Abstract
In the context of plant breeding, bioinformatics can empower genetic and genomic selection to determine the optimal combination of genotypes that will produce a desired phenotype and help expedite the isolation of these new varieties. Bioinformatics is also instrumental in collecting and processing plant phenotypes, which facilitates plant breeding. Robots that use automated and digital technologies to collect and analyze different types of information to monitor the environment in which plants grow, analyze the environmental stresses they face, and promptly optimize suboptimal and adverse growth conditions accordingly, have helped plant research and saved human resources. In this paper, we describe the use of various bioinformatics databases and algorithms and explore their potential applications in plant breeding and for research on plant disease resistance.
1. Introduction
Broadly speaking, bioinformatics covers the interdisciplinary studies of biological objects (including genes, proteins, and physiological indices) using informatics methods, such as various algorithms and statistical methods. Specifically, complex biological data can be processed using computer tools, which is common practice in dedicated databases, such as in nucleic acid databases, protein databases, and custom functional databases [1]. The implementation of bioinformatics tools reduces the cost of complex analyses, thus enhancing research into topics such as sustainable agriculture [2]. Understanding how bioinformatics can be applied to plant biology research is therefore important for researchers in the life sciences, and here, we have provided a description of these tools and their applications, focusing on plant breeding and research on disease resistance. For example, based on the VPg gene sequence of a PVY (Y virus) isolated from potato, combined with all published sequences in GenBank, two things can be inferred: the rate of evolution of PVY and the time to reach the most recent common ancestor using a Bayesian system dynamics framework to advance disease resistance studies in potatoes [3,4,5]. Given that multifactorial traits involved in resistance and quality are extremely difficult to improve, especially in combinations, and some of the genomes of major forage crops, such as maize, rice, wheat, sorghum and barley, and the forage legumes soybean and alfalfa, are too large to be analyzed using whole-genome sequencing, attention has been focused on comparative genomic approaches in order to produce seeds with desirable shapes [6,7,8].
The typical datasets generated by plant researchers contain morphological, physiological, molecular, and genetic information that describes the entire plant life cycle. Bioinformatics process the collected data and extract key indices and trends to quickly and accurately generate hypotheses and then offer solutions. For example, phenotypes and genotypes can be combined to reveal the underlying mechanism, such as the study of plant rejuvenation [9], and the future growth pattern of plants can be predicted according to the growth trend of plants in the past, such as the plant growth pattern prediction system, developed by deep learning [10], and the comparison of multiple genomes can be used for the prediction of evolutionary relationships, such as in the study of Amphicarpaea edgeworthii [11].
In agricultural applications, the wide utilization of bioinformatics can assist with efficient crop breeding and the improvement of plant resistance against pathogens [12]. In particular, scientists are committed to breeding and modifying crop species to improve the yield and quality, as well as creating new varieties with qualities that benefit human nutrition and health. Bioinformatics accelerates the generation and deployment of these new varieties. Indeed, genes associated with specific traits can be analyzed on a computer before being introduced into a plant, and the results can be used to determine what to introduce further into the plant for a precise phenotypic analysis. Maize (Zea mays L.) kernels, rich in lysine [13]; lettuce (Lactuca sativa), high in vitamin C [14]; and the recently developed vitamin D-rich tomato [15] are examples of the implementation of such pipelines.
Bioinformatics plays a critical role in data integration, analysis, and model prediction, as well as in managing the massive amounts of data resulting from new, high-throughput approaches [16]. Classical biological experiments, such as the visualization of mitosis and meiosis and pollen tube growth, are undergoing deeper, higher throughput exploration thanks to bioinformatics and time-lapse microscopy [17,18]. Plant growth can be predicted based on the available wealth of physiological and phenotypic data, enabling the generation of a virtual plant that can accurately predict growth patterns and the consequences of interactions with diseases or pests [19]. Bioinformatics has also wide applications in the analysis of plant resistance to various stresses [20]. The molecular mechanisms underlying plant responses to abiotic stress have been studied in depth, and they can open new avenues in agriculture when combined with the predictive power of bioinformatics [21]. In addition, bioinformatics has been applied in plant pathology, such as identifying and predicting the “effector” proteins produced by plant pathogens in order to manipulate their host plants. The functional annotation of this pathogen’s ability to predict virulence is a critical step in translating the sequence data into potential applications in plant pathology [22]. A bioinformatics framework has been proposed to enable stakeholders to make more informed decisions. In this way, a shared biosecurity infrastructure can be established to cater for sustainable global food and fiber production in the context of global climate change and the increased chances of accidental disease invasions in the global plant trade [23]. To develop new strategies for plant disease control, researchers must elucidate the complex molecular mechanisms underlying pathogen infection. Whole genome sequencing technology has enabled the sequencing of an increasing number of pathogens and the accumulation of large amounts of genetic data. Therefore, bioinformatics tools for analyzing pathogen genomes, effectors, and interspecific interactions have been developed to understand disease infection mechanisms and pathogenic targets, which all contribute to plant pathology [24].
In this review, we focus on the applications of bioinformatics in crop breeding and the study of resistance to various stress factors: to (1) list the applications of bioinformatics in plant research, (2) to clarify the application of bioinformatics in plant breeding, (3) to emphasize the advances made by bioinformatics in the study of plant tolerance and disease resistance, (4) to predict the plant growth by bioinformatics, and (5) to call for a greater use of bioinformatic methods in plant research.
2. Databases Provide Abundant Gene and Pathway Information to Study Plant Biology
Thanks to large-scale sequencing technologies, vast amounts of data are released continuously and are often uploaded to a specific database. Depending on the species they represent, databases can be formally classified as general or species-specific databases.
General databases include those that integrate information about genomes, proteins, and metabolic pathways (Table 1). Genome databases represent a centralized and public collection of all published data, so researchers can easily obtain information concerning their gene or protein of interest. For example, UniProt offers a comprehensive resource for protein sequences and functional annotation. The database can be queried with a specific gene/protein name or with keywords of interest to sort through the catalogued data, but it is also possible to perform a protein BLAST (basic local alignment search tool) and download the sequence of the new protein of interest [25]. In addition, general databases compile various biological pathways, such as those represented in Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), EuKaryotic Orthologous Groups (KOG), and metabolic pathways, which can be used to determine if a candidate protein belongs to one of many known pathways.

Table 1.
General databases used for data integration and presentation.
As one example, a bioinformatic analysis of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) from multiple C3 plant species included an in silico characterization of RuBisCO and its interacting proteins, whose structures and functions were predicted with the ProtParam, SOPMA, Predotar 1.03, SignalP 4.1, TargetP 1.1, and TMHMM 2.0 tools, which are all accessible from the ExPASy database. A MEME and MAST analysis of RuBisCO from all C3 plants, combined with a phylogenetic tree constructed with MEGA 6.06 software based on a sequence alignment obtained with the ClustalW algorithm, illustrated the high-sequence identity shared by RuBisCO from different C3 plant species, supporting the notion that they originated from a common ancestor [26]. A list of these databases and how they are used is provided in Table 1.
The model plant Arabidopsis thaliana is one of a few plant species with its own databases due to its widespread use in plant research (Table 2). These databases are rich in resources and can help researchers quickly obtain the latest Arabidopsis genome information. For example, The Arabidopsis Information Resource (TAIR) database allows users to download gene sequences in bulk, while the SeqViewer in TAIR also provides a simple tool to visualize the genes. In addition, TAIR has a powerful function for displaying various expression maps, each representing expression data during Arabidopsis development or under different growth conditions [27].

Table 2.
Databases specific for Arabidopsis.
Most major crops have dedicated databases, including rice (Oryza sativa), wheat (Triticum aestivum), barley (Hordeum vulgare), maize, soybean (Glycine max), cotton (Gossypium hirsutum), and sorghum (Sorghum bicolor) (Table 3). For example, the Rice Mutant Database (RMD) includes mutants for the identification of new genes and regulatory elements and includes a list of lines for the ectopic expression of target genes in specific tissues or at specific growth stages, providing rich data resources for the study of different rice mutants [28]. The Wheat Genomic Variation Database (WGVD) compiles all published single nucleotide polymorphisms (SNPs), insertion/deletion polymorphisms (InDels), and selection sweeps, together with a BLAST search tool for wheat [29]. Researchers at Huazhong Agricultural University have developed the ZEAMAP database for corn, which includes multiple omics data resources, such as genomes, transcriptomes and genetic variation, phenotypic data, metabolome studies, and genetic maps. The database also provides access to a variety of data on complex traits and boasts rich online capacities for data retrieval, analysis, and visualization [30].

Table 3.
Databases used for major crops.
3. Various Algorithms Create Possibilities for Customized Analysis
Bioinformatics tools or websites can be used to predict protein structure, to look for conserved domains in a protein, or to annotate genes (Table 4). Data visualization and presentation are an integral part of bioinformatics analysis [31]. The biggest advantages of TBtools are batch processing and the visualization of data, and the interactive graphics generated with TBtools are rich with editable features that provide maximum flexibility for users [32]. Protter supports protein data analysis and protein prediction by visualizing the characteristics of an annotated sequence and associated experimental proteomic data in a protein topological environment. Protter is of great use for comprehensive visualization of membrane proteins and the selection of targeted proteomic peptides [33].

Table 4.
Bioinformatics tools and websites that can be used in plant research.
Many tools are also tailored for specific applications (Table 5), including the prediction of transcription factor binding sites and the exploration of large-scale genomic variation data. For example, the PlantPAN database hosts a comprehensive list of transcription factors and their cognate binding sites. TRANSFAC and PlnTFDB are comprehensive databases of plant transcription factors, and AGRIS contains a database of Arabidopsis transcription factors, which can be used to predict transcription factor binding sites in plant promoter regions [34]. SnpHub can be used to retrieve, analyze, and visualize large-scale genomic variation data by specifying samples and lists of specific genomic regions [35].

Table 5.
Bioinformatics tools specific to applications in plant research.
Outside of dedicated web tools, various algorithms can be used to empower data integration and analysis, such as Python, R, and Perl. Python and R are perhaps more widely used in bioinformatics than Perl. R has powerful statistical functions, which are very useful for processing large experimental datasets, together with a graphics solution for data exploration [36]. Python is better suited for building databases and web applications and is better for developing utilities [37]. While the basic introductory programming paradigm in R relies on so-called functions hosted by user-written packages, Python’s programming paradigm is based on design flow. Although R code might not be as human-readable as Python’s, R is overall better suited to biologists with no strong programming background. Based on these programming languages, various scripts have been developed to efficiently analyze data. For example, R uses a k-means function for clustering analysis and can draw Manhattan plots produced from genome-wide association studies (GWASs) with the qqman package [38].
4. Application of Bioinformatics in Plant Breeding
Plant breeding aims to produce new plant varieties. This long-term activity begins with basic research and often takes many years, thus necessitating a significant financial investment [39]. Genomics-assisted breeding is an effective and economical strategy and is thus widely applied in crop breeding. Genomics may help to understand the organization and function of biological systems and has the potential to track the molecular changes during development under different conditions, such as changes in plant physiology, pathogen pressure, or in the environment [40]. Samples for genomics studies can be collected from the same or different individuals from one species or from different species [41]. In addition, comparative genomics allows the study of specific traits in related plants by capitalizing on sequence conservation between species with small genomes (easier to study) and those with large and complex genomes (more difficult to study, but including most current crop species). For example, in Chrysanthemum, GWASs have been used to explore genetic patterns and identify favorable alleles for several ornamental and resistance traits, including plant structural and inflorescence traits, waterlogging tolerance, aphid resistance, and drought tolerance [42]. Su et al. transferred a major SNP co-isolated with waterlogging tolerance in Chrysanthemum to a PCR-based derived cut amplified polymorphism sequence (dCAPS) marker with an accuracy of 78.9%, which was verified in 52 cultivars or progenitors [43]. Chong et al. developed two dCAPS markers associated with the flowering stage and diameter of the head in Chrysanthemum. These dCAPS markers have potential applications in the molecular breeding of Chrysanthemum [44]. These techniques will provide new powerful tools for future Chrysanthemum breeding.
4.1. Bioinformatics Can Be Applied to Breed Germplasm with High Yield and Quality
Bioinformatics can be applied to crop breeding to improve yield and quality (Figure 1) [45]. Through a bioinformatics analysis of the genes related to seed germination, seedling growth and reproductive yield, and artificial interference with relevant genes, crops can be further improved [46]. For example, the adaptability, yield, and quality of rapeseed (Brassica napus) have been the target of genetic improvement via breeding [47]. In addition, bioinformatics methods can help measure the best leaf angle for the highest photosynthetic rates, to create plants with an optimal leaf angle, which may increase the accumulation of organic matter in plants.
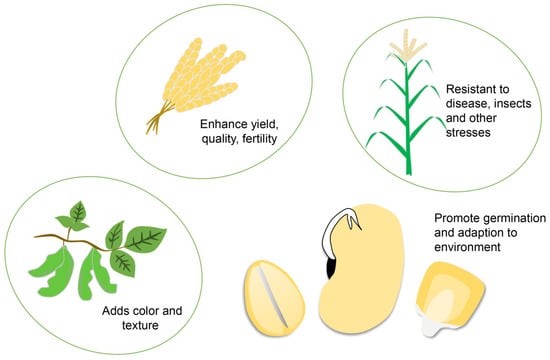
Figure 1.
Breeding indicators that can be improved using bioinformatics. Breeding aims to integrate various indicators such as yield, quality, fertility, disease resistance, insect resistance, collapse resistance, as well as salt resistance and adaptability to adverse environments such as drought, waterlogging, high temperature, low temperature, and salinity to achieve superior varieties.
4.2. Bioinformatics Can Accurately Predict Plant Growth and Conditions
Plant leaf angle (Figure 2) has a great impact on plant photosynthesis. Reasonable close planting is an effective method to increase crop yield by increasing the photosynthetic area. Leaf angle is a key character of plant structure and a target of crop genetic improvement. Under high density planting, upright leaves can better capture light, which improves photosynthetic efficiency, ventilation, and stress tolerance, and ultimately, increases grain yield. Considerable evidence has shown that auxin, gibberellins (GAs), lactones (SLs), and ethylene contribute to leaf angle formation [48]. For example, LsNRL4 deletion in lettuce resulted in chloroplast enlargement, reduced the amount of cell space allocated to chloroplasts, and caused defective secondary cell wall biosynthesis in leaf joints. Overexpression of LsNRL4 significantly decreased leaf angle and improved photosynthesis [49]. In the applications, it is possible to use the bioinformatics method to measure the stronger leaf angle of plant photosynthesis, from the perspective of bioinformatics analysis to create the optimal angle of leaves, and to increase the accumulation of organic matter in plants. For example, the QTL of the opposite leaf angle in maize and the key part of regulating leaf angle in the leaf tongue region were studied [50], and the leaf angle extractor (LAX) developed based on the image-processing framework of MATLAB, which quantifies corn and sorghum leaf angles from image data. LAX can be used to analyze changes in leaf angle across multiple genotypes and measure their response to drought stress, and it is particularly used in tracking individual plants over time [51].
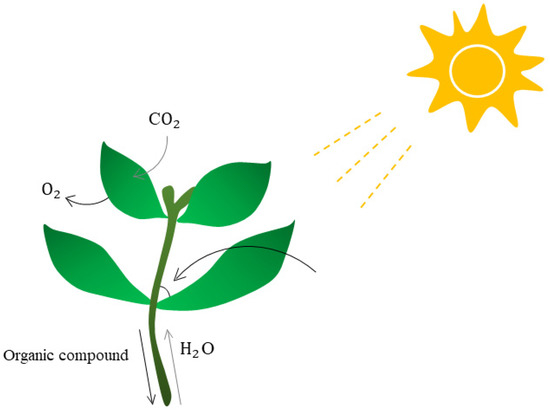
Figure 2.
The effect of plant leaf angle. The angle of plant leaves has a strong influence on many plant activities. When the plant leaf angle is optimal, plants enjoy a high photosynthetic rate, thus producing more organic matter. Creating new species with optimal leaf angles could help plants synthesize more organic matter, leading to better growth and, in turn, increased survival and yield.
Mineral malnutrition has significant effects on plant development (Figure 3) [52], especially the lack of nitrogen, potassium, calcium, phosphorus, and iron, which is a huge problem for agriculture [53]. Watching for early warning signs of deficiency in these elements and knowing how to remedy this problem is of great significance for agriculture. Current methods employed to determine nutritional deficiency in plants rely on the analysis of mineral contents in the soil and/or in the plant. However, these methods are expensive and time consuming. The physiological state of legumes changes when plants experience deficiency in any of these macro- and microelements, which can be reflected in the changes of the chlorophyll fluorescence transient [54]. Nutritional deficiency in the above elements is accompanied by damage to the electron transport chains on the donor and acceptor sides of photosystem II (PSII) and PSI [55]. Based on chlorophyll fluorescence data, one study used back propagation within artificial neural networks to identify missing elements [56]. From this analysis, researchers then proposed a new method for determining plant nutrient deficiencies based on rapid chlorophyll fluorescence measurements, which can accurately predict whether legumes lack N, P, K, Ca, and Fe before the plants start to show obvious signs of a deficiency. This new method clearly illustrates the potential for incorporating bioinformatics into the early detection and prevention of plant nutritional deficiency.
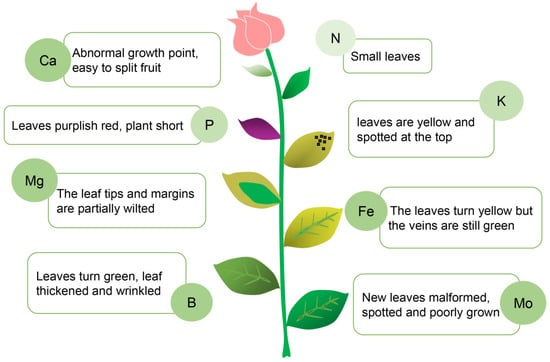
Figure 3.
The effects of specific mineral nutrient deficiency on plants. The nutritional status of a plant is reflected in leaf shape and leaf color. Based on the information collected by visualizing the leaves, the appropriate nutrients can be supplied to the plant in a targeted manner to achieve precise fertilization. This chart is an example of a moonflower.
4.3. Automation for Agriculture
The advancement of automation and digital technologies has led to the development and use of automated robots. The development of smart agriculture has changed the time-consuming and labor-intensive nature of traditional agriculture and has improved the efficiency of agricultural practices [57,58]. For instance, an electric sprayer can be assembled on a robot that can calculate the leaf density of the plants beneath, which is then used by the controller to adjust the air flow, water rate, and the water density of the sprayer for optimal watering and the precise application of pesticides, thus limiting the potential for chemical residues in the soil [59]. An automated irrigation system was later developed that provides both efficient water use and real-time monitoring of the environment. The irrigation system uses a NodeMCU ESP32 microcontroller to collect environmental data such as humidity, temperature, and soil moisture levels through sensors that can irrigate plants at specific times and is also equipped with a passive infrared sensor to detect intruders in the vicinity of the farmland and warn the farmer if severe conditions, such as extreme temperatures, are detected. This system can therefore automatically irrigate farmland without human intervention and allows farmers to monitor and manually control irrigation with the use of a smartphone app [60].
Some researchers have applied bioinformatics to breeding and developed a bioinformation breeder, which can transfer the good traits of donor crops to recipient crops after processing, so that the good traits of recipient crops coincide with the good traits of donor crops. A large number of experimental results show that the biological energy breeding machine can realize the transfer of biological information across space, realize the donor plants biofield through a biological information transfer machine across space in a relatively short time, and influence or induce the change of the receptor plant’s genetic traits. This new breeding method operation is simple, low cost, and does not destroy the organism’s own genes. To meet the health needs of human beings, new varieties with high yield, high quality, and harsh environment resistance are produced at the same time, thus creating a new stage of breeding industry [61]. However, this bioinformation breeder is based on a special kind of bioenergy—biomicrowave. Although the magnitude of biomicrowave is much lower than an electron volt, a large number of experiments have shown that this weak energy can not only transmit biological information, but also affect the protein activities of biological receptors across space. However, because the biological microwave (approximately 4–20 μm) is the lowest energy state in nature, which is involved in quantum, biology, electronics, microwave, and many other scientific and technological fields, it has not been developed and widely applied [62].
4.4. Accurate Prediction of Experimental Results and Transgenic Phenotypes
In plant research, genotype-phenotype prediction has traditionally used statistical methods. For example, two statistical methods, autoregressive (AR) and Markov chain (MCMC), are used to predict the growth trends of plants by using the Normalized Difference Vegetation Index (NDVI) [63]. Importantly, the application of machine learning to genotype–phenotype prediction will facilitate the study of the roles played by various molecular components in shaping plant phenotypes. The advantages of machine learning over traditional statistical methods are that machine learning can distinguish between different types of genomic regions, and also predict the location of genomic crossovers, which expands the application of machine learning to population genetics [64].
Plant breeders increasingly rely on genomic selection, that is, the selection of favorable alleles at specific loci, which requires the mapping and localization of quantitative trait loci (QTL) to describe the underlying genetic architecture of a given trait and clone the causal alleles. For example, QTLs have been identified in durum wheat for protein grain content [65], high grain yield [66], disease resistance [67,68], and quality traits [69]. In Chrysanthemum, several QTLs controlling flower color, flowering time, ray floret number, and disc floret number were identified by multiallelic QTL analysis. Similarly, seven QTLs affecting tuber shape were detected in the potato (Solanum tuberosum) [70]. In maize, several QTLs were described for both insect resistance [71] and for multiple drug resistance, relating to disease resistance research [72]. QTLs were also reported for thrips resistance in pepper (Capsicum annuum) [73] and to explore the genetic basis of cooking time and protein concentration of dried beans from Phaseolus vulgaris L. [74]. Several researchers have explored machine learning methods for QTL localization (mainly for pre-screening), but their use is still limited. Alternatively, deep learning has been successfully applied to plant phenotype identification. For example, a Convolutional Neural Network (CNN) was used to detect and classify spikes and spikelets in wheat images to study plant development [75].
5. Application of Bioinformatics in Research on Plant Disease Resistance and Various Abiotic Stresses
Plants face many environmental challenges, such as diseases and insect pests, light, extreme temperatures, water availability, soil salinity, and other stresses [76]. Plant tolerance to biotic and abiotic stresses is controlled by cross adaptation. Different stresses cause distinct changes in plants (Figure 4). Under high light or drought conditions, perturbations in the Calvin–Benson–Bassham (CBB) cycle generate reactive oxygen species (ROS) and activate chloroplast-to-nucleus retrograde signaling to confer drought tolerance [77]. Under high-temperature stress conditions, the steady-state levels of the nucleocytoplasmic immune regulators ENHANCED DISEASE SUSCEPTIBILITY 1 and PHYTOALEXIN DEFICIENT 4 also decrease [78]. At low temperatures, cold signals lead to an increased accumulation of salicylic acid and pathogenesis-related (PR) proteins [79]. In addition, the transcription factor ELONGATED HYPOCOTYL 5 (HY5) accumulates to promote COLD-REGULATED gene expression and an acclimation to cold [80]. Following its cold-induced monomerization and nuclear translocation, NONEXPRESSER OF PR GENES 1 functions with the heat shock factor HSFA1 to confer freezing tolerance [81]. The cell wall undergoes softening and remodeling under high salinity [82]. The fluidity, integrity, and function of phospholipid membranes are affected by different stresses [83]. Chrysanthemum dwarf viroids (CSVd) can invade the leaf primordium and cells very close to the apical dome or even the outermost layer of the apical dome. The lack of CSVd in shoot tip seriously affected the asexual propagation of chrysanthemum [84]. Some researchers have developed a plant disease resistance protein predictor (RD-RFPDR), which showed to be sensitive and specific in identifying DR proteins after excluding data imbalance. This can provide a method for predicting plant disease resistance proteins [85].
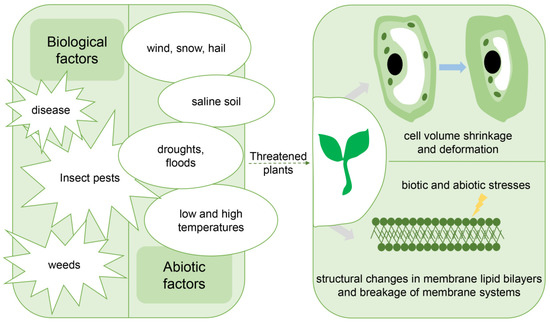
Figure 4.
Some plant resistance factors and their effects. Plants are continuously exposed to biotic and abiotic stresses. Plant tolerance to biotic and abiotic stresses is controlled by cross tolerance. Although each stress will impose distinct physical and chemical effects on plants, most stresses will often directly or indirectly lead to water stress at the whole-plant scale, causing water loss, cell volume shrinkage and deformation, structural changes in the membrane lipid bilayer, and degradation of the membrane system. Therefore, a common basis for plant resistance is osmoregulation, changes in the interaction between membrane lipids and membrane proteins, and repair activities.
5.1. Predicting Plant Resistance Based on Key Indicators
The molecular regulatory networks underlying plant stress resistance and adaptation can be studied in conjunction with genomics and proteomics [86]. Technologies such as transcriptome deep sequencing (RNA-seq) can provide a wealth of information on differential gene expression to study key genes involved in plant stress tolerance. Bioinformatics, next-generation sequencing, and genomics help us to better understand the molecular mechanisms of plant tolerance to different stress conditions and can aid in breeding novel plant varieties and improving crop quality [87].
Several groups used RNA-seq analysis to investigate the transcriptional changes in different crop plants [88]. For example, genes whose transcript levels increased in response to salt and drought stress were identified in chickpea (Cicer arietinum) at different developmental stages [89]; differentially expressed genes were described in cold-tolerant and cold-sensitive varieties of sorghum in response to cold stress [90] Likewise, abiotic stress was shown to induce transcriptional reprogramming in poplar (Populus trichocarpa) [91]; changes in gene expression were reported in heat-resistant rice [92]; and genes potentially related to heat stress tolerance were proposed in spinach (Spinacia oleracea) [93]. Genomics is a powerful tool to study the molecular mechanism behind plant stress tolerance, as recently illustrated by the studies of salt stress in plants [94] and of drought tolerance in rice [95]. In addition, a comparative analysis of transcriptome datasets in tobacco revealed the genes of resistance and susceptibility to Phytophthora nicotiana, which provided valuable resources for breeding resistant tobacco plants [96]. As for the study on sorghum, differential gene expression analysis identified more than 3000 specific genes in two sorghum cultivars with resistance and susceptibility to anthracnose disease, and showed significant changes in the expression of these genes after inoculation with anthracnose [97].
5.2. Genome Annotation Is Available and Feasible
Identifying genes from the mass of genomic sequences is an important task in bioinformatics. The annotation of genome sequences can encounter one of two scenarios: (1) gene annotation is performed for a few target sequences with the aim of understanding the possible complement of functional genes, for example, the members of a specific gene family; and (2) gene annotation is performed at the whole-genome level for a newly sequenced genome [98]. In the first scenario, gene annotation of the target sequences can be performed using free online gene prediction platforms and search platforms; the second scenario usually calls upon ab initio prediction algorithms.
Bioinformatics is instrumental for many experiments that explore how plants respond to adverse growth conditions and adapt accordingly. Eelgrass (Zostera marina), for example, lacks stomata because the key genes responsible for stomatal developmental are missing in this species, perhaps as an adaptation to life in the ocean. Variation in the CAZyme protein family resulted in a thickening of the cuticle of eelgrass. Changes in sucrose synthase and transport genes also led to changes in the metabolic pathways [99]. These studies were only made possible by bioinformatics approaches.
To adapt to a high salinity environment, halophytes have evolved many unique characteristics. Bioinformatics has also greatly facilitated the comparative analysis of halophytes to reveal their adaptations to saline-alkali soils [100]. Selaginella is a kind of xerophyte that has adapted to arid environments by thickening the cuticles of leaves, the causes of which were analyzed using bioinformatics [101]. Carnivorous plants use animals and insects as their source of nutrition; bioinformatics revealed the molecular basis that led to the evolution of carnivorous plants [102]. With more genomes available, bioinformatics also uncovered the evolutionary lineages of plants. For example, in the study of water lilies (Nymphaea colorata), a phylogenetic analysis determined that Amborellales and Nymphaeales are the successive sister lineages to all other extant angiosperms [103].
5.3. Multiple Genes Can Be Merged to Analyze Their Roles in Various Resistance
Ab initio methods are an important research area in bioinformatics, and many prediction algorithms and corresponding procedures have been proposed and applied successively. Unlike homology-based comparison methods, ab initio prediction methods are based on the statistical characteristics of coding regions and gene signals for the prediction of gene structure [104].
The transfer of exogenous DNA into plant genomes has greatly promoted the progress of basic and applied plant research [105]. Plant genome engineering can be harnessed to alter plant metabolism and produce the desired metabolites, as well as improving crop traits. Different transformation methods and strategies have been developed to allow the simultaneous production of multiple plant- or non-plant-derived recombinant proteins in transgenic plant hosts [106]. Future studies on improving plant stress resistance should focus more on the combination of multiple approaches, such as the introduction of multiple genes simultaneously into transgenic plants. An alternative to stacking multiple genes in transgenic plants is the use of iterative or serial transformation strategies, whereby the genes of interest are introduced one at a time through successive rounds of transformation [107,108] or by sexual crossing of transgenic lines, carrying different transgenes to bring them together in the same background [109,110,111,112]. For example, genes involved in osmoprotectant biosynthesis have been co-expressed with other stress resistance-related genes, such as ion transporters and transcription factors.
6. Perspective
In the era of big data, bioinformatics faces opportunities and challenges for its application to agriculture. Learning and developing more bioinformatics tools will help integrate all existing bioinformation resources and provide support for effective breeding and plant resistance analysis [113].
Food production systems are under tremendous pressure due to the continuous growth of the human population. Many of the world’s ecosystems are already overexploited, and meeting the growing demand for food by expanding arable land use is not possible [114]. Indeed, according to the Food and Agriculture Organization (FAO), only 10% of future growth in agricultural production will come from the expansion of acreage, while the remaining 90% must come from yield hikes [115]. The development of genomics technology has provided huge technical support for breeders, who have been able to continuously breed new varieties that are more adaptable to the environment and have higher yields, leading to the continuous improvement of seed replacement rate.
The bioinformatics era initiated by NGS has revolutionized the design of experiments in molecular biology, substantially contributing to the growth of scientific knowledge while influencing relevant applications in many different aspects of agriculture [116]. Data from different research areas support the co-development and advancement of molecular knowledge through extensive efforts, with bioinformatics being the driving force. Organization, detection, integration of data, and data sharing are facilitating multidisciplinary interactions, expanding resources, and disseminating common methods [117]. Bioinformatics is thus revolutionizing agricultural practices and production, providing knowledge and tools to improve product quality, and improving strategies to counteract environmental stresses, diseases, and parasites [8,118]. Bioinformatics is evolving, and we have great hopes for the integration of bioinformatics in plant research.
Author Contributions
Conceptualization, F.Y.; Writing—Original Draft, H.M.; Writing—Review & Editing, F.Y. and B.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Research Foundation of China (NSFC, project nos. 32170301, 31770288 and 31600200), and the MOE Layout Foundation of Humanities and Social Sciences (21YJAZH108).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Conflicts of Interest
The authors declare no competing interests.
References
- Chen, C.; Huang, H.; Wu, C.H. Protein Bioinformatics Databases and Resources. Methods Mol. Biol. 2017, 1558, 3–39. [Google Scholar] [CrossRef] [PubMed]
- Małyska, A.; Jacobi, J. Plant breeding as the cornerstone of a sustainable bioeconomy. New Biotechnol. 2018, 40, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Sun, X.; Shen, J.; Gao, F.; Qiu, G.; Wang, T.; Nie, X.; Zhang, W.; Gao, Y.; Bai, Y. Molecular Evolutionary Analysis of Potato Virus Y Infecting Potato Based on the VPg Gene. Front. Microbiol. 2019, 10, 1708. [Google Scholar] [CrossRef] [PubMed]
- Blätke, M.A.; Szymanski, J.J.; Gladilin, E.; Scholz, U.; Beier, S. Advances in Applied Bioinformatics in Crops. Front. Plant Sci. 2021, 12, 640394. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, U.K.S.; Deo, I.; Jaiswal, J.P.; Prasad, B. Role of Bioinformatics in Crop Improvement. Glob. J. Sci. Front. Res. 2017, 17, 13–23. [Google Scholar]
- Alemu, K. The role and application of bioinformatics in plant disease management. Adv. Life Sci. Technol. 2015, 28, 28–33. [Google Scholar]
- Khan, A.; Singh, S.; Singh, V.K. Bioinformatics in Plant Pathology. In Emerging Trends in Plant Pathology; Singh, K.P., Jahagirdar, S., Sarma, B.K., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Vassilev, D.; Leunissen, J.; Atanassov, A.; Nenov, A.; Dimov, G. Application of Bioinformatics in Plant Breeding. Biotechnol. Biotechnol. Equip. 2005, 19, 139–152. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Y.; Li, Y. Plant rejuvenation: From phenotypes to mechanisms. Plant Cell Rep. 2020, 39, 1249–1262. [Google Scholar] [CrossRef]
- Yasrab, R.; Zhang, J.; Smyth, P.; Pound, M.P. Predicting Plant Growth from Time-Series Data Using Deep Learning. Remote Sens. 2021, 13, 331. [Google Scholar] [CrossRef]
- Song, T.; Zhou, M.; Yuan, Y.; Yu, J.; Cai, H.; Li, J.; Chen, Y.; Bai, Y.; Zhou, G.; Cui, G. First high-quality reference genome of Amphicarpaea edgeworthii. bioRxiv 2020. [Google Scholar] [CrossRef]
- Gomez-Casati, D.F.; Busi, M.V.; Barchiesi, J.; Peralta, D.A.; Hedin, N.; Bhadauria, V. Applications of Bioinformatics to Plant Biotechnology. Curr. Issues Mol. Biol. 2018, 27, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Houmard, N.M.; Mainville, J.L.; Bonin, C.P.; Huang, S.; Luethy, M.H.; Malvar, T.M. High-lysine corn generated by endosperm-specific suppression of lysine catabolism using RNAi. Plant Biotechnol. J. 2007, 5, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, R.H.; Fu, X.; Sun, X.; Tang, K. Over-expression of l-galactono-γ-lactone dehydrogenase increases vitamin C, total phenolics and antioxidant activity in lettuce through bio-fortification. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 114, 225–236. [Google Scholar] [CrossRef]
- Li, J.; Scarano, A.; Gonzalez, N.M.; D’Orso, F.; Yue, Y.; Nemeth, K.; Saalbach, G.; Hill, L.; de Oliveira Martins, C.; Moran, R.; et al. Biofortified tomatoes provide a new route to vitamin D sufficiency. Nat. Plants 2022, 8, 611–616. [Google Scholar] [CrossRef]
- Ko, G.; Kim, P.-G.; Yoon, J.; Han, G.; Park, S.-J.; Song, W.; Lee, B. Closha: Bioinformatics workflow system for the analysis of massive sequencing data. BMC Bioinform. 2018, 19, 43. [Google Scholar] [CrossRef]
- Luciano, A.M.; Peluso, J.J. PGRMC1 and the faithful progression through mitosis and meiosis. Cell Cycle 2016, 15, 2239–2240. [Google Scholar] [CrossRef]
- Hoffmann, R.D.; Portes, M.T.; Olsen, L.I.; Damineli, D.S.C.; Hayashi, M.; Nunes, C.O.; Pedersen, J.T.; Lima, P.T.; Campos, C.; Feijó, J.A.; et al. Plasma membrane H+-ATPases sustain pollen tube growth and fertilization. Nat. Commun. 2020, 11, 2395. [Google Scholar] [CrossRef]
- Kim, T.; Lee, S.-H.; Kim, J.-O. A Novel Shape Based Plant Growth Prediction Algorithm Using Deep Learning and Spatial Transformation. IEEE Access 2022, 10, 37731–37742. [Google Scholar] [CrossRef]
- İncili, Ç.Y.; Arslan, B.; Çelik, E.N.Y.; Ulu, F.; Horuz, E.; Baloglu, M.C.; Çağlıyan, E.; Burcu, G.; Bayarslan, A.U.; Altunoglu, Y.C. Comparative bioinformatics analysis and abiotic stress responses of expansin proteins in Cucurbitaceae members: Watermelon and melon. Protoplasma 2022. [Google Scholar] [CrossRef]
- Aditya Shastry, K.; Sanjay, H.A. Hybrid prediction strategy to predict agricultural information. Appl. Soft Comput. 2020, 98, 106811. [Google Scholar] [CrossRef]
- Cock, P.J.A.; Grüning, B.A.; Paszkiewicz, K.; Pritchard, L. Galaxy tools and workflows for sequence analysis with applications in molecular plant pathology. PeerJ 2013, 1, e167. [Google Scholar] [CrossRef] [PubMed]
- Lucaciu, R.; Pelikan, C.; Gerner, S.M.; Zioutis, C.; Köstlbacher, S.; Marx, H.; Herbold, C.W.; Schmidt, H.; Rattei, T. A Bioinformatics Guide to Plant Microbiome Analysis. Front. Plant Sci. 2019, 10, 1313. [Google Scholar] [CrossRef] [PubMed]
- Dong, A.-Y.; Wang, Z.; Huang, J.-J.; Song, B.-A.; Hao, G.-F. Bioinformatic tools support decision-making in plant disease management. Trends Plant Sci. 2021, 26, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Pundir, S.; Martin, M.J.; O’Donovan, C.; null, n. UniProt Tools. Curr. Protoc. Bioinform. 2016, 53, 1.29.1–1.29.15. [Google Scholar] [CrossRef]
- Darabi, M.; Seddigh, S. Computational study of biochemical properties of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) enzyme in C 3 plants. J. Plant Biol. 2017, 60, 35–47. [Google Scholar] [CrossRef]
- Reiser, L.; Subramaniam, S.; Li, D.; Huala, E. Using the Arabidopsis Information Resource (TAIR) to Find Information About Arabidopsis Genes. Curr. Protoc. Bioinform. 2017, 60, 1.11.1–1.11.45. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Wu, C.; Xiong, L.; Chen, G.; Zhang, Q.; Wang, S. RMD: A rice mutant database for functional analysis of the rice genome. Nucleic Acids Res. 2006, 34, D745–D748. [Google Scholar] [CrossRef]
- Wang, J.; Fu, W.; Wang, R.; Hu, D.; Cheng, H.; Zhao, J.; Jiang, Y.; Kang, Z. WGVD: An integrated web-database for wheat genome variation and selective signatures. Database J. Biol. Databases Curation 2020, 2020, baaa090. [Google Scholar] [CrossRef]
- Gui, S.; Yang, L.; Li, J.; Luo, J.; Xu, X.; Yuan, J.; Chen, L.; Li, W.; Yang, X.; Wu, S.; et al. ZEAMAP, a Comprehensive Database Adapted to the Maize Multi-Omics Era. iScience 2020, 23, 101241. [Google Scholar] [CrossRef]
- Liu, W.; Xie, X.; Ma, X.; Li, J.; Chen, J.; Liu, Y.-G. DSDecode: A Web-Based Tool for Decoding of Sequencing Chromatograms for Genotyping of Targeted Mutations. Mol. Plant 2015, 8, 1431–1433. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Omasits, U.; Ahrens, C.H.; Müller, S.; Wollscheid, B. Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 2013, 30, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-C.; Lee, T.-Y.; Huang, H.-D.; Huang, H.-Y.; Pan, R.-L. PlantPAN: Plant promoter analysis navigator, for identifying combinatorial cis-regulatory elements with distance constraint in plant gene groups. BMC Genom. 2008, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Z.; Li, X.; Ni, Z.; Hu, Z.; Xin, M.; Peng, H.; Yao, Y.; Sun, Q.; Guo, W. SnpHub: An easy-to-set-up web server framework for exploring large-scale genomic variation data in the post-genomic era with applications in wheat. GigaScience 2020, 9, giaa060. [Google Scholar] [CrossRef] [PubMed]
- Ristić, M.M. R programming for bioinformatics. J. Appl. Stat. 2009, 36, 925. [Google Scholar] [CrossRef]
- Bioinformatics: Biological models in Python. Nat. Methods 2013, 10, 384. [CrossRef]
- Chen, X.-W.; Gao, J.X. Big Data Bioinformatics. Methods 2016, 111, 1–2. [Google Scholar] [CrossRef]
- Smith, A.M. FAO should focus on real not nominal food prices. Nature 2022, 602, 33. [Google Scholar] [CrossRef]
- Nordin, S.M.; Zolkepli, I.A.; Rizal, A.R.A.; Tariq, R.; Mannan, S.; Ramayah, T. Paving the way to paddy food security: A multigroup analysis of agricultural education on Circular Economy Adoption. J. Clean. Prod. 2022, 375, 134089. [Google Scholar] [CrossRef]
- Esposito, A.; Colantuono, C.; Ruggieri, V.; Chiusano, M.L. Bioinformatics for agriculture in the Next-Generation sequencing era. Chem. Biol. Technol. Agric. 2016, 3, 9. [Google Scholar] [CrossRef]
- Li, P.; Su, J.; Guan, Z.; Fang, W.; Chen, F.; Zhang, F. Association analysis of drought tolerance in cut chrysanthemum (Chrysanthemum morifolium Ramat.) at seedling stage. 3 Biotech 2018, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, F.; Chong, X.; Song, A.; Guan, Z.; Fang, W.; Chen, F. Genome-wide association study identifies favorable SNP alleles and candidate genes for waterlogging tolerance in chrysanthemums. Hortic. Res. 2019, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Chong, X.; Su, J.; Wang, F.; Wang, H.; Song, A.; Guan, Z.; Fang, W.; Jiang, J.; Chen, S.; Chen, F.; et al. Identification of favorable SNP alleles and candidate genes responsible for inflorescence-related traits via GWAS in chrysanthemum. Plant Mol. Biol. 2019, 99, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, K.; Arora, S.; Silva, P.; Sánchez-Martín, J.; Horsnell, R.; Gao, L.; Brar, G.S.; Widrig, V.; John Raupp, W.; Singh, N.; et al. Population genomic analysis of Aegilops tauschii identifies targets for bread wheat improvement. Nat. Biotechnol. 2021, 40, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Mascher, M.; Schreiber, M.; Scholz, U.; Graner, A.; Reif, J.C.; Stein, N. Genebank genomics bridges the gap between the conservation of crop diversity and plant breeding. Nat. Genet. 2019, 51, 1076–1081. [Google Scholar] [CrossRef]
- Hu, J.; Chen, B.; Zhao, J.; Zhang, F.; Xie, T.; Xu, K.; Gao, G.; Yan, G.; Li, H.; Li, L.; et al. Genomic selection and genetic architecture of agronomic traits during modern rapeseed breeding. Nat. Genet. 2022, 54, 694–704. [Google Scholar] [CrossRef]
- Cao, Y.; Zhong, Z.; Wang, H.; Shen, R. Leaf angle: A target of genetic improvement in cereal crops tailored for high-density planting. Plant Biotechnol. J. 2022, 20, 426–436. [Google Scholar] [CrossRef]
- An, G.; Qi, Y.; Zhang, W.; Gao, H.; Qian, J.; Larkin, R.M.; Chen, J.; Kuang, H. LsNRL4 enhances photosynthesis and decreases leaf angles in lettuce. Plant Biotechnol. J. 2022, 20, 1956–1967. [Google Scholar] [CrossRef]
- Peng, B.; Zhao, X.; Wang, Y.; Li, C.; Li, Y.; Zhang, D.; Shi, Y.; Song, Y.; Wang, L.; Li, Y.; et al. Genome-wide association studies of leaf angle in maize. Mol. Breed. 2021, 41, 50. [Google Scholar] [CrossRef]
- Kenchanmane Raju, S.K.; Adkins, M.; Enersen, A.; Santana de Carvalho, D.; Studer, A.J.; Ganapathysubramanian, B.; Schnable, P.S.; Schnable, J.C. Leaf Angle eXtractor: A high-throughput image processing framework for leaf angle measurements in maize and sorghum. Appl. Plant Sci. 2020, 8, e11385. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Alzarah, M.I. Macro and micro-elements concentrations in Calligonum comosum wild grazing plant through its growth period. Saudi J. Biol. Sci. 2021, 28, 6992–6999. [Google Scholar] [CrossRef] [PubMed]
- Holušová, K.; Vrána, J.; Šafář, J.; Šimková, H.; Balcárková, B.; Frenkel, Z.; Darrier, B.; Paux, E.; Cattonaro, F.; Berges, H.; et al. Physical Map of the Short Arm of Bread Wheat Chromosome 3D. Plant Genome 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The Impacts of Phosphorus Deficiency on the Photosynthetic Electron Transport Chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef]
- Aleksandrov, V. Identification of nutrient deficiency in plants by artificial intelligence. Acta Physiol. Plant. 2022, 44, 29. [Google Scholar] [CrossRef]
- Bochtis, D.D.; Sørensen, C.G.C.; Busato, P. Advances in agricultural machinery management: A review. Biosyst. Eng. 2014, 126, 69–81. [Google Scholar] [CrossRef]
- Hyde, J.; Engel, P. Investing in a robotic milking system: A Monte Carlo simulation analysis. J. Dairy Sci. 2002, 85, 2207–2214. [Google Scholar] [CrossRef]
- Baltazar, A.R.; Santos, F.N.d.; Moreira, A.P.; Valente, A.; Cunha, J.B. Smarter Robotic Sprayer System for Precision Agriculture. Electronics 2021, 10, 2061. [Google Scholar] [CrossRef]
- Suhaimi, A.F.; Yaakob, N.; Saad, S.A.; Sidek, K.A.; Elshaikh, M.E.; Dafhalla, A.K.; Lynn, O.B.; Almashor, M. IoT Based Smart Agriculture Monitoring, Automation and Intrusion Detection System. J. Phys. Conf. Ser. 2021, 1962, 012016. [Google Scholar] [CrossRef]
- Wurtzel, E.T.; Kutchan, T.M. Plant metabolism, the diverse chemistry set of the future. Science 2016, 353, 1232–1236. [Google Scholar] [CrossRef]
- Thackston, K.A.; Deheyn, D.D.; Sievenpiper, D.F. Limitations on electromagnetic communication by vibrational resonances in biological systems. Phys. Rev. E 2020, 101, 062401. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Fang, S.; Gao, J.; Zhang, Y.; Jin, G.; Wang, S.; Zhu, Y.; Xu, J. Could Vegetation Index be Derive from Synthetic Aperture Radar?—The Linear Relationship between Interferometric Coherence and NDVI. Sci. Rep. 2020, 10, 6749. [Google Scholar] [CrossRef] [PubMed]
- Guzzetta, G.; Jurman, G.; Furlanello, C. A machine learning pipeline for quantitative phenotype prediction from genotype data. BMC Bioinform. 2010, 11, S3. [Google Scholar] [CrossRef] [PubMed]
- Nigro, D.; Gadaleta, A.; Mangini, G.; Colasuonno, P.; Marcotuli, I.; Giancaspro, A.; Giove, S.L.; Simeone, R.; Blanco, A. Candidate genes and genome-wide association study of grain protein content and protein deviation in durum wheat. Planta 2019, 249, 1157–1175. [Google Scholar] [CrossRef]
- Blanco, A.; Mangini, G.; Giancaspro, A.; Giove, S.; Colasuonno, P.; Simeone, R.; Signorile, A.; De Vita, P.; Mastrangelo, A.M.; Cattivelli, L.; et al. Relationships between grain protein content and grain yield components through quantitative trait locus analyses in a recombinant inbred line population derived from two elite durum wheat cultivars. Mol. Breed. 2012, 30, 79–92. [Google Scholar] [CrossRef]
- Maccaferri, M.; Mantovani, P.; Tuberosa, R.; Deambrogio, E.; Giuliani, S.; Demontis, A.; Massi, A.; Sanguineti, M.C. A major QTL for durable leaf rust resistance widely exploited in durum wheat breeding programs maps on the distal region of chromosome arm 7BL. Theor. Appl. Genet. 2008, 117, 1225–1240. [Google Scholar] [CrossRef]
- Zhu, K.; Li, M.; Wu, H.; Zhang, D.; Dong, L.; Wu, Q.; Chen, Y.; Xie, J.; Lu, P.; Guo, G.; et al. Fine mapping of powdery mildew resistance gene MlWE74 derived from wild emmer wheat (Triticum turgidum ssp. dicoccoides) in an NBS-LRR gene cluster. Theor. Appl. Genet. 2022, 135, 1235–1245. [Google Scholar] [CrossRef]
- Blanco, A.; Colasuonno, P.; Gadaleta, A.; Mangini, G.; Schiavulli, A.; Simeone, R.; Digesù, A.M.; De Vita, P.; Mastrangelo, A.M.; Cattivelli, L. Quantitative trait loci for yellow pigment concentration and individual carotenoid compounds in durum wheat. J. Cereal Sci. 2011, 54, 255–264. [Google Scholar] [CrossRef]
- Hara-Skrzypiec, A.; Śliwka, J.; Jakuczun, H.; Zimnoch-Guzowska, E. QTL for tuber morphology traits in diploid potato. J. Appl. Genet. 2018, 59, 123–132. [Google Scholar] [CrossRef]
- Badji, A.; Otim, M.; Machida, L.; Odong, T.; Kwemoi, D.B.; Okii, D.; Agbahoungba, S.; Mwila, N.; Kumi, F.; Ibanda, A.; et al. Maize Combined Insect Resistance Genomic Regions and Their Co-localization With Cell Wall Constituents Revealed by Tissue-Specific QTL Meta-Analyses. Front. Plant Sci. 2018, 9, 895. [Google Scholar] [CrossRef]
- Martins, L.B.; Rucker, E.; Thomason, W.; Wisser, R.J.; Holland, J.B.; Balint-Kurti, P. Validation and Characterization of Maize Multiple Disease Resistance QTL. G3 Genes Genomes Genet. 2019, 9, 2905–2912. [Google Scholar] [CrossRef] [PubMed]
- Maharijaya, A.; Vosman, B.; Steenhuis-Broers, G.; Pelgrom, K.; Purwito, A.; Visser, R.G.F.; Voorrips, R.E. QTL mapping of thrips resistance in pepper. Theor. Appl. Genet. 2015, 128, 1945–1956. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.; Izquierdo, P.; Jeffery, H.; Shaw, S.; Nchimbi-Msolla, S.; Cichy, K. QTL analysis of cooking time and quality traits in dry bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2020, 133, 2291–2305. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, A.D.J.; Kootstra, G.; Kruijer, W.; de Ridder, D. Machine learning in plant science and plant breeding. iScience 2020, 24, 101890. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Loo, E.P.-I. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Savchenko, T.; Baidoo, E.E.; Chehab, W.E.; Hayden, D.M.; Tolstikov, V.; Corwin, J.A.; Kliebenstein, D.J.; Keasling, J.D.; Dehesh, K. Retrograde signaling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 2012, 149, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Parker, J.E. The impact of temperature on balancing immune responsiveness and growth in Arabidopsis. Trends Plant Sci. 2011, 16, 666–675. [Google Scholar] [CrossRef]
- Griffith, M.; Yaish, M.W.F. Antifreeze proteins in overwintering plants: A tale of two activities. Trends Plant Sci. 2004, 9, 399–405. [Google Scholar] [CrossRef]
- Catalá, R.; Medina, J.; Salinas, J. Integration of low temperature and light signaling during cold acclimation response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 16475–16480. [Google Scholar] [CrossRef]
- Olate, E.; Jiménez-Gómez, J.M.; Holuigue, L.; Salinas, J. NPR1 mediates a novel regulatory pathway in cold acclimation by interacting with HSFA1 factors. Nat. Plants 2018, 4, 811–823. [Google Scholar] [CrossRef]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.-C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA Receptor Kinase Maintains Cell-Wall Integrity during Salt Stress through Ca2+ Signaling. Curr. Biol. 2018, 28, 666–675.e5. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, G.; Ferrari, S.; Cervone, F.; Okun, E. Extracellular DAMPs in Plants and Mammals: Immunity, Tissue Damage and Repair. Trends Immunol. 2018, 39, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, T.; Matsushita, Y.; Hosokawa, M. Chrysanthemum Stunt Viroid Resistance in Chrysanthemum. Viruses 2018, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Simón, D.; Borsani, O.; Filippi, C.V. RFPDR: A random forest approach for plant disease resistance protein prediction. PeerJ 2022, 10, e11683. [Google Scholar] [CrossRef]
- Longuespée, R.; Casadonte, R.; Schwamborn, K.; Reuss, D.; Kazdal, D.; Kriegsmann, K.; von Deimling, A.; Weichert, W.; Schirmacher, P.; Kriegsmann, J.; et al. Proteomics in Pathology. Proteomics 2018, 18, 1700361. [Google Scholar] [CrossRef]
- Knief, C. Analysis of plant microbe interactions in the era of next generation sequencing technologies. Front. Plant Sci. 2014, 5, 216. [Google Scholar] [CrossRef]
- Bashir, K.; Matsui, A.; Rasheed, S.; Seki, M. Recent advances in the characterization of plant transcriptomes in response to drought, salinity, heat, and cold stress. F1000Research 2019, 8, 658. [Google Scholar] [CrossRef]
- Garg, R.; Shankar, R.; Thakkar, B.; Kudapa, H.; Krishnamurthy, L.; Mantri, N.; Varshney, R.K.; Bhatia, S.; Jain, M. Transcriptome analyses reveal genotype- and developmental stage-specific molecular responses to drought and salinity stresses in chickpea. Sci. Rep. 2016, 6, 19228. [Google Scholar] [CrossRef]
- Chopra, R.; Burow, G.; Hayes, C.; Emendack, Y.; Xin, Z.; Burke, J. Transcriptome profiling and validation of gene based single nucleotide polymorphisms (SNPs) in sorghum genotypes with contrasting responses to cold stress. BMC Genom. 2015, 16, 1040. [Google Scholar] [CrossRef]
- Filichkin, S.A.; Hamilton, M.; Dharmawardhana, P.D.; Singh, S.K.; Sullivan, C.; Ben-Hur, A.; Reddy, A.S.N.; Jaiswal, P. Abiotic Stresses Modulate Landscape of Poplar Transcriptome via Alternative Splicing, Differential Intron Retention, and Isoform Ratio Switching. Front. Plant Sci. 2018, 9, 5. [Google Scholar] [CrossRef]
- González-Schain, N.; Dreni, L.; Lawas, L.M.F.; Galbiati, M.; Colombo, L.; Heuer, S.; Jagadish, K.S.V.; Kater, M.M. Genome-Wide Transcriptome Analysis During Anthesis Reveals New Insights into the Molecular Basis of Heat Stress Responses in Tolerant and Sensitive Rice Varieties. Plant Cell Physiol. 2015, 57, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yu, L.; Xuan, J.; Lu, Y.; Lu, S.; Zhu, W. De novo transcriptome sequencing and gene expression profiling of spinach (Spinacia oleracea L.) leaves under heat stress. Sci. Rep. 2016, 6, 19473. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, M.G.; Ishizaki, T.; Valencia, M.; Ogawa, S.; Dedicova, B.; Ogata, T.; Yoshiwara, K.; Maruyama, K.; Kusano, M.; Saito, K.; et al. Overexpression of an Arabidopsis thaliana galactinol synthase gene improves drought tolerance in transgenic rice and increased grain yield in the field. Plant Biotechnol. J. 2017, 15, 1465–1477. [Google Scholar] [CrossRef]
- Meng, H.; Sun, M.; Jiang, Z.; Liu, Y.; Sun, Y.; Liu, D.; Jiang, C.; Ren, M.; Yuan, G.; Yu, W.; et al. Comparative transcriptome analysis reveals resistant and susceptible genes in tobacco cultivars in response to infection by Phytophthora nicotianae. Sci. Rep. 2021, 11, 809. [Google Scholar] [CrossRef]
- Natarajan, P.; Ahn, E.; Reddy, U.K.; Perumal, R.; Prom, L.K.; Magill, C. RNA-Sequencing in Resistant (QL3) and Susceptible (Theis) Sorghum Cultivars Inoculated With Johnsongrass Isolates of Colletotrichum sublineola. Front. Genet. 2021, 12, 722519. [Google Scholar] [CrossRef]
- Lewis, S.E.; Searle, S.M.J.; Harris, N.; Gibson, M.; Lyer, V.; Richter, J.; Wiel, C.; Bayraktaroglu, L.; Birney, E.; Crosby, M.A.; et al. Apollo: A sequence annotation editor. Genome Biol. 2003, 3, 1–14. [Google Scholar] [CrossRef]
- Olsen, J.L.; Rouzé, P.; Verhelst, B.; Lin, Y.-C.; Bayer, T.; Collen, J.; Dattolo, E.; De Paoli, E.; Dittami, S.; Maumus, F.; et al. The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea. Nature 2016, 530, 331–335. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, X.; Zhao, B.; Xu, X.; Shi, M.; Leng, B.; Dong, X.; Lu, C.; Feng, Z.; Guo, J.; et al. The genome of the recretohalophyte Limonium bicolor provides insights into salt gland development and salinity adaptation during terrestrial evolution. Mol. Plant 2022, 15, 1024–1044. [Google Scholar] [CrossRef]
- VanBuren, R.; Wai, C.M.; Ou, S.; Pardo, J.; Bryant, D.; Jiang, N.; Mockler, T.C.; Edger, P.; Michael, T.P. Extreme haplotype variation in the desiccation-tolerant clubmoss Selaginella lepidophylla. Nat. Commun. 2018, 9, 13. [Google Scholar] [CrossRef]
- Fukushima, K.; Fang, X.; Alvarez-Ponce, D.; Cai, H.; Carretero-Paulet, L.; Chen, C.; Chang, T.-H.; Farr, K.M.; Fujita, T.; Hiwatashi, Y.; et al. Genome of the pitcher plant Cephalotus reveals genetic changes associated with carnivory. Nat. Ecol. Evol. 2017, 1, 0059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, F.; Zhang, X.; Li, Z.; Zhao, Y.; Lohaus, R.; Chang, X.; Dong, W.; Ho, S.Y.W.; Liu, X.; et al. The water lily genome and the early evolution of flowering plants. Nature 2019, 577, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Meyer, I.M.; Durbin, R. Comparative ab initio prediction of gene structures using pair HMMs. Bioinformatics 2002, 18, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Fraley, R.T.; Rogers, S.G.; Horsch, R.B.; Sanders, P.R.; Flick, J.S.; Adams, S.P.; Bittner, M.L.; Brand, L.A.; Fink, C.L.; Fry, J.S.; et al. Expression of bacterial genes in plant cells. Proc. Natl. Acad. Sci. USA 1983, 80, 4803–4807. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, A.; Arró, M.; Manzano, D.; Altabella, T. Strategies and Methodologies for the Co-expression of Multiple Proteins in Plants. Adv. Exp. Med. Biol. 2016, 896, 263–285. [Google Scholar] [CrossRef]
- Jobling, S.A.; Westcott, R.J.; Tayal, A.; Jeffcoat, R.; Schwall, G.P. Production of a freeze-thaw-stable potato starch by antisense inhibition of three starch synthase genes. Nat. Biotechnol. 2002, 20, 295–299. [Google Scholar] [CrossRef]
- Qi, B.; Fraser, T.; Mugford, S.; Dobson, G.; Sayanova, O.; Butler, J.; Napier, J.A.; Stobart, A.K.; Lazarus, C.M. Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat. Biotechnol. 2004, 22, 739–745. [Google Scholar] [CrossRef]
- Ma, J.K.C.; Hiatt, A.; Hein, M.; Vine, N.D.; Wang, F.; Stabila, P.; van Dolleweerd, C.; Mostov, K.; Lehner, T. Generation and assembly of secretory antibodies in plants. Science 1995, 268, 716–719. [Google Scholar] [CrossRef]
- van Erp, H.; Kelly, A.A.; Menard, G.; Eastmond, P.J. Multigene engineering of triacylglycerol metabolism boosts seed oil content in Arabidopsis. Plant Physiol. 2014, 165, 30–36. [Google Scholar] [CrossRef]
- Datta, K.; Baisakh, N.; Thet, K.M.; Tu, J.; Datta, S.K. Pyramiding transgenes for multiple resistance in rice against bacterial blight, yellow stem borer and sheath blight. Theor. Appl. Genet. 2003, 106, 1–8. [Google Scholar] [CrossRef]
- Houshyani, B.; Assareh, M.; Busquets, A.; Ferrer, A.; Bouwmeester, H.J.; Kappers, I.F. Three-step pathway engineering results in more incidence rate and higher emission of nerolidol and improved attraction of Diadegma semiclausum. Metab. Eng. 2013, 15, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Magi, A.; Benelli, M.; Gozzini, A.; Girolami, F.; Torricelli, F.; Brandi, M.L. Bioinformatics for Next Generation Sequencing Data. Genes 2010, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.K.; Kumar, S.; Jaiswal, A.; Prasad, M.; Gandomi, A.H. Towards Precision Agriculture: IoT-Enabled Intelligent Irrigation Systems Using Deep Learning Neural Network. IEEE Sens. J. 2021, 21, 17479–17491. [Google Scholar] [CrossRef]
- Deusch, S.; Tilocca, B.; Camarinha-Silva, A.; Seifert, J. News in livestock research—Use of Omics-technologies to study the microbiota in the gastrointestinal tract of farm animals. Comput. Struct. Biotechnol. J. 2015, 13, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Rott, M.; Xiang, Y.; Boyes, I.; Belton, M.; Saeed, H.; Kesanakurti, P.; Hayes, S.; Lawrence, T.; Birch, C.; Bhagwat, B.; et al. Application of Next Generation Sequencing for Diagnostic Testing of Tree Fruit Viruses and Viroids. Plant Dis. 2017, 101, 1489–1499. [Google Scholar] [CrossRef]
- Cao, Y.; Geddes, T.A.; Yang, J.Y.H.; Yang, P. Ensemble deep learning in bioinformatics. Nat. Mach. Intell. 2020, 2, 500–508. [Google Scholar] [CrossRef]
- Coleman-Derr, D.; Tringe, S.G. Building the crops of tomorrow: Advantages of symbiont-based approaches to improving abiotic stress tolerance. Front. Microbiol. 2014, 5, 283. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
