossRef]
- Guittian, N.J.; Navarro, L. Allocation of reproductive resources within inflorescences of Petrocoptis grandiflora (Caryophyllaceae). Can. J. Bot. 1996, 74, 1482–1486. [Google Scholar] [CrossRef]
- Navarro, L. Fruit-set and seed weight variation in Anthyllis vulneraria subsp. vulgaris (Fabaceae). Plant Syst. Evol. 1996, 201, 139–148. [Google Scholar] [CrossRef]
- Mendez, M. Sources of variation in seed mass in Arum italicum. Int. J. Plant Sci. 1997, 158, 298–305. [Google Scholar] [CrossRef]
- Thomson, J.D. Pollination and seed set in Diervilla lonicera (Caprifoliaceae): Temporal patterns of flower and ovule development. Am. J. Bot. 1985, 72, 737–740. [Google Scholar] [CrossRef]
- Goldingay, R.L.; Whelan, R.J. The influence of pollinators on fruit positioning in the Australian shrub Telopea speciosissima (Proteaceae). Oikos 1993, 68, 501–509. [Google Scholar] [CrossRef]
- Brunet, J.; Charlesworth, D. Floral sex allocation in sequentially blooming plants. Evolution 1995, 49, 70–79. [Google Scholar]
- Solomon, B.P. Patterns of pre- and postfertilization resource allocation within an inflorescence: Evidence for interovary competition. Am. J. Bot. 1988, 75, 1074–1079. [Google Scholar] [CrossRef]
- Berry, P.E.; Calvo, R.N. Pollinator limitation and position dependent fruit set in the high Andean orchid Myrosmodes cochleare (Orchidaceae). Plant Syst. Evol. 1991, 174, 93–101. [Google Scholar] [CrossRef]
- Herrera, J. Allocation of reproductive resources within and among inflorescences of Lavandula stoechas (Lamiaceae). Am. J. Bot. 1991, 78, 789–794. [Google Scholar] [CrossRef]
- Ashman, T.L.; Hitchens, M.S. Dissecting the causes of variation in intra-inflorescence allocation in a sexually polymorphic species, Fragaria virginiana (Rosaceae). Am. J. Bot. 2000, 87, 197–204. [Google Scholar] [CrossRef]
- Grubb, P.J.; Coomes, D.A. Seed mass and nutrient content in nutrient starved tropical rainforest in Venezuela. Seed Sci. Res. 1997, 7, 269–280. [Google Scholar] [CrossRef]
- Wulff, R.D. Seed size variation in Desmodium paniculatum. I. Factors affecting seed size. J. Ecol. 1986, 74, 87–97. [Google Scholar] [CrossRef]
- McGinley, M.A.; Temme, D.H.; Geber, M.A. Parental investment in offspring in variable environments: Theoretical and empirical considerations. Am. Nat. 1987, 130, 370–398. [Google Scholar] [CrossRef]
- Khan, M.L. Effects of seed mass on seedling success in Artocarpus heterophyllus L., a tropical tree species of north-east India. Acta Oecol. 2004, 25, 103–110. [Google Scholar] [CrossRef]
- Harper, J.L. Population Biology of Plants; Academic Press: London, UK, 1977. [Google Scholar]
- Meyer, S.E.; Carlson, S.L. Achene mass variation in Ericameria nauseosus (Asteraceae) in relation to dispersal ability and seedling fitness. Funct. Ecol. 2001, 15, 274–281. [Google Scholar] [CrossRef]
- Talbot, M.J.; White, R.G. Methanol fixation of plant tissue for scanning electron microscopy improves preservation of tissue morphology and dimensions. Plant Methods 2013, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, K.; Josefsson, B.; Nyquist, G. Analysis of Lignin Products by Fluorescence Spectroscopy. Holzforschung 1978, 32, 27–32. [Google Scholar] [CrossRef]
- Kovach, W. MVSP—A Multivariate Statistical Package for Windows; Version 3.1; Kovach Computing Services: Wales, UK, 1999. [Google Scholar]


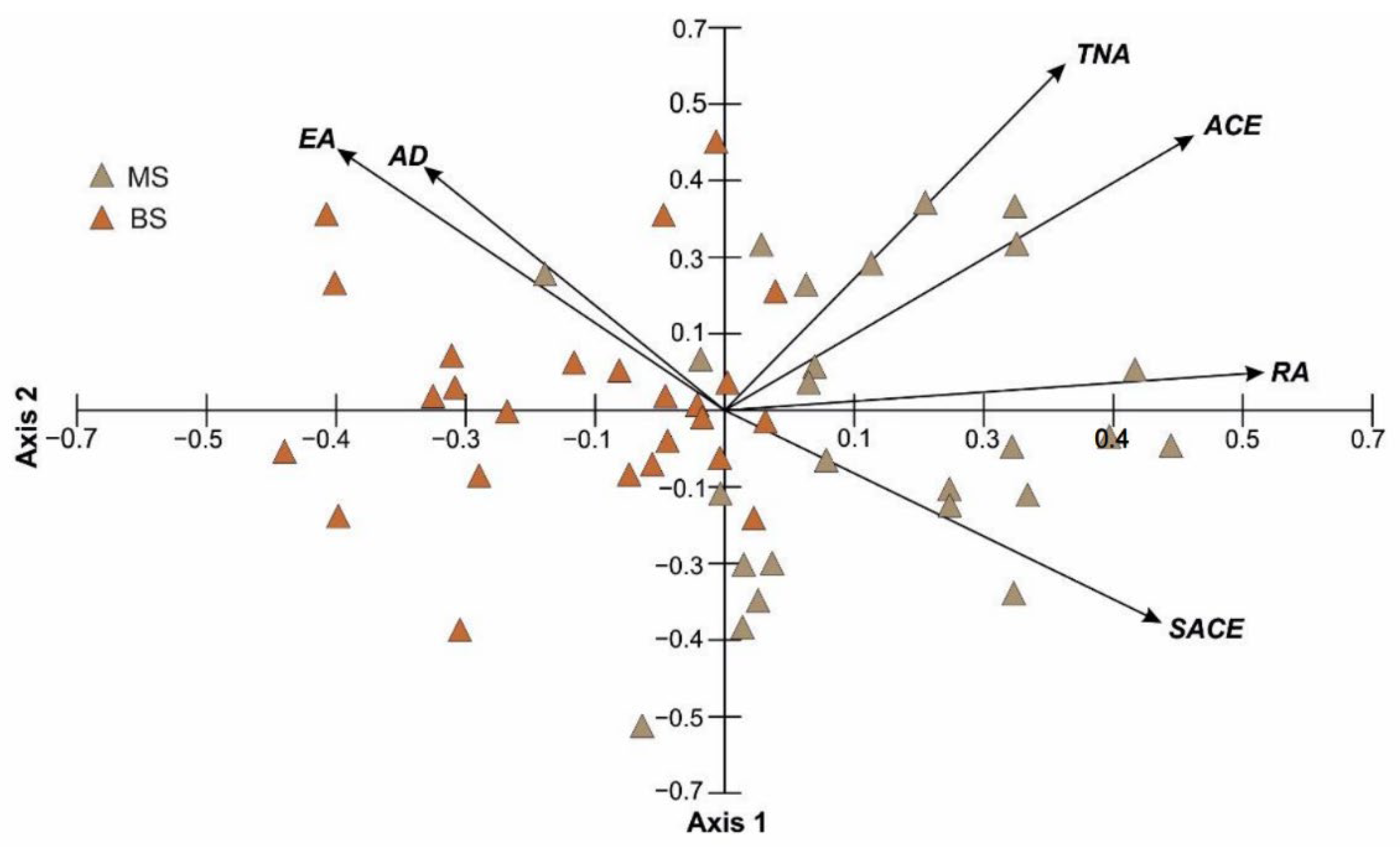

| Axis 1 | Axis 2 | |
|---|---|---|
| Eigenvalues | 2.35 | 2.26 |
| Percentage | 39.21 | 37.69 |
| Cum. Percentage | 39.21 | 76.90 |
| TNA | 0.331 | 0.57 |
| ACE | 0.456 | 0.452 |
| EA | −0.377 | 0.43 |
| SACE | 0.424 | −0.349 |
| RA | 0.524 | 0.062 |
| AD | −0.293 | 0.401 |
| LAP | LA | PL | WA | TA | AW | I | |
|---|---|---|---|---|---|---|---|
| API | F = 268.72 | F = 184.64 | F = 135.41 | F = 7.09 | F = 25.54 | F = 36.51 | F = 40.086 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| PIP | F = 202.48 | F = 168.77 | F = 115.99 | F = 19.74 | F = 24.19 | F = 782.09 | F = 0.463 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.496 | |
| API × PIP | F = 2.47 | F = 0.81 | F = 3.07 | F = 0.07 | F = 0.23 | F = 1.76 | F = 0.521 |
| p = 0.060 | p = 0.485 | p < 0.05 | p = 0.786 | p = 0.878 | p = 0.153 | p = 0.668 |
| PW | EW | EW/AW | G | |
|---|---|---|---|---|
| API | F = 1.62 | F = 5.74 | F = 2.74 | F = 6.97 |
| p = 0.185 | p < 0.001 | p < 0.05 | p < 0.001 | |
| PIP | F = 101.94 | F = 25.78 | F = 0.22 | F = 1.26 |
| p < 0.001 | p < 0.001 | p = 0.639 | p = 0.265 | |
| PIP × API | F = 3.88 | F = 2.19 | F = 1.39 | F = 2.92 |
| p < 0.01 | p = 0.088 | p = 0.245 | p < 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugier, P.; Rysiak, A.; Sugier, D.; Winiarczyk, K.; Wołkowycki, D.; Kołos, A. Differentiation and Propagation Potential of Arnica montana L. Achenes as a Consequence of the Morphological Diversity of Flowers and the Position of Flower Heads on the Plant. Plants 2022, 11, 3424. https://doi.org/10.3390/plants11243424
Sugier P, Rysiak A, Sugier D, Winiarczyk K, Wołkowycki D, Kołos A. Differentiation and Propagation Potential of Arnica montana L. Achenes as a Consequence of the Morphological Diversity of Flowers and the Position of Flower Heads on the Plant. Plants. 2022; 11(24):3424. https://doi.org/10.3390/plants11243424
Chicago/Turabian StyleSugier, Piotr, Anna Rysiak, Danuta Sugier, Krystyna Winiarczyk, Dan Wołkowycki, and Aleksander Kołos. 2022. "Differentiation and Propagation Potential of Arnica montana L. Achenes as a Consequence of the Morphological Diversity of Flowers and the Position of Flower Heads on the Plant" Plants 11, no. 24: 3424. https://doi.org/10.3390/plants11243424
APA StyleSugier, P., Rysiak, A., Sugier, D., Winiarczyk, K., Wołkowycki, D., & Kołos, A. (2022). Differentiation and Propagation Potential of Arnica montana L. Achenes as a Consequence of the Morphological Diversity of Flowers and the Position of Flower Heads on the Plant. Plants, 11(24), 3424. https://doi.org/10.3390/plants11243424






