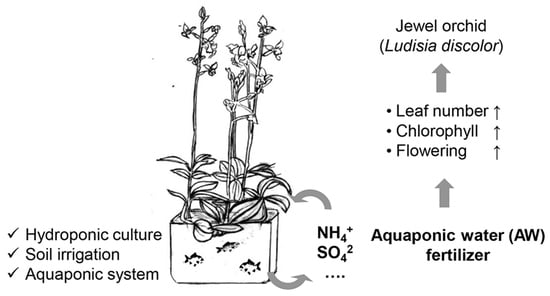
Jewel Orchid’s Biology and Physiological Response to Aquaponic Water as a Potential Fertilizer
Abstract
1. Introduction
2. Results and Discussion
2.1. Taxonomic Identification of L. discolor via DNA Barcoding
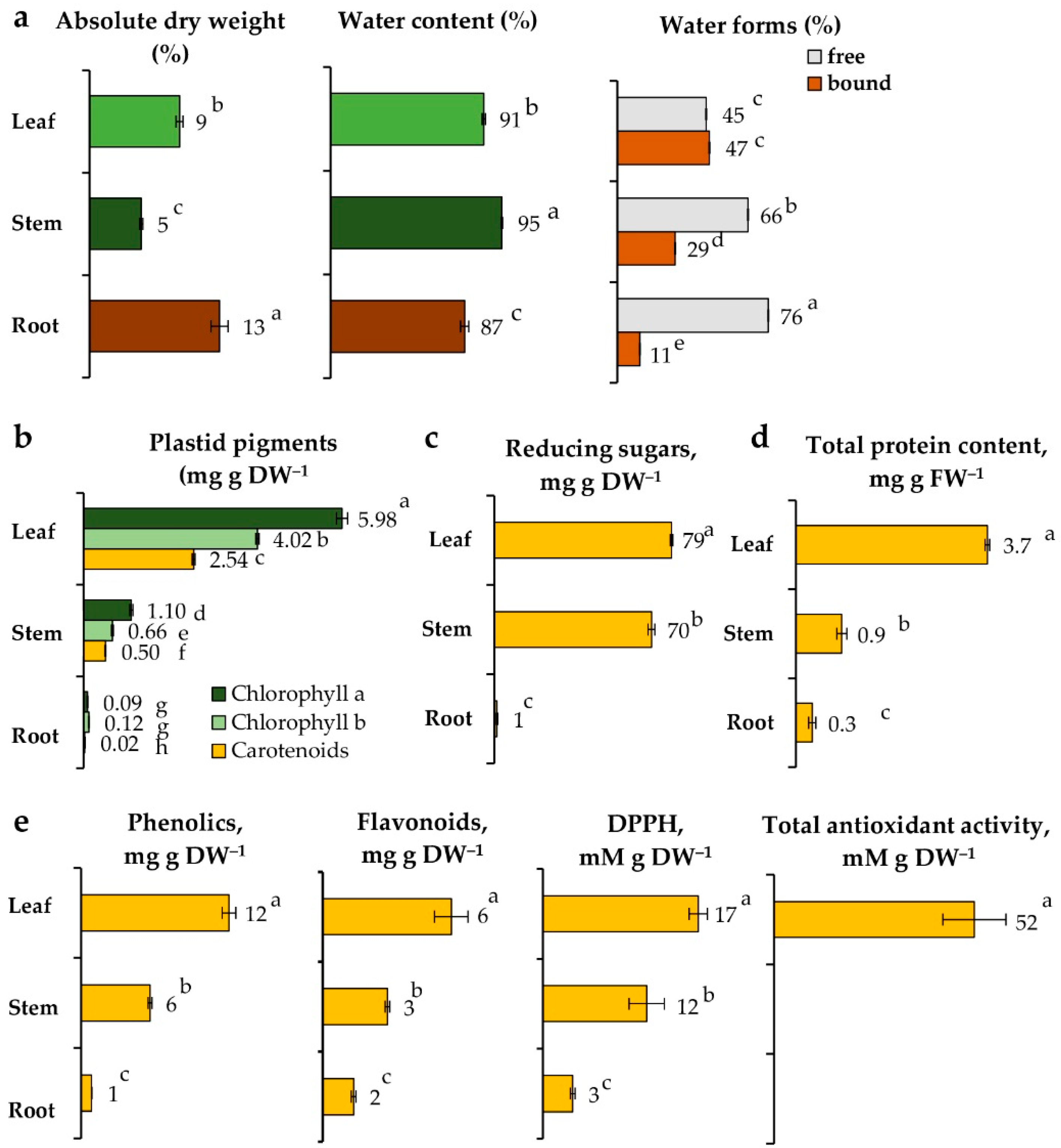
2.2. Biological Traits of Vegetative Organs of L. discolor Grown in Soil
2.2.1. Water Content
2.2.2. Plant Anatomy
2.2.3. Pigments, Reducing Sugars, Proteins, and Antioxidants
2.2.4. Macro- and Microelements
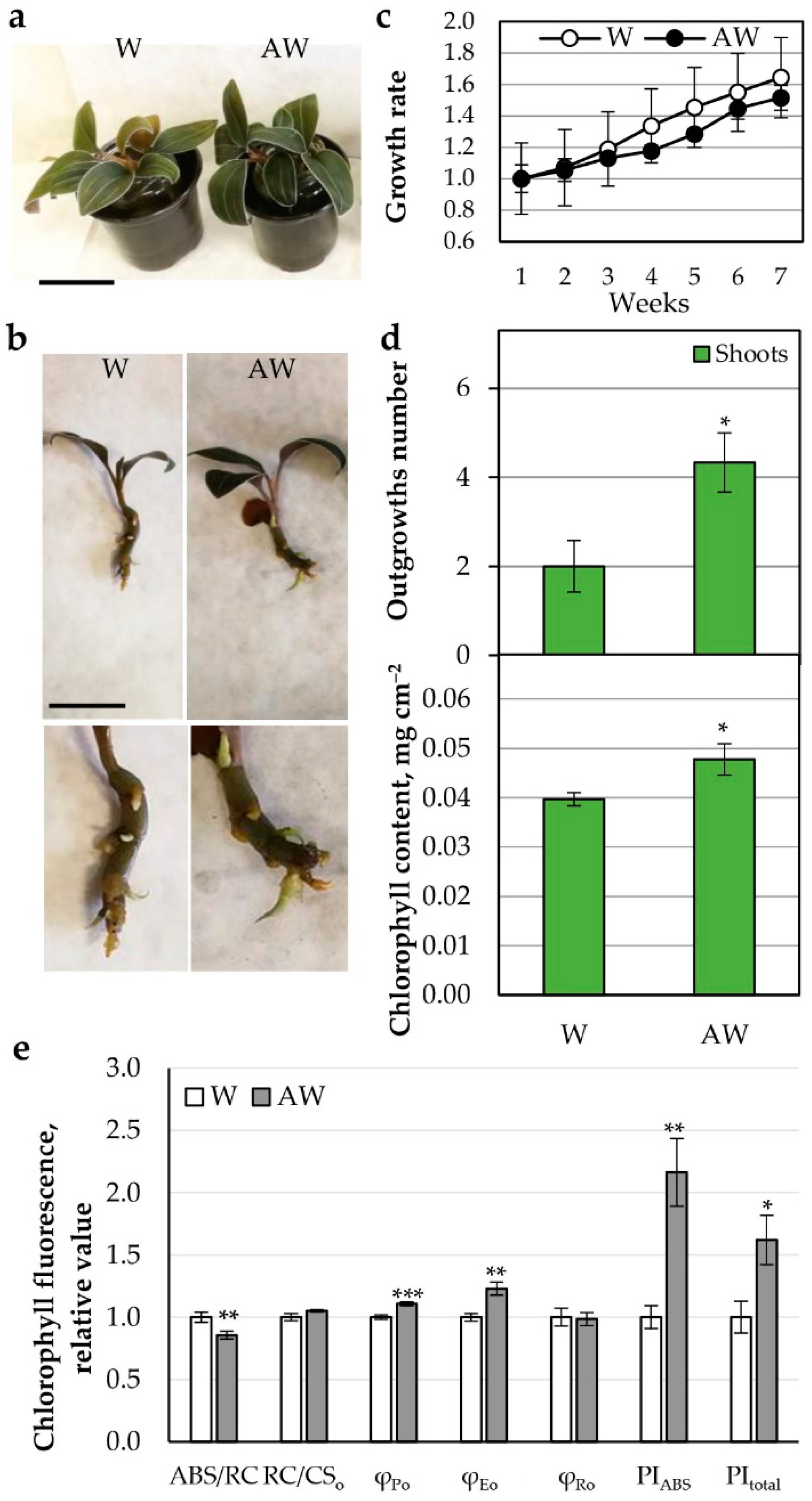
2.3. Aquaponic Water (AW) as a Fertilizer for L. discolor
2.3.1. AW Characteristics
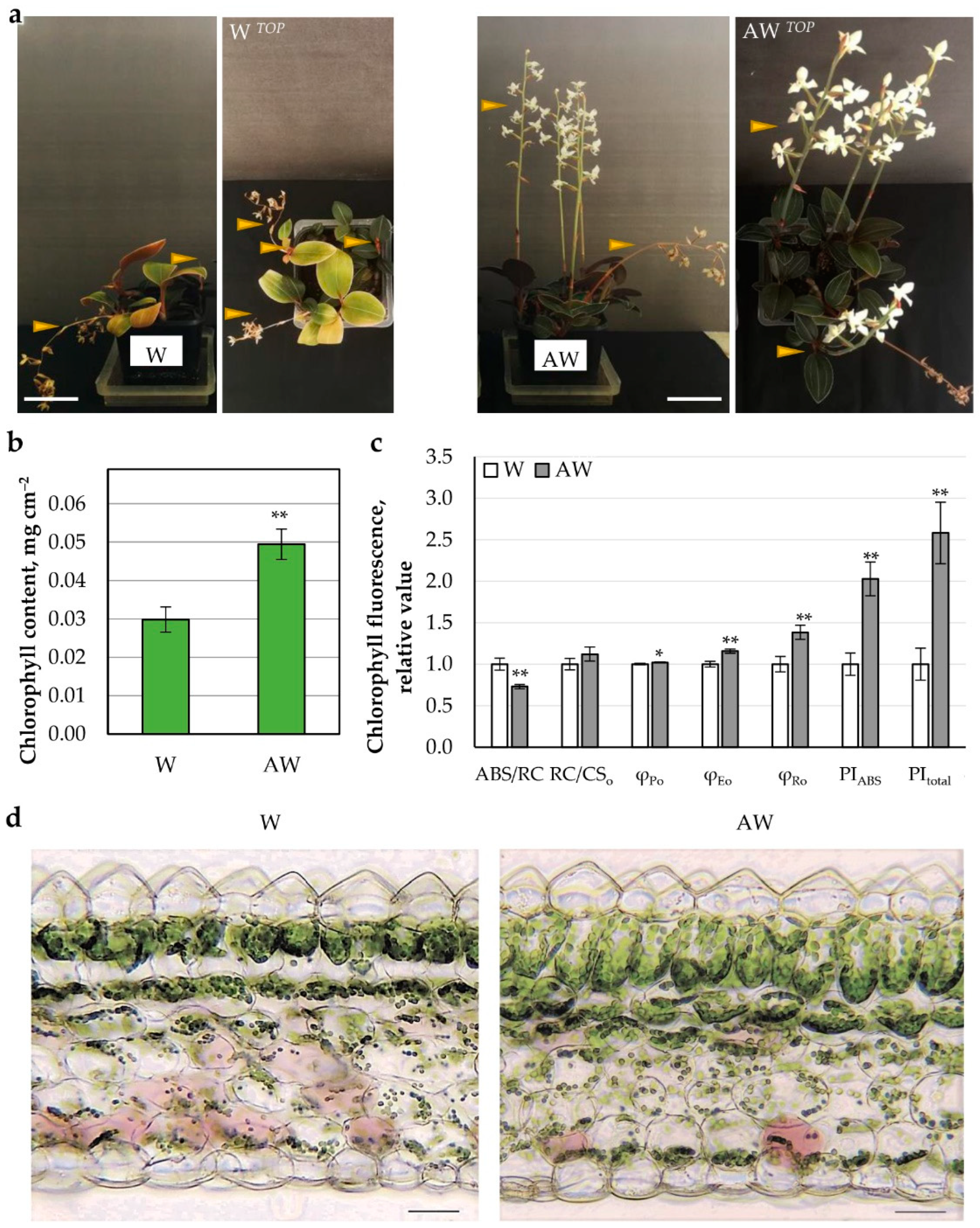
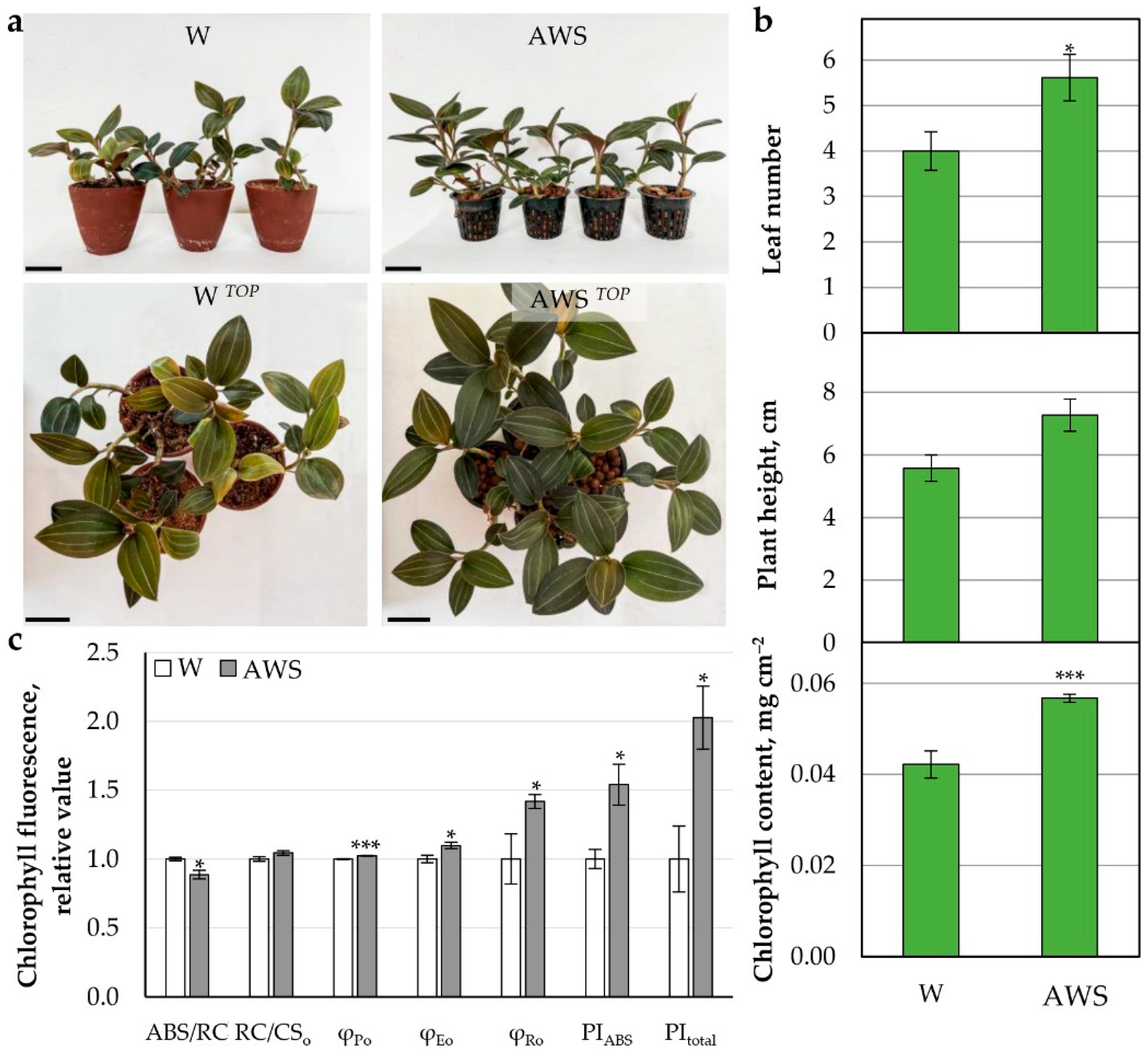
2.3.2. AW Effect on Growth and Photosynthetic Parameters of L. discolor Plants
AW Week 4 Hydroponics
AW Week 4 Fertilized Soil
AW System (AWS)
3. Conclusions
4. Materials and Methods
4.1. Plant Material and Cultivation Conditions
4.2. DNA Extraction and DNA Barcoding Analysis
4.3. Physiological Parameters
4.3.1. Water Content
4.3.2. Histological Analyses
4.3.3. Biochemical Analyses
4.3.4. Plant Growth
4.3.5. Flow Cytometry
4.3.6. Mineral Element Assay
4.4. Chlorophyll Content and Fluorescense
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramírez, S.R.; Gravendeel, B.; Singer, R.B.; Marshall, C.R.; Pierce, N.E. Dating the origin of the Orchidaceae from a fossil orchid with its pollinator. Nature 2007, 448, 1042–1045. [Google Scholar] [CrossRef] [PubMed]
- Krumov, N.P.; Hristova, V.A.; Bonchev, G.N.; Nedelcheva, A.M.; Tomov, A.A.; Zhiponova, M.K. Review on Biological and Biotechnological Characteristics of the Terrestrial Orchid Ludisia discolor. Annu. Sofia Univ. “St. Kliment Ohridski” Fac. Biol. Book 2—Bot. 2022, 106, 13–30. [Google Scholar]
- Ye, B.; Wu, Y.; Zhai, X.; Zhang, R.; Wu, J.; Zhang, C.; Rahman, K.; Qin, L.; Han, T.; Zheng, C. Beneficial effects of endophytic fungi from the Anoectochilus and Ludisia species on the growth and secondary metabolism of Anoectochilus roxburghii. ACS Omega 2020, 5, 3487–3497. [Google Scholar] [CrossRef] [PubMed]
- Tomov, A.; Petrova, D.; Yocheva, L.; Georgieva, Z.; Karcheva, Z.; Chaneva, G. Antioxidant status of hydroponically and aquaponically grown plant species. Oxid. Commun. 2021, 44, 397–408. [Google Scholar]
- Tsoumalakou, E.; Mente, E.; Kormas, K.A.; Katsoulas, N.; Vlahos, N.; Kapsis, P.; Levizou, E. Precise monitoring of lettuce functional responses to minimal nutrient supplementation identifies aquaponic system’s nutrient limitations and their time-course. Agriculture 2022, 12, 1278. [Google Scholar] [CrossRef]
- Bethe, L.A. Effect of foliar spray of compost tea on water spinach (Ipomoea aquatica) in Aquaponic System. Master’s Thesis, Department of Aquaculture, Bangladesh Agricultural University, Mymensingh, Bangladesh, 2014. [Google Scholar]
- Azad, K.N.; Salam, M.A.; Azad, K.N. Aquaponics in Bangladesh: Current status and future prospects. J. Biosci. Agric. Res. 2016, 7, 669–677. [Google Scholar] [CrossRef]
- Oladimeji, A.S.; Olufeagba, S.O.; Ayuba, V.O.; Sololmon, S.G.; Okomoda, V.T. Effects of different growth media on water quality and plant yield in a catfish-pumpkin aquaponics system. J. King Saud. Univ. Sci. 2018. [Google Scholar] [CrossRef]
- Pantanella, E.; Cardarelli, M.; Colla, G.; Marcucci, E. Aquaponics vs. Hydroponics: Production and quality of lettuce crop. Acta Hortic. 2012, 927, 887–894. [Google Scholar] [CrossRef]
- Roosta, H.R.; Hamidpour, M. Effects of foliar application of some macro-and micro-nutrients on tomato plants in aquaponic and hydroponic systems. Sci. Hortic. 2011, 129, 396–402. [Google Scholar] [CrossRef]
- Nozzi, V.; Graber, A.; Shmautz, Z. Nutrient management in aquaponics: Comparison of three approaches for cultivating lettuce, mint and mushroom Herb. Agronomy 2018, 8, 27. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Jukes, T.H.; Cantor, C.R. Evolution of protein molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; pp. 21–132. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transi-tion-transversion and G + C-content biases. Molecular Biology and Evolution 9:678-687. Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [CrossRef]
- Poobathy, R.; Zakaria, R.; Murugaiyah, V.; Subramaniam, S. Autofluorescence study and selected cyanidin quantification in the Jewel orchids Anoectochilus sp. and Ludisia discolor. PLoS ONE 2018, 13, e0195642. [Google Scholar] [CrossRef]
- Chandler, R.C. Bound water in plant sap and some effects of temperature and nutrition thereon. Plant Physiol. 1941, 16, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Fueki, T.; Tanaka, K.; Obara, K.; Kawahara, R.; Namiki, T.; Makino, T. The acrid raphides in tuberous root of Pinellia ternata have lipophilic character and are specifically denatured by ginger extract. J. Nat. Med. 2020, 74, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Stern, W.L.; Morris, M.W.; Judd, W.S.; Pridgeon, A.M.; Dressler, R.L. Comparative vegetative anatomy and systematics of Spiranthoideae (Orchidaceae). Bot. J. Linn. Soc. 1993, 113, 161–197. [Google Scholar] [CrossRef]
- Poobathy, R.; Zakaria, R.; Hamzah, S.M.E.S.; Subramaniam, S. Early studies on protoplast isolation of Ludisia discolor, a wild orchid. Trop. Life Sci. Res. 2016, 27, 17–19. [Google Scholar] [CrossRef]
- Hatier, J.H.B.; Gould, K.S. Anthocyanin function in vegetative organs. In Anthocyanins; Winefield, C., Davies, K., Gould, K., Eds.; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- De Schepper, V.; De Swaef, T.; Bauweraerts, I.; Steppe, K. Phloem transport: A review of mechanisms and controls. J. Exp. Bot. 2013, 64, 4839–4850. [Google Scholar] [CrossRef]
- Zhiponova, M.; Yordanova, Z.; Pavlova, D.; Rogova, M.; Dimitrova, M.; Dragolova, D.; Tasheva-Terzieva, E.; Kapchina-Toteva, V. Importance of phenolics in populations of Teucrium chamaedrys (Lamiaceae) from serpentine soils. Aust. J. Bot. 2020, 68, 352–362. [Google Scholar] [CrossRef]
- Ma, Z.; Li, S.; Zhang, M.; Jiang, S.; Xiao, Y. Light intensity affects growth, photosynthetic capability, and total flavonoid accumulation of Anoectochilus plants. HortScience 2010, 45, 863–867. [Google Scholar] [CrossRef]
- Saxena, M.; Saxena, J.; Pradhan, A. Flavonoids and phenolic acids as antioxidants in plants and human health. Int. J. Pharm. Sci. Rev. Res. 2012, 16, 130–134. [Google Scholar]
- Bert, D.; Danjon, F. Carbon concentration variations in the roots, stem and crown of mature Pinus pinaster (Ait.). Forest Ecol. Manag. 2006, 222, 279–295. [Google Scholar] [CrossRef]
- Yao, F.Y.; Chen, Y.H.; Yan, Z.B.; Li, P.; Han, W.X.; Fang, J.Y. Biogeographic patterns of structural traits and C:N:P stoichiometry of tree twigs in China’s forests. PLoS ONE 2015, 10, e0116391. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Smith, F.; Mudge, S.; Rae, A.; Glassop, D. Phosphate transport in plants. Plant Soil 2003, 248, 71–83. [Google Scholar] [CrossRef]
- White, P.J.; Karley, A.J. Potassium. In Cell Biology of Metals and Nutrients; Hell, R., Mendel, R.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 17, pp. 199–224. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Ruiz, J.; Romero, L. Relationship between potassium fertilisation and nitrate assimilation in leaves and fruits of cucumber (Cucumis sativus) plants. Ann. Appl. Biol. 2002, 140, 241–245. [Google Scholar] [CrossRef]
- Biško, A. The effect of NaCl on the uptake and distribution of Na and Cl in plant organs of three strawberry cultivars (Fragaria × ananassa). Acta Hortic. 2017, 1177, 369–376. [Google Scholar] [CrossRef]
- Vatansever, R.; Ozyigit, I.; Filiz, E. Essential and beneficial trace elements in plants, and their transport in roots: A Review. Appl. Biochem. Biotechnol. 2017, 181, 464–482. [Google Scholar] [CrossRef]
- Kotzen, B.; Emerenciano, M.G.C.; Moheimani, N.; Burnell, G.M. Aquaponics: Alternative types and approaches. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Suwardi. Utilization and Improvement of Marginal Soils for Agricultural Development in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 383, 012047. [Google Scholar] [CrossRef]
- MediaMatic.com. Available online: http://www.mediamatic.net/321620/en/mediamatic-ibc-strongvertical-strong-lt-strong-gt (accessed on 23 October 2022).
- Sun, Y.; Skinner, D.Z.; Liang, G.H.; Hulbert, S.H. Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theor. Appl. Genet. 1994, 89, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.A.; Wagner, W.L.; Hoch, P.C.; Nepokroeff, M.; Pires, J.C.; Zimmer, E.A.; Sytsma, K.J. Family-level relationships of Onagraceae based on chloroplast rbcL and ndhF data. Am. J. Bot. 2003, 90, 107–115. [Google Scholar] [CrossRef]
- Fazekas, A.J.; Burgess, K.S.; Kesanakurti, P.R.; Graham, S.W.; Newmaster, S.G.; Husband, B.C.; Percy, D.M.; Hajibabaei, M.; Barrett, S.C. Multiple multilocus DNA barcodes from the plastid genome discriminate plant species equally well. PLoS ONE 2008, 3, e2802. [Google Scholar] [CrossRef] [PubMed]
- Kuzmina, M.L.; Johnson, K.L.; Barron, H.R.; Hebert, P.D. Identification of the vascular plants of Churchill, Manitoba, using a DNA barcode library. BMC Ecol. 2012, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.A.; Simpson, B.B. Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. Syst. Bot. 2003, 28, 723–737. [Google Scholar] [CrossRef]
- Sang, T.; Crawford, D.J.; Stuessy, T.F. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). Am. J. Bot. 1997, 84, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Zhiponova, M.K.; Vanhoutte, I.; Boudolf, V.; Betti, C.; Dhondt, S.; Coppens, F.; Mylle, E.; Maes, S.; González-García, M.P.; Caño-Delgado, A.I.; et al. Brassinosteroid production and signaling differentially control cell division and expansion in the leaf. New Phytol. 2013, 197, 490–502. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- McKinney, G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Plummer, D. An Introduction to Practical Biochemistry; McGraw Hill: New York, NY, USA; University of California: Los Angeles, CA, USA, 1988; Volume 16, pp. 98–100. [Google Scholar]
- Asare-Brown, E.; Bullock, C. Simple practical investigation using invertase. Biochem. Educ. 1988, 16, 98–100. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb Protoc. 2020, 4, 102269. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Nesterenko, M.V.; Tilley, M.; Upton, S.J. A simple modification of Blum’s silver stain method allows for 30 minute detection of proteins in polyacrylamide gels. J. Biochem. Biophys. Methods 1994, 28, 239–242. [Google Scholar] [CrossRef]
- Petrova, D.; Gašić, U.; Yocheva, L.; Hinkov, A.; Yordanova, Z.; Chaneva, G.; Mantovska, D.; Paunov, M.; Ivanova, L.; Rogova, M.; et al. Catmint (Nepeta nuda L.) Phylogenetics and Metabolic Responses in Variable Growth Conditions. Front. Plant Sci. 2022, 13, 866777. [Google Scholar] [CrossRef]
- Singleton, V.; Orthofer, R.; Lamuela-Raventys, R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Dolezel, J.; Greilhuber, J.; Suda, J. Flow cytometry with plants: An overview. In Flow Cytometry with Plant Cells: Analysis of Genes, Chromosomes and Genomes; Dolezel, J., Greilhuber, J., Suda, J., Eds.; WILEYVCH Verlag GmbH & Co.: Berlin, Germany, 2007; pp. 41–65. [Google Scholar]
- Novichonok, E.; Novichonok, A.; Kurbatova, J.; Markovskaya, E. Use of the atLEAF+ chlorophyll meter for a nondestructive estimate of chlorophyll content. Photosynthetica 2016, 54, 130–137. [Google Scholar] [CrossRef]
- Zhiponova, М.; Paunov, M.; Anev, S.; Petrova, N.; Krumova, S.; Raycheva, A.; Goltsev, V.; Tzvetkova, N.; Taneva, S.; Sapunov, K.; et al. JIP-test as a tool for early diagnostics of plant growth and flowering upon selected light recipe. Photosynthetica 2020, 58, 399–408. [Google Scholar] [CrossRef]
| 1 | C | N | P | 2 | K | Na | Mg | Fe | Mn | Zn | Ca | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | 35.6 | 2.8 | 0.5 | 45.1 | 3.0 | 1.4 | 105 | 31.5 | 45.5 | 2.8 | ||
| Stem | 29.9 | 2.5 | 0.8 | 43.9 | 6.4 | 3.7 | 165 | 31.5 | 54.5 | 7.4 | ||
| Root | 31.3 | 1.4 | 0.3 | 4.4 | 0.9 | 2.3 | 4095 | 216.8 | 164.5 | 11.3 |
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | |
|---|---|---|---|---|---|
| pH | 6.42 | 6.32 | 5.89 | 5.71 | 5.35 |
| Conductivity, µS.m−1 | 650 | 750 | 1150 | 1390 | 1900 |
| 1 | NH4+ | NO3− | P2O5 | SO42− | K | Na | Mg | Fe | Mn | Zn | Ca |
| AW Week 4 | 23.43 | 7.73 | 12.12 | 6.11 | 7.93 | 2.03 | 1.33 | 0.02 | 0.04 | 0.26 | 5.50 |
| Lactofol 2 | 200 | 150 | 150 | 270 | n/a 3 | 100 | n/a | n/a | n/a | n/a |
| Parameter | W | AW |
|---|---|---|
| Leaf lamina | 370.38 ± 2.40 | 405.34 ** ± 17.84 |
| Mesophyll | 265.54 ± 6.78 | 295.85 * ± 26.59 |
| Palisade parenchyma | 76.66 ± 6.52 | 91.91 * ± 0.99 |
| Spongy parenchyma | 197.23 ± 2.29 | 214.22 * ± 1.28 |
| Adaxial epidermis | 75.76 ± 9.79 | 61.97 * ± 8.60 |
| Abaxial epidermis | 57.87 ± 0.13 | 55.95 ± 13.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaneva, G.; Tomov, A.; Paunov, M.; Hristova, V.; Ganeva, V.; Mihaylova, N.; Anev, S.; Krumov, N.; Yordanova, Z.; Tsenov, B.; et al. Jewel Orchid’s Biology and Physiological Response to Aquaponic Water as a Potential Fertilizer. Plants 2022, 11, 3181. https://doi.org/10.3390/plants11223181
Chaneva G, Tomov A, Paunov M, Hristova V, Ganeva V, Mihaylova N, Anev S, Krumov N, Yordanova Z, Tsenov B, et al. Jewel Orchid’s Biology and Physiological Response to Aquaponic Water as a Potential Fertilizer. Plants. 2022; 11(22):3181. https://doi.org/10.3390/plants11223181
Chicago/Turabian StyleChaneva, Ganka, Alexander Tomov, Momchil Paunov, Viktoria Hristova, Valentina Ganeva, Nikolina Mihaylova, Svetoslav Anev, Nikolay Krumov, Zhenya Yordanova, Boris Tsenov, and et al. 2022. "Jewel Orchid’s Biology and Physiological Response to Aquaponic Water as a Potential Fertilizer" Plants 11, no. 22: 3181. https://doi.org/10.3390/plants11223181
APA StyleChaneva, G., Tomov, A., Paunov, M., Hristova, V., Ganeva, V., Mihaylova, N., Anev, S., Krumov, N., Yordanova, Z., Tsenov, B., Vassileva, V., Bonchev, G., & Zhiponova, M. (2022). Jewel Orchid’s Biology and Physiological Response to Aquaponic Water as a Potential Fertilizer. Plants, 11(22), 3181. https://doi.org/10.3390/plants11223181