Abstract
Genista etnensis is a remarkable and well-known tree endemic to Sicily, Sardinia, and Corsica (Mediterranean Basin). Nevertheless, its morphological variability and its native status throughout its range need to be further investigated. In this study, we aim to clarify some aspects of this infraspecific variability by molecular means. Sequences of one nuclear and five plastid markers were analyzed under maximum parsimony by using TCS software. Plastid data were also time-calibrated under a Bayesian Inference framework. Plastid data revealed strong isolation between the populations from the Cyrno-Sardinian biogeographical province, which are also the most diverse and presumably the most archaic, and those from Sicily and Southern Italy (in this latter area, the species is naturalized). The calibration analysis indicates that the last common ancestor between G. etnensis and its sister group G. fasselata dates back to the middle Pliocene or slightly later, when sclerophyllous Mediterranean vegetation spread, whereas G. etnensis itself might have originated in the middle Pleistocene. The current, rather unusual distribution of G. etnensis could be explained by long-range seed dispersal from the western part of the range or by anthropogenic introduction into Sicily, with extinctions of transported haplotypes in the region of origin. Interestingly, the Vesuvius population, introduced from Sicily in recent times and locally naturalized, shows private genotypes, and was richer in both genotypes and haplotypes than the Sicilian ones.
1. Introduction
The genus Genista L. (Fabaceae, Genisteae) includes about 150 species and over 180 taxa, including subspecies [], of spiny or harmless shrubs (rarely small trees) (see [] for a detailed morphological description). It is widespread from the Euro-Mediterranean region, where the genus is greatly diversified, with over than 120 species [], to western Asia []. A greater concentration can be observed in the central and western sectors of the Mediterranean Basin (e.g., [,,,,]). As known, its basin is one of the richest mega hot spots of plant biodiversity worldwide [,,,], and numerous taxa are narrow endemic and often limited to islands [,]. In various cases, distributions show disjunctions that are suggestive of past tectonic or climatic events [,,,,,,]. This scenario has been reported for several Genista species [,], but, at least in G. anglica L. s.l. [] and in some representatives of the G. ephedroides DC. group [], the distribution has been partly attributed to anthropogenic dispersal in protohistoric or historical times. The well-known arboreal broom G. etnensis (Raf.) DC. ([], and the nomenclatural section therein, for the correct name of the taxon) shows one of the most intriguing native distributions within the genus, as it occurs only in the three largest islands of the Mediterranean: Sicily, Sardinia, and Corsica []. In Sicily, it was discovered on Mt. Etna in 1810 or before by Bivona ([] p. 27, sub Spartium), where it grows on lavas up to 2000 m a.s.l.; but it had been introduced for forestry purposes on the Peloritani massif a long time ago [] and later in some neighboring localities of the Nebrodi Massif and in Lachea (Cyclopean Isles) []. Moris [] first observed G. etnensis in Sardinia, where it is known for several siliceous mounts of the central and northern sectors of the island (rarely descending to the basal plain along riverbeds) []. Only in 1961 was the species reported as present in Corsica [], where it occurs very locally along the south-eastern coasts [,,]. For a detailed distribution, see Table 1. In addition, this plant was first introduced into Campania (southern Italy), i.e., in Ischia Island, the Sorrentine Peninsula, and, especially, Vesuvius [,], where it has become invasive []. Nowadays, as a naturalized or casual alien, it is also reported for several other regions of Italy, from Emilia-Romagna to Calabria [].

Table 1.
Investigated samples of Genista etnensis and the outgroup.
On the basis of ecological considerations, some authors [] hypothesized that G. etnensis originated in Sardinia, and only later spread to Sicily. Indeed, Valsecchi [] seemed to suggest that the plant had been introduced to the latter island, where apparently it occurs mainly in man-influenced habitats. Despite this, Spada et al. [] regard G. etnensis as an archaic species characterized by a relic distribution both in Sardinia and Sicily; in this latter island, it may have survived on the relatively recent Mt. Etna on account of the peculiar activity of this volcano, which periodically regenerates its cacuminal vegetation. In Corsica, it was believed to be introduced (e.g., [,,,,]), but this statement has been vigorously confuted by the most recent studies [,]. In addition to its atypical native range, the species shows a remarkable taxonomic distinctness, which is suggested also macroscopically by its arboreal habitus, which is very unusual within the genus. This feature inspired Spach ([] p. 152) in publishing the new monotypic genus Dendrospartum Spach, including D. etnense (Raf.) Spach (“aetnense”), and also characterized by several other features, among which is the calyx shape. Immediately after, Presl ([] p. 568) segregated D. etnensis s.l. into the new genus Drymospartum, including D. purgans (L.) C. Presl, D. etnense (Raf.) C. Presl (“aetnense”), and the new species D. sardum C. Presl, which included the Sardinian population described by Moris as G. etnensis (see below). Valsecchi [] kept the species in Genista, but in the monotypic section Aureospartum Vals., belonging to the subgenus Spartocarpus Spach (see also [] for the treatment under Cytisanthus). This assessment has been supported by molecular analyses [,], according to which G. etnensis falls in a distinct subclade of the Genista clade, with several autapomorphies. From a karyological viewpoint, G. etnensis populations also show an unusual chromosome complement in Sicily and Sardinia (2n = 52) [,]. Remarkably, the populations from Corsica show 2n = 54 ([] p. 263), which, in turn, is atypical as well.
If the phylogenetic position of G. etnenis appears as well defined [], this is less true about the variability of the species throughout its distributional range. Indeed, several authors reported morphological differences between the Sardinian populations and those of Sicily. As reported above, already Presl [] proposed to separate the plants of Sardinia and Sicily into two species, on the basis of the measures of flowers and legumes. A similar opinion was expressed both by Spach and Walpers ([] p. 220), who adopted the name Dendrospartum sardum (C.Presl) Walp. (“sardoum”) for the Sardinian plant. In addition, Rizzi Longo and Feoli Chiapella [] emphasized that the Sicilian populations show relevant differences in the micro-morphology of pollen grains when compared those of Sardinia. As far as the populations from Corsica are concerned, they have been recently described as a distinct subspecies, i.e., subsp. fraisseorum Fridl., on the basis of morphological, cytological, and ecological data ([] p. 77). The same author provided a new combination, at the same rank, for most of the Sardinian populations, namely, subsp. sarda (C. Presl) Fridl. ([] p. 80), stating, however, that those occurring near the coast of this latter island belong to subsp. fraisseorum.
As investigations are urgently needed to clarify the variability and nativeness of G. etnensis throughout its whole range, in this work we propose population-level molecular analyses using Sanger sequencing of nuclear and plastid markers. Indeed, these latter analyses have proven useful when applied to other Mediterranean taxa (e.g., [,,,,,]). Populations of G. etnensis from representative localities from Sardinia, Corsica, and Sicily were sampled. To these plants, we added a population from Mt. Vesuvius, in Campania, in light of the fact that G. etnensis was introduced there at the beginning of the twentieth century directly from Sicily ([] p. 58), in order to compare variation in this allochthonous population with that of the native Sicilian populations.
2. Materials and Methods
2.1. Sampling and DNA Extraction
Eighty-three individuals of G. etnensis (Table 1) were sampled from Sicily (two localities; codes B and F), Sardinia (three localities; codes G, L, and N), Corsica (three localities; codes P, S + Ss, and T), and Campania (one locality; code V). Plant material was collected from ten individuals per population, except for the populations from Solaro (T), for which only three individuals were found. In addition, the ten sampled individuals from the Solenzara area were collected from two locations (Solenzara, six individuals; road between Sari and Solenzara, four individuals), which were separately recorded (codes S and SS). For the investigations requiring outgroups, five individuals of Genista fasselata Decne. (Israel) were employed (code GF; Table 1); the choice of this outgroup was carried out according to unpublished results by P. Caputo, aiming a molecular phylogeny of several sections of the genus Genista. DNAs were extracted by using the GeneAll Exgene Plant SV kit (GeneAll Biotechnology), according to the manufacturer’s instructions.
2.2. Molecular Markers Selection
A preliminary investigation was carried out on two samples per population by using 42 plastid DNA markers (Pr) and one nuclear DNA marker (N) in the search of variable DNA traits (Supplementary File S1). PCR amplification sequence reactions conditions were optimized during this initial step of the research.
This screening allowed selection of six variable DNA regions (Table 2), namely, trnQ(UUG)–psbK intergenic spacer (labelled as Pr1), trnS(UGA)–psbZ intergenic spacer (Pr8), trnV(UAC)–atpE intergenic spacer (Pr18), psbA–trnH(GUG) intergenic spacer (Pr38), and first ycf3 intron (Pr11) from plastid DNA, and Internal Transcribed Spacers 1 and 2 plus the intervening 5.8S (N43) from the nuclear ribosomal DNA. Amplifications of the genomic DNAs were carried out by using Q5 Hot Start High-Fidelity DNA Polymerase (New England Biolabs); several recalcitrant samples were amplified by using AmpONE Fast Pfu DNA Polymerase (GeneAll Biotechnology), in all cases according to the manufacturer’s instructions. Amplified fragments were purified by using DNA Clean & Concentrator-5 kit (Zymo Research). Information on the employed molecular markers is reported in Table 2.
2.3. DNA Sequencing
DNA sequences were obtained through modified Sanger chemistry by using the Bright Dye Terminator (iCloning), according to the manufacturer’s specification, purified by using the BigDye XTerminator Purification Kit (Applied Biosystems, Thermo Fisher Scientific), and loaded onto a 3130 Genetic Analyzer (Applied Biosystems, Thermo Fisher Scientific). Electropherograms were analyzed by AB DNA Sequencing Analysis ver. 5.2 software (Applied Biosystems, Thermo Fisher Scientific), edited by employing Chromas lite ver. 2.6.6 software (Technelysium Pty Ltd., http://technelysium.com.au/?page_id=13; accessed on 1 May 2021), and assembled by using ChromasPro ver. 2.1.8 software (Technelysium Pty Ltd.), which was used to detect the mutations (single nucleotide polymorphisms (SNP) or length mutations), generating the corresponding haplotypes and genotypes. The edited sequences were aligned by using ClustalW [], as implemented in Mega ver. 10.1.8 software []; the latter software generated the matrices employed for the further analyses. The resulting sequences were submitted to GenBank (G. etnensis: nrDNA, OP794491–OP794496; cpDNA, OP830512-OP830515, OP830517-OP830518, OP830520-OP830521, OP830523-OP830524, OP830526-OP830528, G. fasselata: nrDNA, OP794497–OP794498; cpDNA, OP830516, OP830519, OP830522, OP830525, OP830529- OP830530).
2.4. Data Analysis
A genealogical investigation of the haplotypes and genotypes was carried out under a maximum parsimony (MP) framework, by using TCS ver. 1.21 software []. Plastid DNA sequence data for the five markers (i.e., Pr1, Pr8, Pr11, Pr18, and Pr38; Table 2) were merged into a single dataset, which was analyzed separately from the nuclear DNA sequence data (N43).


Table 2.
Information on the molecular markers employed.
Table 2.
Information on the molecular markers employed.
| Code | Locus | (Name) Primer Sequence 5′-3′ | Ta (°C) | Ref. |
|---|---|---|---|---|
| cpDNA | ||||
| Pr1 | trnQ(UUG)-psbK IGS | (F: trnQ-IGSR)§ ACC CGT TGC CTT ACC GCT TGG (R: psbK-IGSR)§ ATC GAA AAC TTG CAG CAG CTT G | 58 | 1 |
| Pr8 | trnS(UGA)-psbZ IGS | (F: psbZ-IGS) AAT AGC CAA TTG AAA AGC (R: trnS_UGA-IGSR)§ ATC AAC CAC TCG GCC ATC | 55 | 1 |
| Pr11 | ycf3 intron-1 | (F: ycf3-E1F)§ CAT TTA CCT ATT ACA GAG ATG G (R: ycf3-E2R) TTC CGC GTA ATT TCC TTC | 50 | 1 |
| Pr18 | trnV(UAC)-atpE IGS | (F: atpE-IGSF)§ AGT GAC ATT GAT CCR CAA GAA GC (R: trnV_UAC-E1R) GTG TAA ACG AGG TGC TCT AC | 57 | 1 |
| Pr38 | psbA-trnH(GUG) IGS | (F: psbA3f) GTT ATG CAT GAA CGT AAT GCT C (R: trnHf)§ CGC GCA TGG TGG ATT CAC AATCC | 55 | 2, 3 |
| nrDNA | ||||
| N43 | ITS1+5.8S+ITS2 | (F: JK14)§ GGA GAA GTC GTA ACA AGG TTT CCG (R: SN3)§ TTC GCT CGC CGT TAC TAA GGG | 55 | 4, 5 |
IGS = intergenic spacer; F = forward primer; R = reverse primer; Ta = annealing temperature; § = primer used for sequencing; Ref. = reference, 1 = []; 2 = []; 3 = []; 4 = []; 5 = [].
Mononucleotide repeats from the cpDNA sequences were excluded from this analysis, as they may produce artifacts owing to possible homoplasy in the polyN sequences [,,]. Analyses were run treating indels as missing data. When analyzing only G. etnensis haplotypes/genotypes, the parsimony threshold was set at 99%, whereas when G. fasselata was used to polarize the data, the parsimony threshold was set at 95%.
Plastid DNA data, the only one showing a detectable phylogenetic signal, were also investigated and time-calibrated under a Bayesian Inference framework. In the analyses described below, the most likely substitution model was computed by using jModeltest ver. 2.1.7 software [] and applying the Akaike Information Criterion []. Given the comparatively small variation of our sequences, we preferred not to partition data in order to avoid over parametrization. In the absence of known macrofossils in the lineage of our species (or of the entire sect. Spartocarpus), calibration information (i.e., dates) obtained by Fiz-Palacios and Valcárcel [] were employed. To the cpDNA sequences from the said paper ([]; Supplementary Materials, Figure S2a), which originated from De Castro et al. [], one sequence of G. fasselata was added (GenBank n. AJ890986), for a total of 26 taxa. A time-calibrated Bayesian analysis was carried out by using Beast ver. 2.5.1 software [], hypothesizing a relaxed lognormal clock and a calibrated Yule prior and the chains were run for 5,000,000 generations. The dates from Fiz-Palacios and Valcárcel [] employed for this analysis included that for the ingroup used in that paper (9.06 Ma) and that for the clade, including G. radiata (L.) Scop. and G. tyrrhena Vals. Clade (6.07 Ma), both with a lognormal prior. The date recovered for the node G. fasselata–G. etnensis was employed for a further calibration attempt within G. etnensis. A final calibrated Bayesian analysis was then carried out on our G. etnensis dataset; i.e., all our G. etnensis samples and the five specimens of G. fasselata for all our cpDNA markers, namely, Pr1, Pr8, Pr11, Pr18, and Pr38; Table 2. The Bayesian analysis was run for 50,000,000 generations, hypothesizing a relaxed lognormal clock, with a coalescent skyline prior and a lognormal prior for the date recovered from the preliminary calibration. The resulting trees and their associate information, such as their Posterior Probability (PP), 95% Highest Posterior Density (95%HPD), Node ages (in Ma), were explored by using Tracer ver. 1.7.1 software [].
3. Results
3.1. Plastid Sequences
All the selected DNA sequences were polymorphic in G. etnensis. The point mutations and length variation in the cpDNA markers (Pr1, Pr8, Pr11, Pr18, and Pr38) yielded seven haplotypes (codes P1–P7; Table 3), when considering indels as a fifth state, or six, and scoring the indels as missing data (in this case, haplotype P6 from Vesuvius merges into haplotype P2). The length of the combined plastid dataset was of 1913 bp, with eight variable sites, of which five were parsimony-informative sites; considering the outgroup, the combined dataset was of 1929 bp with 30 variable sites of which 24 were parsimony-informative sites. In the outgroup two haplotypes were observed (GF_PA in three and GF_PB in two samples). The geographic distribution of the G. etnensis haplotypes is shown in Figure 1a. Each geographical region in which our sample is partitioned (i.e., Sardinia, Corsica, and Sicily/southern Italy) shows at least one regionally unique haplotype (Figure 1a), but only the Vesuvius population displays one (or two) private haplotype(s) in the strictest sense (P7, by considering the length mutations as missing data; P6 and P7, when scoring them as a fifth state).

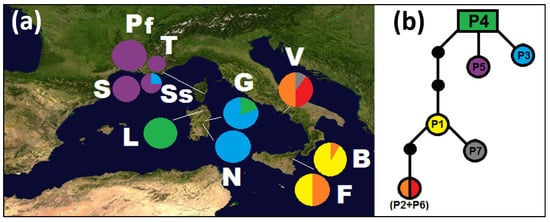
Figure 1.
(a) Distribution map of the populations of Genista etnensis and the relative frequency of haplotypes (cpDNA) (populations labels: B = Mount Baracca, F = Fornazzo, G = Genna Silana, L = Mount Limbara, N = Villagrande Strisaili, Pf = Serra Fiumorbu, S = Solenzara, Ss = road between Sari and Solenzara, T = Solaro, V = Mount Vesuvius); (b) haplotype genealogies as inferred by TCS software (indels scored as missing data); black dots represent undetected haplotypes.
The MP network of Figure 1b indicates close relationships between the Sicilian and continental southern Italian haplotypes, which are separated from the Cyrno-Sardinian ones; the latter are plesiomorphic in G. etnensis. In particular, when analyzing G. etnensis sequences only, the estimated outgroup haplotype is an exclusively Sardinian haplotype, P4, present in all the individuals at Mount Limbara and several individuals of Genna Silana (Figure 1a,b). When using G. fasselata as well, the haplotype directly connected to G. fasselata is P5, which is widespread in Corsica and absent from Sardinia.
3.2. Nuclear Sequences
The nuclear ITS1, 5.8S, and ITS2 (650 bp) showed a lower genotypic variability, as compared to the cpDNA haplotype variation, and no length mutations (indels) were detected (Supplementary File S2). Six genotypes were detected (codes N1–N6; Figure 2a), one of which (N2) was widely distributed across all populations, with an overall frequency of 75% (from approx. 35% to 100% according to population). All mutations occur in the ITS2 region, either as individual mutations, as in the cases of genotypes N2 and N5, or as paralogues, in genotypes N1, N3, N4, and N6 (Figure 2b). Sardinia has the largest genotypic diversity, with four genotypes (N3, N4, N5, and N6), followed by Corsica, with three, represented also in Sardinia (N2, N4, and N6) and the Vesuvius area (N1, N2, and N3). In Sicily only two genotypes occur (N2 and N4). Private genotypes occur in Sardinia, in the Mount Limbara population (N5) and in the Vesuvius population (N1 and N3). The four detected paralogous genotypes may derive from crosses between unambiguous genotypes (e.g., see Figure 2c) and were not subjected to genealogical analysis.
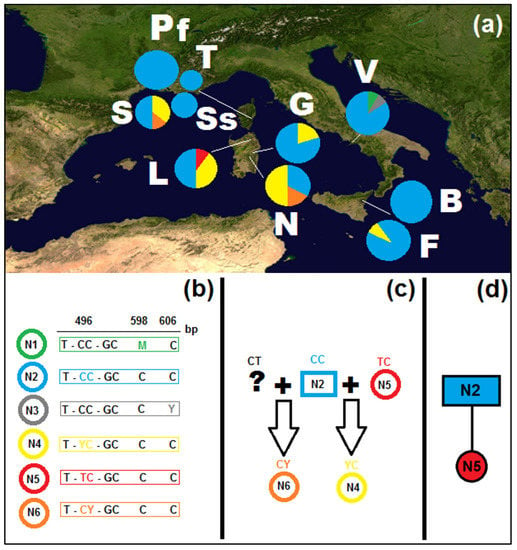
Figure 2.
(a) Distribution map of the populations of Genista etnensis and relative frequency of the ITS genotypes (nrDNA) (populations labels: B = Mount Baracca, F = Fornazzo, G = Genna Silana, L = Mount Limbara, N = Villagrande Strisaili, Pf = Serra Fiumorbu, S = Solenzara, Ss = road between Sari and Solenzara, T = Solaro, V = Mount Vesuvius); (b) genotype and relative SNPs (see Supplemental File S2 for the alignment, GenBank accessions N1–N6: OP794491–OP794496); (c) possible crosses between genotypes; (d) reconstructed genealogy of unambiguous genotypes.
In the outgroup, two genotypes were observed and correlated with the same specimens for the same haplotypes (GF_NA in three and GF_NB in two samples). The MP genealogy reconstruction for the two unambiguous genotypes (Figure 2d), both with and without the outgroup, indicates that the ancestral genotype is the widespread N2. As the investigated nuclear sequences included comparatively little information when compared to the cpDNA markers and given the paralogues did add a further measure of uncertainty, they were not further employed, and the following investigations were carried out exclusively on cpDNA sequences.
3.3. Molecular Dating
Bayesian calibration was carried out in two steps, as indicated in Materials and Methods. The best-fitting substitution model for the 26-terminal matrix for the preliminary first calibration on the literature data was the General Time Reversible (GTR) model. This investigation yielded a phylogram (data not shown) in which G. fasselata is a sister group to G. etnensis. The date for this sister group relationship is 3.365 MA (p.p = 0.935; 1.051-5.530 MA 95% HPD). This latter date was employed for the final calibration, including all 83 cpDNA sequences produced in this work for G. etnensis and the sequences of G. fasselata. Both the results of the preliminary and the final Bayesian analyses were convergent and all Estimated Sample Sizes were >> 100. The investigation on all the samples of G. etnensis recovered this taxon as monophyletic (PP = 0.9987), with a date of 0.718 MA (0.2157-1.3827 95% HPD). The species is divided into two major groups, one including all Sicilian and southern Italian samples (PP = 0.9967), with a date of 0.278 MA, and the other one including the Sardinian and Corsican samples (PP = 0.997), with a date of 0.277 MA. In both groups, several clades with high posterior probability can be observed (Figure 3), although no one corresponds to a single population. The only subgroup associated to a single geographic area is the one associated to the Corsican haplotype, P5.
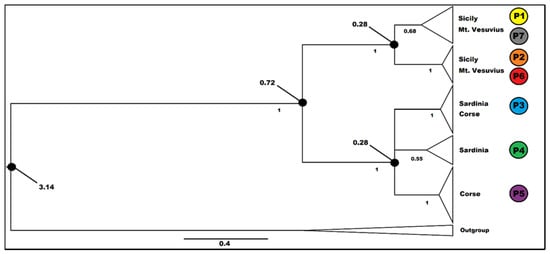
Figure 3.
Calibrated Bayesian analysis on the plastid sequences of the inclusive group in the study of Genista etnensis populations (outgroup = G. fasselata). Posterior probabilities are indicated below the branches (PP > 0.5 are shown); relevant dates (Ma) on the nodes (black circle). Triangles are proportional to the number of individuals. For haplotype distribution and information, see Figure 1.
4. Discussion
Biogeographically, Genista etnensis is an element of endemic to the Italo-Tyrrhenian superprovince [,]. The analysis of cpDNA data indicates strong isolation between the populations belonging to the province Cyrno-Sardinian province [] and those from Sicily and Southern Italy; no haplotype, in fact, is shared between the two areas (Figure 1). Stronger affinities are on the contrary observed within the two lineages, with one haplotype shared between Sardinia and Corsica, and another between Sicily and Southern Italy. Sardinia and Corsica are home to three different haplotypes, Sicily to two and the Vesuvius area to three. The haplotype genealogy analysis carried out with TCS, when employing only the G. etnensis samples, reconstructs haplotype P4, which is unique to Sardinia, as plesiomorphic.
According to this pattern of descent, the populations of G. etnensis located in the areas between Supramonte (the historical Sardinian subregion including the Genna Silana mountain pass) and southern Gallura, i.e., central to northern Sardinia, retain the most archaic molecular features. This area, however, may only tentatively be regarded as coincident with that of the origin of the living populations of G. etnensis, given the fact that, when polarizing our TCS analysis by using G. fasselata, the most archaic haplotype is indeed P5, which is exclusive to Corsica. However, haplotype P4 is also present in Corsica at Solenzara. For this reason, we regard the whole Cyrno-Sardinian area as the location of the most plesiomorphic living populations of G. etnensis.
The six genotypes detected in nuclear ITS and 5.8S sequences were less informative than plastid DNA given their little variation, distribution, and the presence of paralogous sites, in addition to the possible problems related to incomplete concerted evolution when dealing with such sequences (e.g., []). However, differently from plastid DNA, the most widespread genotype (N2) is ubiquitous, and the second most frequent one (N4) is present in Corsica, Sardinia, and Sicily, being absent only from the Vesuvius area (Figure 2). The largest genotypic diversity is present in the Cyrno-Sardinian biogeographical province, with four genotypes (N2, N4, N5, and N6), three of which are present in both islands (N5 being represented only in the Limbara population of Sardinia); Sicily accounts for genotypes N2 and N4, both present also in Corsica and Sardinia. The Vesuvius area, on the contrary, in addition to the ubiquitous N2 genotype, has two private ones (N1 and N3). Overall, only two genotypes are unambiguous (N2 and N5); one (N4) may be accounted as a consequence of a cross between N2 and N5 and the other three as a consequence of crosses with unsampled or extinct genotypes.
The median date recovered in the calibration analysis for the sister group relationship between G. etnensis and G. fasselata (3.365 MA; 1.051–5.530 MA 95% HPD; Figure 3) indicates that their last common ancestor dates back to the middle Pliocene, roughly at the time of the appearance of the Mediterranean climate and its associated precipitation rhythm [], or immediately after its onset, when a great reduction in subtropical taxa and a corresponding increase in sclerophyllous vegetation took place [,]. The age in which present-day G. etnensis populations started their diversification is 0.718 MA. Even when considering the interval of Highest Posterior Density (0.2157–1.3827 MA), the majority of this time interval is included in the so called “mid-Pleistocene revolution”, and its median value is almost perfectly coincident with the early-middle Pleistocene transition [], when the first major glacial episode had just occurred, and a conspicuous fraction of the Tertiary subtropical flora had become finally extinct from Italy [,]. Justifying the present distribution pattern of G. etnensis is difficult. In fact, the range of G. etnensis is rather uncommon. By perusing the regional distribution of the taxa of the Italian Flora ([], Appendix 2), only about ten species or subspecies are exclusively native to Sicily and Sardinia, constituting a rather disparaged assemblage of annuals or short-lived perennials, some of which have potential for epizoochorous or anemochorous long-range seed dispersal, the record in Sardinia for at least one of which (i.e., Ranunculus pratensis C. Presl) may be the result of confusion (see [] p. 230). If we include also neighboring continental Southern Italy, only a few more species are added.
The Cyrno-Sardinian plate did not share a general tectonic history in common with Sicily [,,], barring the existence of relationships between Sardinia, Kabilie (Algeria), and the Calabrian-Peloritan arc, much earlier than the proposed time frame for the origin of G. etnensis (see, for example, []). In addition, eustatic sea level variations, given the depth of the sea surrounding Sardinia and Corsica, only justify the biogeographic connections between these islands and maritime Tuscany, or more generally north-western Italy [,]. However, in spite of the great geologic and biogeographic separation between the Cyrno-Sardinian and Sicilian provinces [], Schmitt et al. [], in a comprehensive study based on over 100 research papers dealing with animals and plants, recovered definite biogeographic relationships between the two regions []. These relationships, however, are likely a consequence of the distribution of vertebrates more than plant species.
In light of the above, the present-day distribution of our species does not depend upon vicariance. Dispersal, however, calls into question a possible mechanism and a direction. For the first issue, no information is available for G. etnensis (barring a vague reference to heath-mediated pod explosion by Paiero et al., [] p. 160) and the paucity in literature on the genus indicates that probably myrmecochory is involved in at least G. tinctoria L. []; a report is also available that non-native ants gather seeds from two species of the related Teline Medik. In the Iberian Peninsula [], barochory is also suggested for an unidentified species of Genista from Pantelleria island []. Therefore, no long-range dispersal mechanism is known. Indeed, an event of exceptional, naturally occurring long-range dispersal may be envisaged (albeit unlikely). Given the dates recovered for the two main groups into which G. etnensis samples are divided (slightly over 250 thousand years for both), anthropogenic dispersal would be ruled out, unless our calibration data are incorrect as a consequence of an unusually fast mutation rate (which, in particular, would easily justify the different haplotypic pattern of the plants from Vesuvius). Nevertheless, without hypothesizing a fast mutation rate, a recent, man-mediated dispersal event may be hypothesized, by supposing that introduced haplotypes became extinct in the regions of origin. The direction of such an event is easier to envisage, as said above; our TCS analyses would indicate either a Sardinian or a Corsican haplotype as plesiomorphic and, therefore, our species of interest dispersed from the Cyrno-Sardinian province to Sicily. In this regard, we recall that Sicily and Sardinia have a long, if not intense, history of economic and social contacts which, within the limits of written history, date back to the times in which Carthaginians fought against Greeks for the control of Sicily and kept coastal colonies in Sardinia, from roughly the seventh century BCE and until the Punic wars (e.g., []). In addition, this would not be the only species of genus Genista for which recent anthropogenic dispersal has been hypothesized to justify unusual distributions. In fact, the existence of G. cilentina Vals., endemic to the southern coasts of Campania, has been regarded as the consequence of a deliberate introduction from a population of G. numidica Spach from Algeria [,]. In the same way, the Genista of the island Mezzu Mare (Ajaccio gulf, Corsica) is not a Corsican microendemic species (G. mezzumarensis Coulot & Rabaute), as assumed by Coulot and Rabaute ([] pp. 786–793). An investigation conducted by Paradis and Chiappe [], within the local population, showed that it is G. tyrrhena Vals. subsp. pontiana Brullo & De Marco. A few individuals were collected by a fisherman, born on Ponza Island (Pontine Islands, Latium, Italy) and living in Corsica, and were then planted in Ajaccio around 1982 or 1984, and whose seeds were sown on Mezzu Mare Island, probably in April 1987. The expansion of this broom on the island, initially very slow, seems to have become rapid from 2003 []. This example of voluntary introduction followed by rapid progression of a non-indigenous taxon on a given territory illustrates once again the importance of the human factor for understanding the chorology of many plant entities. In the light of the comparative homogeneity of haplotypes within the two areas (three very similar haplotypes in the Sardinian and Corsican areas and two for Sicily), the variation found in the Vesuvius area, with one (or two) private haplotypes, is indeed puzzling, given that the population is, as was already said, a consequence of purposeful anthropogenic dispersal in very recent times. Moreover, this population is distinct not only from a molecular but also from a morphological point of view (in flowers, legumes, and seeds; S. Brullo, unpublished data). At present, is would be very difficult to understand whether variation has increased in the new environment, with plants undergoing rapid diversification, or whether the plants that now are found in the Vesuvius area originate from an unsampled (or extinct) haplotype from Sicily.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11223171/s1, Supplementary File S1: Information and results on the molecular markers tested (+ = variable sequence; 0 = non-variable sequence; x = amplified with abnormal structure; − = low amplification efficiency) (Pr = plastid DNA; N = nuclear DNA); Supplementary File S2: Alignment of N43 molecular marker (ITS) for all dataset; Supplementary File S3: Alignment of Pr1 molecular marker (trnQ(UUG)–psbK IGS) for all dataset; Supplementary File S4: Alignment of Pr8 molecular marker (trnS(UGA)–psbZ IGS) for all dataset; Supplementary File S5: Alignment of Pr11 molecular marker (ycf3 intron 1) for all dataset; Supplementary File S6: Alignment of Pr18 molecular marker (trnV(UAC)–atpE IGS) for all dataset; Supplementary File S7: Alignment of Pr38 molecular marker (psbA–trnH(GUG) IGS) for all dataset. Refs. [,,,,,] have been cited in supplementary materials.
Author Contributions
Conceptualization of the study, O.D.C., G.B. and P.C.; conceptualization of the molecular study, O.D.C.; collection of fresh samples, G.B., S.B., E.D.G. and C.P.; conducted molecular laboratory analyses, O.D.C. and E.D.I.; performed bioinformatic molecular analyses, O.D.C., and P.C.; statistical analyses, O.D.C. and P.C.; writing the first draft of the manuscript, O.D.C., P.C. and E.D.G.; writing the molecular section of the manuscript, O.D.C. and P.C.; all authors contributed critically to the draft and gave final approval for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The datasets generated in the current study are available from the corresponding author on reasonable request.
Acknowledgments
For the field sampling of Genista fasselata, a special thanks go to Hagar Leschner (The Herbarium, The Natural History Collections, The Hebrew University of Jerusalem, Israel).
Conflicts of Interest
No potential conflict of interest was provided by the authors.
References
- POWO. “Plants of the World Online”. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 7 March 2021).
- Gibbs, P.E. A revision of the genus Genista L. Notes Roy. Bot. Gard. Edinb. 1966, 27, 11–99. [Google Scholar]
- ILDIS. International Legume Database & Information Service. Available online: http://ww2.bgbm.org/EuroPlusMed/ (accessed on 7 March 2021).
- Duran, A.; Dural, H. Genista vuralii (Fabaceae), a new species from Turkey. Ann. Bot. Fenn. 2003, 40, 113–116. [Google Scholar]
- Gibbs, P.E. Genista L. In Flora Europaea 2; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1968; pp. 94–100. [Google Scholar]
- Greuter, W.; Burdet, R.M.; Long, G. Med-Checklist 4; Conservatoire et Jardin botaniques de la Ville de Genève: Berlin, Germany; Gèneve, Switzerland, 1989. [Google Scholar]
- Talavera, S. Genista L. In Flora Iberica; Talavera, S., Aedo, C., Castroviejo, S., Romero Zarco, C., Sáez, L., Salgueiro, F.J., Velayos, M., Eds.; CSIC: Madrid, Spain, 1999; Volume 7, pp. 45–119. [Google Scholar]
- Bacchetta, G.; Brullo, S.; Cusma Velari, T.; Feoli Chiapella, L.; Kosovel, V. Taxonomic notes on the Genista ephedroides Group (Fabaceae) from the Mediterranean area. Novon J. Bot. Nomencl. 2011, 21, 4–19. [Google Scholar] [CrossRef]
- Greuter, W. Botanical diversity, endemism, rarity, and extinction in the Mediterranean area: An analysis based on the published volumes of Med-Checklist. Bot. Chron. 1991, 10, 63–79. [Google Scholar]
- Thompson, J.D. Plant Evolution in the Mediterranean; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Mittermeier, R.A.; Robles Gil, P.; Hoffman, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.G.; Lamoreux, J.; da Fonseca, G.A.B. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions; University of Chicago Press: Chicago, IL, USA, 2005. [Google Scholar]
- Cañadas, E.M.; Fenu, G.; Peñas, J.; Lorite, J.; Mattana, E.; Bacchetta, G. Hotspots within hotspots: Endemic plant richness, environmental drivers, and implications for conservation. Biol. Conserv. 2014, 170, 282–291. [Google Scholar] [CrossRef]
- Greuter, W. Diversity of Mediterranean Island Floras; Bocconea: Milano, Italy, 2001; Volume 13, pp. 55–64. [Google Scholar]
- Junikka, L.; Uotila, P.; Lahti, T. A phytogeographical comparison of the major Mediterranean islands on the basis of Atlas Florae Europaeae. Willdenowia 2006, 36, 379–388. [Google Scholar] [CrossRef]
- Medail, F.; Quezel, P. Biodiversity Hotspots in the Mediterranean Basin: Setting Global Conservation Priorities. Conserv. Biol. 2001, 13, 1510–1513. [Google Scholar] [CrossRef]
- Mansion, G.; Rosenbaum, G.; Schoenenberger, N.; Bacchetta, G.; Rosselló, J.A.; Conti, E. Phylogenetic Analysis Informed by Geological History Supports Multiple, Sequential Invasions of the Mediterranean Basin by the Angiosperm Family Araceae. Syst. Biol. 2008, 57, 269–285. [Google Scholar] [CrossRef]
- Gianguzzi, L.; Cusimano, D.; Bonventre, V.; Romano, S.; Ilardi, V. Bio-ecological, phytosociological and conservation aspects of relictual and disjointed populations of Simethis mattiazzi (Vandelli) Sacc. (Xanthorrhoeaceae) in the Channel of Sicily. Acta Bot. Gall. 2012, 159, 303–318. [Google Scholar] [CrossRef]
- Chen, C.; Qi, Z.; Xu, X.; Comes, H.P.; Koch, M.A.; Jin, X.; Fu, C.; Qiu, Y. Understanding the formation of Mediterranean–African–Asian disjunctions: Evidence for Miocene climate-driven vicariance and recent long-distance dispersal in the Tertiary relict Smilax aspera (Smilacaceae). New Phytol. 2014, 204, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Hardion, L.; Dumas, P.-J.; Abdel-Samad, F.; Kharrat, M.B.D.; Surina, B.; Affre, L.; Médail, F.; Bacchetta, G.; Baumel, A. Geographical isolation caused the diversification of the Mediterranean thorny cushion-like Astragalus L. sect. Tragacantha DC. (Fabaceae). Mol. Phylogenetics Evol. 2016, 97, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Affenzeller, M.; Kadereit, J.W.; Comes, H.P. Parallel bursts of recent and rapid radiation in the Mediterranean and Eritreo-Arabian biodiversity hotspots as revealed by Globularia and Campylanthus (Plantaginaceae). J. Biogeogr. 2018, 45, 552–566. [Google Scholar] [CrossRef]
- Bacchetta, G.; Brullo, S.; Chiapella, L.F.; Velari, T.C.; Fenu, G.; Del Galdo, G.G. Taxonomic remarks on Genista salzmannii group (Fabaceae) in Sardinia and Corsica. Phytotaxa 2020, 449, 31–51. [Google Scholar] [CrossRef]
- Fernández Prieto, J.A.; Sanna, M.; Bueno, Á.; Pérez, M. Genista anglica (Fabaceae): One very diverse species or one species complex? J. Plant Res. 2016, 129, 411–422. [Google Scholar] [CrossRef]
- De Castro, O.; Vallariello, R.; Del Guacchio, E. Integration of morphology, genetics, historical and ethnobotanical data: A case of an enigmatic Genista (Fabaceae) from Ischia Island (southern Italy). Phytotaxa 2013, 82, 64–68. [Google Scholar] [CrossRef]
- Bacchetta, G.; Brullo, S.; Caputo, P.; De Castro, O.; Del Guacchio, E.; Dettori, C.A.; Giusso del Galdo, G.; Grillo, O.; Piazza, C. Morphological and micro-morphological comparative study of Genista etnensis populations. Not. Soc. Bot. Ital. 2016, 0, 27–28. [Google Scholar]
- Ortolani, E.O.; Rafinesque-Schmaltz, C.S. Statistica Generale di Sicilia; Dalla Reale stamperia: Palermo, Italy, 1810. [Google Scholar]
- Inferrera, G. La Genista aetnensis nel messinese. Boll. Soc. Bot. Ital. 1904, 6, 270–272. [Google Scholar]
- La Mantia, T.; Rühl, J.; Massa, B.; Pipitone, S.; Verde, G.L.; Bueno, R.S. Vertebrate-mediated seed rain and artificial perches contribute to overcome seed dispersal limitation in a Mediterranean old field. Restor. Ecol. 2019, 27, 1393–1400. [Google Scholar] [CrossRef]
- Moris, J.H. Stirpium Sardoarum Elenchus; Ex Typis Regiis: Carali, Belgium, 1827; Volume 1. [Google Scholar]
- Arrigoni, P.V. Flora dellIsola di Sardegna, 3rd ed.; Carlo Delfino Editore: Sassari, Italy, 2010. [Google Scholar]
- Vivant, J. Sur quelques plantes de Corse. Monde des Plantes 1966, 351, 12–14. [Google Scholar]
- Paradis, G. Notes et Contributions à la Flore de Corse, IX. Candollea 1993, 48, 551–555. [Google Scholar]
- Piazza, C.; Paradis, G. Précisions sur les stations d’une espèce très rare en Corse: Genista aetnensis. «Etat des lieux» en 1995. Monde des Plantes 1996, 456, 9–12. [Google Scholar]
- Fridlender, A. Observations sur le Genêt de l’Etna en Corse: Genista etnensis (Biv.) DC. subsp. fraisseorum subsp. nova. Bull. Mens. Soc. Linn. Lyon. 2018, 87, 73–95. [Google Scholar]
- Agostini, R. Alcuni reperti interessanti la flora della Campania. Delpinoa 1959, 1, 42–68. [Google Scholar]
- Del Guacchio, E.; La Valva, V. The non-native vascular flora of Campania (southern Italy) [Appendices]. Plant Biosyst. 2017, 52, 767–779. [Google Scholar] [CrossRef]
- Stinca, A.; Conti, P.; Menegazzi, G.; Chirico, G.B.; Bonanomi, G. Invasion Impact of the Nitrogen-fixing Shrub Genista aetnensis on Vesuvius Grand Cone. Procedia Environ. Sci. 2013, 19, 865–874. [Google Scholar] [CrossRef][Green Version]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Arrigoni, P.V.; Vannelli, S. La «Genista aetnensis» (Raf.) DC. in Sardegna. Webbia 1967, 22, 1–20. [Google Scholar] [CrossRef]
- Valsecchi, F. Il genere Genista L. in Italia: I. Le specie delle sezioni Erinacoides Spach, Ephedrospartum Spach, Aureospartum sect. nova. Webbia 1993, 48, 779–884. [Google Scholar]
- Spada, F.; Cutini, M.; Paura, B. Considerazioni fitostoriche sulla zonazione altitudinale della vegetazione di alcuni rilievi dell’Appennino meridionale e della Sicilia. Biogeographia 2011, 30, 95–112. [Google Scholar] [CrossRef]
- Vivant, J. Quelques notes à propos de plantes vasculaires de la Corse. Bull. Soc. Bot. Fr. 1974, 121, 27–36. [Google Scholar] [CrossRef][Green Version]
- Deschâtres, R. À propos du Genista etnensis (Biv.) DC. en Corse. Rev. Sci. Bourbon. Cent. Fr. 1980, 1979, 4–10. [Google Scholar]
- Verlaque, R.; Contandriopoulos, J.; Aboucaya, A. Cytotaxonomie et conservation de la flore insulaire: Les espèces endémiques ou rares de Corse. Ecol. Mediterr. 1995, 21, 257–268. [Google Scholar] [CrossRef]
- Jeanmonod, D.; Gamisans, J. Flora corsica, ed. 2. Bull. Soc. Bot. Cent. Ouest 2013, 39, 1–1074. [Google Scholar]
- Nery, L.; Delage, A. Notes à la Flore de Corse, XXV. Candollea 2015, 70, 109–140. [Google Scholar]
- Spach, E. Revisio generis Genista. Ann. Des Sci. Nat. Bot. Ser. 3. 1845, 3, 102–158. [Google Scholar]
- Presl, C.B. Botanische Bemerkungen. Abh. Königl. Böhm. Ges. Wiss. ser. 5. 1845, 3, 431–584. [Google Scholar]
- Cristofolini, G.; Feoli Chiapella, L. Serological systematics of the tribe Genisteae (Fabaceae). Taxon 1977, 26, 43–56. [Google Scholar] [CrossRef]
- De Castro, O.; Cozzolino, S.; Jury, S.L.; Caputo, P. Molecular relationships in Genista L. sect. Spartocarpus Spach (Fabaceae). Plant Syst. Evol. 2002, 231, 91–108. [Google Scholar] [CrossRef]
- Pardo, C.; Cubas, P.; Tahiri, H. Molecular phylogeny and systematics of Genista (Leguminosae) and related genera based on nucleotide sequences of nrDNA (ITS region) and cpDNA (trnL-trnF intergenic spacer). Plant Syst. Evol. 2004, 244, 93–119. [Google Scholar] [CrossRef]
- Bedini, G.; Peruzzi, L. Chrobase.it—Chromosome Numbers for the Italian Flora v. 2.0. Available online: http://bot.biologia.unipi.it/chrobase/ (accessed on 4 February 2022).
- Cusma Velari, T.; Feoli Chiapella, L.; Kosovel, V. A karyological study of Genista sect. Spartocarpus Spach (Cytiseae-Fabaceae). Webbia 2013, 66, 57–68. [Google Scholar] [CrossRef]
- Walpers, G.G. Annales Botanices Systematicae; Sumtibus F. Hofmeister: Lipsiae, Germany, 1848; Volume 1. [Google Scholar]
- Rizzi Longo, L.; Feoli Chiapella, L. Contribution to the systematics of Genista L. sect. Spartocarpus Spach (Genisteae, Fabaceae) with emphasis on palynological data. Studia Geobot. 1994, 14, 41–62. [Google Scholar]
- Cennamo, P.; Del Guacchio, E.; Jury, S.L.; Caputo, P. Molecular markers in Viola L. subsect. Viola: Application and taxonomic implications for the identification of dubious herbarium specimens. Plant Biosyst. 2011, 145, 306–323. [Google Scholar] [CrossRef]
- Cennamo, P.; Del Guacchio, E.; Paino, L.; De Castro, O.; Menale, B.; Vazquez-Torres, M.; Caputo, P. Genetic structure of Ipomoea imperati (Convolvulaceae) in the Mediterranean region and implications for its conservation. Phytotaxa 2013, 141, 40–54. [Google Scholar] [CrossRef][Green Version]
- De Castro, O.; Innangi, M.; Di Maio, A.; Menale, B.; Bacchetta, G.; Pires, M.; Noble, V.; Gestri, G.; Conti, F.; Peruzzi, L. Disentangling phylogenetic relationships in a hotspot of diversity: The butterworts (Pinguicula L., Lentibulariaceae) endemic to Italy. PLoS ONE 2016, 11, e0167610. [Google Scholar] [CrossRef] [PubMed]
- De Castro, O.; Geraci, A.; Mannino, A.M.; Mormile, N.; Santangelo, A.; Troia, A. A Contribution to the Characterization of Ruppia drepanensis (Ruppiaceae), a Key Species of Threatened Mediterranean Wetlands. Ann. Mo. Bot. Gard. 2021, 106, 1–9. [Google Scholar] [CrossRef]
- De Castro, O.; Innangi, M.; Menale, B. Message in a bottle: The Mediterranean Sea currents acted as protagonists in shaping the distribution of the sea daffodil (Pancratium maritimum, Amaryllidaceae). Bot. J. Linn. Soc. 2020, 194, 207–220. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef]
- Prince, L.M. Plastid Primers for Angiosperm Phylogenetics and Phylogeography. Appl. Plant Sci. 2015, 3, 1400085. [Google Scholar] [CrossRef]
- Sang, T.; Crawford, D.J.; Stuessy, T.F. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). Am. J. Bot. 1997, 84, 1120–1136. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.A.; Simpson, B.B. Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. Syst. Bot. 2003, 28, 723–737. [Google Scholar] [CrossRef]
- Aceto, S.; Caputo, P.; Cozzolino, S.; Gaudio, L.; Moretti, A. Phylogeny and Evolution of Orchis and Allied Genera Based on ITS DNA Variation: Morphological Gaps and Molecular Continuity. Mol. Phylogenetics Evol. 1999, 13, 67–76. [Google Scholar] [CrossRef] [PubMed]
- De Castro, O.; Di Maio, A.; García, J.A.L.; Piacenti, D.; Vázquez-Torres, M.; De Luca, P. Plastid DNA sequencing and nuclear SNP genotyping help resolve the puzzle of central American Platanus. Ann. Bot. 2013, 112, 589–602. [Google Scholar] [CrossRef] [PubMed][Green Version]
- JJarne, P.; Lagoda, P.J. Microsatellites, from molecules to populations and back. Trends Ecol. Evol. 1996, 11, 424–429. [Google Scholar] [CrossRef]
- Goldstein, D.G.; Pollock, D.D. Launching microsatellites: A review of mutation processes and methods of phylogenetic inference. J. Hered. 1997, 88, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Provan, J.; Powell, W.; Hollingsworth, P.M. Chloroplast microsatellites: New tools for studies in plant ecology and evolution. Trends Ecol. Evol. 2001, 16, 142–147. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. System identification and time-series analysis. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Fiz-Palacios, O.; Valcárcel, V. From Messinian crisis to Mediterranean climate: A temporal gap of diversification recovered from multiple plant phylogenies. Perspect. Plant Ecol. Evol. Syst. 2013, 15, 130–137. [Google Scholar] [CrossRef]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.-H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Fenu, G.; Fois, M.; Cañadas, E.M.; Bacchetta, G. Using endemic-plant distribution, geology and geomorphology in biogeography: The case of Sardinia (Mediterranean Basin). Syst. Biodiver. 2014, 12, 181–193. [Google Scholar] [CrossRef]
- Fois, M.; Farris, E.; Calvia, G.; Campus, G.; Fenu, G.; Porceddu, M.; Bacchetta, G. The Endemic Vascular Flora of Sardinia: A Dynamic Checklist with an Overview of Biogeography and Conservation Status. Plants 2022, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Poczai, P.; Hyvönen, J. Nuclear ribosomal spacer regions in plant phylogenetics: Problems and prospects. Mol. Biol. Rep. 2009, 37, 1897–1912. [Google Scholar] [CrossRef] [PubMed]
- Suc, J.P. Origin and evolution of the Mediterranean vegetation and climate in Europe. Nature 1984, 307, 429–432. [Google Scholar] [CrossRef]
- Tzedakis, P.C. Cenozoic climate and vegetation changes in the Mediterranean. In The Physical Geography of the Mediterranean; Woodward, J., Ed.; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Head, M.J.; Gibbard, P.L. Early-Middle Pleistocene transitions: An overview and recommendation for the defining boundary. In Early-Middle Pleistocene Transitions: The Land-Ocean Evidence; Head, M.J., Gibbard, P.L., Eds.; Geological Society, Special Publications; The Geological Society of London: London, UK, 2005; Volume 247, pp. 1–18. [Google Scholar]
- Magri, D.; Di Rita, F.; Aranbarri, J.; Fletcher, W.; González-Sampériz, P. Quaternary disappearance of tree taxa from Southern Europe: Timing and trends. Quat. Sci. Rev. 2017, 163, 23–55. [Google Scholar] [CrossRef]
- Magri, D.; Palombo, M.R. Early to Middle Pleistocene dynamics of plant and mammal communities in South West Europe. Quat. Int. 2013, 288, 63–72. [Google Scholar] [CrossRef]
- Arrigoni, P.V. Flora dell’Isola di Sardegna; Carlo Delfino Editore: Sassari, Italy, 2006; Volume 1. [Google Scholar]
- Boccaletti, M.; Ciaranfi, N.; Cosentino, D.; Deiana, G.; Gelati, R.; Lentini, F.; Massari, F.; Moratti, G.; Pescatore, T.; Lucchi, F.R.; et al. Palinspastic restoration and paleogeographic reconstruction of the peri-Tyrrhenian area during the Neogene. Palaeogeogr. Palaeoclim. Palaeoecol. 1990, 77, 1–41. [Google Scholar] [CrossRef]
- Rook, L.; Gallai, G.; Torre, D. Lands and endemic mammals in the Late Miocene of Italy: Constrains for paleogeographic outlines of Tyrrhenian area. Palaeogeogr. Palaeoclim. Palaeoecol. 2006, 238, 263–269. [Google Scholar] [CrossRef]
- Advokaat, E.L.; van Hinsbergen, D.J.; Maffione, M.; Langereis, C.G.; Vissers, R.L.; Cherchi, A.; Schroeder, R.; Madani, H.; Columbu, S. Eocene rotation of Sardinia, and the paleogeography of the western Mediterranean region. Earth Planet. Sci. Lett. 2014, 401, 183–195. [Google Scholar] [CrossRef]
- Schmitt, T.; Fritz, U.; Delfino, M.; Ulrich, W.; Habel, J.C. Biogeography of Italy revisited: Genetic lineages confirm major phylogeographic patterns and a pre-Pleistocene origin of its biota. Front. Zool. 2021, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Paiero, P.; Martini, F.; Colpi, C. Leguminose Arboree E Arbustive in Italia; Edizioni Lint: Trieste, Italy, 1993. [Google Scholar]
- Pemberton, R.W.; Irving, D.W. Elaiosomes on Weed Seeds and the Potential for Myrmecochory in Naturalized Plants. Weed Sci. 1990, 38, 615–619. [Google Scholar] [CrossRef]
- Devenish, A.J.M.; Gomez, C.; Bridle, J.R.; Newton, R.J.; Sumner, S. Invasive ants take and squander native seeds: Implications for native plant communities. Biol. Invasions 2018, 21, 451–466. [Google Scholar] [CrossRef]
- Van Dommelen, P. Colonial interactions and hybrid practices: Phoenician and Carthaginian Settlement in the Ancient Mediterranean. In The Archaeology of Colonial; Encounters Stein, G.J., Ed.; Comparative Perspectives: Oxford, UK, 2005; pp. 109–142. [Google Scholar]
- De Marco, G.; Altieri, A.; Estabrook, G.F. Relazioni evolutive e biogeografiche dei popolamenti ad areale disgiunto di Genista ephedroides DC. Biogeographia 1987, 11, 115–130. [Google Scholar] [CrossRef]
- De Castro, O.; Véla, E.; Vendramin, G.G.; Gargiulo, R.; Caputo, P. Genetic structure in the Genista ephedroides complex (Fabaceae) and implications for its present distribution. Bot. J. Linn. Soc. 2015, 177, 607–618. [Google Scholar] [CrossRef]
- Coulot, P.; Rabaute, P. Monographie des Leguminosae de France: Tribus des Fabeae, des Cicereae et des Genisteae. Bull. Soc. Bot. Centre-Ouest 2016, 46, 1–902. [Google Scholar]
- Paradis, G.; Chiappe, M. Origine du Genista de l’île Mezzu Mare (Corse): Une énigme résolue. Bull. Soc. Bot. Centre-Ouest 2017, 48, 143–146. [Google Scholar]
- Paradis, G.; Appietto, A.; Piazza, C. Répartition en 2018 sur l’île Mezzu Mare (Corse) du genêt introduit Genista tyrrhena subsp. pontiana. Monde ses Plantes 2015, 517, 3–5. [Google Scholar]
- Kress, W.J.; Erickson, D.L. A Two-Locus Global DNA Barcode for Land Plants: The Coding rbcL Gene Complements the Non-Coding trnH-psbA Spacer Region. PLoS ONE 2007, 2, e508. [Google Scholar] [CrossRef] [PubMed]
- Calviño, C.I.; Downie, S.R. Circumscription and phylogeny of Apiaceae subfamily Saniculoideae based on chloroplast DNA sequences. Mol. Phylogenet. Evol. 2007, 44, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.B. Four primer pairs for the amplification of chloroplast intergenic regions with intraspecific variation. Mol. Ecol. 1999, 8, 521–523. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).