Climatic Variability Caused by Topographic Barrier Prevents the Northward Spread of Invasive Ageratina adenophora
Abstract
1. Introduction
2. Results
2.1. Spatial–Temporal Distribution of Ageratina adenophora
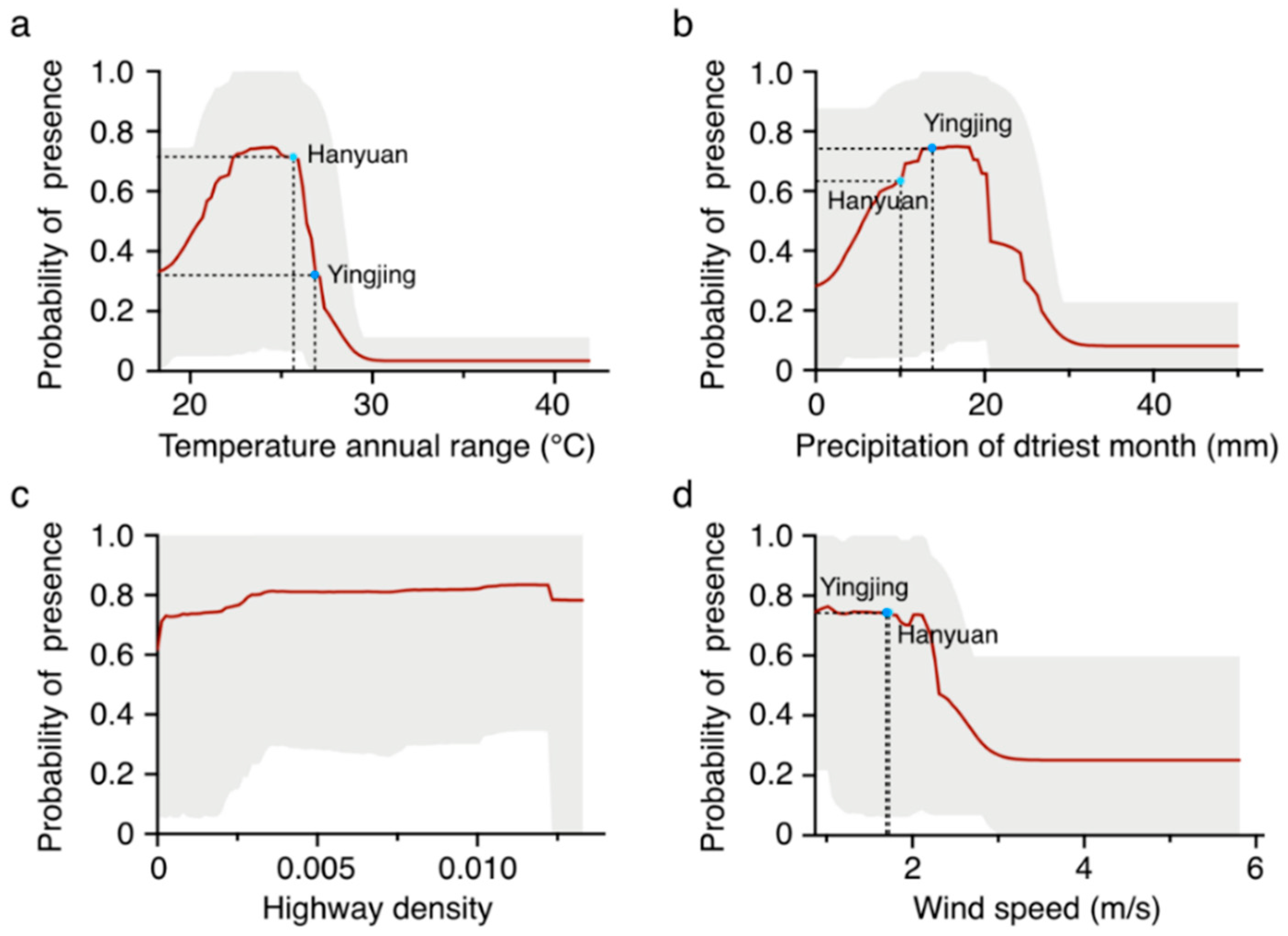
2.2. Determination of the Key Environmental Factors
2.3. Comparison of Environmental Variables between Northern and Southern Sides of Mount Nyba
2.4. Reconstruction of A. adenophora Expansion History in Study Area
3. Discussion
3.1. Distribution and Spread Prediction of A. adenophora
3.2. Key Driving Factors
3.3. Possible Prevention Mechanism of A. adenophora Invasion by Mount Nyba
3.4. Prevention and Control Methods
4. Materials and Methods
4.1. Occurrence Data of Ageratina adenophora
4.2. Environmental Data
4.3. Habitat Suitability Simulation
4.4. Identification of Key Environmental Factors Influencing A. adenophora Distribution
4.5. Spread Simulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceschin, S.; Ferrante, G.; Mariani, F.; Traversetti, L.; Ellwood, N. Habitat change and alteration of plant and invertebrate communities in waterbodies dominated by the invasive alien macrophyte Lemna minuta Kunth. Biol. Invasions 2020, 22, 1325–1337. [Google Scholar] [CrossRef]
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Brondizio, E.S., Settele, J., Díaz, S., Ngo, H.T., Eds.; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- Rai, P.K.; Singh, J. Invasive alien plant species: Their impact on environment, ecosystem services and human health. Ecol. Indic. 2020, 111, 106020. [Google Scholar]
- Vaz, A.S.; Kueffer, C.; Kull, C.A.; Richardson, D.M.; Schindler, S.; Muñoz-Pajares, A.J.; Vicente, J.R.; Martins, J.; Hui, C.; Kühn, I. The progress of interdisciplinarity in invasion science. Ambio 2017, 46, 428–442. [Google Scholar] [CrossRef]
- Yu, F.K.; Akin-Fajiye, M.; Thapa Magar, K.; Ren, J.; Gurevitch, J. A global systematic review of ecological field studies on two major invasive plant species, Ageratina adenophora and Chromolaena odorata. Divers. Distrib. 2016, 22, 1174–1185. [Google Scholar] [CrossRef]
- Poudel, A.S.; Jha, P.K.; Shrestha, B.B.; Muniappan, R. Biology and management of the invasive weed Ageratina adenophora (Asteraceae): Current state of knowledge and future research needs. Weed Res. 2019, 59, 79–92. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, H.; Ding, H. Impacts of invasive alien plant Eupatorium adenophorum on soil animal communities in Kunming. J. Ecol. Rural Environ. 2006, 22, 31–35. [Google Scholar]
- Yu, X.J. Studies on Biological Invasion by Eupatorium adenophorum; Wuhan University: Wuhan, China, 2005. [Google Scholar]
- Wang, C.; Lin, H.; Feng, Q.; Jin, C.; Cao, A.; He, L. A new strategy for the prevention and control of Eupatorium adenophorum under climate change in China. Sustainability 2017, 9, 2037. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, W.; Wan, F.; Li, Z. Competitive effects between Ageratina adenophora (Asteraceae) and Setaria sphacelata (Gramineae). Sci. Agric. Sin. 2008, 41, 1347–1354. [Google Scholar]
- Sun, X.Y.; Lu, Z.H.; Sang, W.G. Review on studies of Eupatorium adenophorum an important invasive species in China. J. For. Res. 2004, 15, 319–322. [Google Scholar]
- Tererai, F.; Wood, A.R. On the present and potential distribution of Ageratina adenophora (Asteraceae) in South Africa. S. Afr. J. Bot. 2014, 95, 152–158. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.Z. Invasion dynamics and potential spread of the invasive alien plant species Ageratina adenophora (Asteraceae) in China. Divers. Distrib. 2006, 12, 397–408. [Google Scholar] [CrossRef]
- Yang, G.; Gui, F.; Liu, W.; Wan, F. Crofton weed Ageratina adenophora (Sprengel). In Biological Invasions and Its Management in China; Wan, F., Jiang, M., Zhan, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 2, pp. 111–129. [Google Scholar]
- Wang, R.; Wang, J.F.; Qiu, Z.J.; Meng, B.; Wan, F.H.; Wang, Y.Z. Multiple mechanisms underlie rapid expansion of an invasive alien plant. New Phytol. 2011, 191, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Horvitz, N.; Wang, R.; Zhu, M.; Wan, F.H.; Nathan, R. A simple modeling approach to elucidate the main transport processes and predict invasive spread: River-mediated invasion of Ageratina adenophora in China. Water Resour. Res. 2014, 50, 9738–9747. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, X.; Wei, H.; Wang, D.; Chen, R.; Wang, L.; Gu, W. Predicting the invasive trend of exotic plants in China based on the ensemble model under climate change: A case for three invasive plants of Asteraceae. Sci. Total Environ. 2021, 756, 143841. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.Q.; Xiong, Q.L.; Qiu, X.P.; Zhang, Y.M. Dynamics of invasive alien plant species in China under climate change scenarios. Ecol. Indic. 2021, 129, 107919. [Google Scholar] [CrossRef]
- Engler, R.; Hordijk, W.; Guisan, A. The MIGCLIM R package—Seamless integration of dispersal constraints into projections of species distribution models. Ecography 2012, 35, 872–878. [Google Scholar] [CrossRef]
- Sun, X.Y.; Lu, Z.H.; Yu, X.J.; Sang, W.G. Age structure dynamics of Eupatorium adenophorum populations and its implications for control. Acta Phytoecol. Sin. 2005, 29, 373–379. [Google Scholar]
- Wang, W.Q. Invasion Mechanisms of Alien Species, Eupatorium adenophorum Spreng; Southwest University: Chongqing, China, 2006. [Google Scholar]
- Yu, X.J.; Yu, D.; Lu, Z.J.; Ma, K.P. A new mechanism of invader success: Exotic plant inhibits natural vegetation restoration by changing soil microbe community. Chin. Sci. Bull. 2005, 50, 1105–1112. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Bergström, P.; Thorngren, L.; Lindegarth, M. Recent change in spatial distribution of the European flat oyster (Ostrea edulis) inferred from field data and empirical models of living oysters and empty shells. Ecol. Evol. 2022, 12, e8925. [Google Scholar] [CrossRef]
- Xiong, Q.; Xiao, Y.; Halmy, M.W.A.; Dakhil, M.A.; Liang, P.; Liu, C.; Zhang, L.; Pandey, B.; Pan, K.; El Kafraway, S.B. Monitoring the impact of climate change and human activities on grassland vegetation dynamics in the northeastern Qinghai-Tibet Plateau of China during 2000–2015. J. Arid Land 2019, 11, 637–651. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Sun, P.; Wang, T.; Wang, G.; Wang, L.; Zhang, X. Using DEM to predict Abies faxoniana and Quercus aquifolioides distributions in the upstream catchment basin of the Min River in southwest China. Ecol. Indic. 2016, 69, 91–99. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Tobin, P.C. Population ecology of insect invasions and their management. Annu. Rev. Entomol. 2008, 53, 387–408. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zha, Y.; Zhang, J.; Li, Y.; Lyu, H. Effect of terrestrial vegetation growth on climate change in China. J. Environ. Manag. 2020, 262, 110321. [Google Scholar] [CrossRef]
- Scheiter, S.; Kumar, D.; Corlett, R.T.; Gaillard, C.; Langan, L.; Lapuz, R.S.; Martens, C.; Pfeiffer, M.; Tomlinson, K.W. Climate change promotes transitions to tall evergreen vegetation in tropical Asia. Glob. Change Biol. 2020, 26, 5106–5124. [Google Scholar] [CrossRef]
- Büyüktahtakın, İ.E.; Haight, R.G. A review of operations research models in invasive species management: State of the art, challenges, and future directions. Ann. Oper. Res. 2018, 271, 357–403. [Google Scholar] [CrossRef]
- Zhu, L.; Sun, O.J.; Sang, W.; Li, Z.; Ma, K. Predicting the spatial distribution of an invasive plant species (Eupatorium adenophorum) in China. Landsc. Ecol. 2007, 22, 1143–1154. [Google Scholar] [CrossRef]
- Wu, X.W.; Luo, J.; Chen, J.K.; Li, B. Spatial patterns of invasive alien plants in China and its relationship with environmental and anthropological factors. Chin. J. Plant Ecol. 2006, 30, 576. [Google Scholar]
- Dong, S.; Cui, B.; Yang, Z.; Liu, S.; Liu, J.; Ding, Z.; Zhu, J.; Yao, W.; Wei, G. The role of road disturbance in the dispersal and spread of Ageratina adenophora along the Dian–Myanmar International Road. Weed Res. 2008, 48, 282–288. [Google Scholar] [CrossRef]
- Yu, S.; Fan, X.; Gadagkar, S.R.; Albright, T.P.; Li, J.; Xue, T.; Xu, H.; Huang, Y.; Shao, X.; Ding, W.; et al. Global ore trade is an important gateway for non-native species: A case study of alien plants in Chinese ports. Divers. Distrib. 2020, 26, 1409–1420. [Google Scholar] [CrossRef]
- Milanovic, M.; Knapp, S.; Pysek, P.; Kuehn, I. Trait-environment relationships of plant species at different stages of the introduction process. Neobiota 2020, 58, 55–74. [Google Scholar] [CrossRef]
- Yang, Z.C. Study on the Seed Ecological Characteristics of Ageratina Adenophora; Yunnan University: Kunming, China, 2020. [Google Scholar]
- Shen, Y.X.; Zhao, C.Y.; Liu, W.Y. Seed vigor and plant competitiveness resulting from seeds of Eupatorium adenophorum in a persistent soil seed bank. Flora Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 935–942. [Google Scholar] [CrossRef]
- Lu, P.; Sang, W.G.; Ma, K.P. Effects of environmental factors on germination and emergence of crofton weed (Eupatorium adenophorum). Weed Sci. 2006, 54, 452–457. [Google Scholar] [CrossRef]
- Wang, H.M.; Jiang, Y.; Li, Y.H.; Wang, W.J.; Zu, Y.G. Light-sensitive features of seed germination in the invasive species Ageratina adenophora (syn. Eupatorium adenophorum) in china. Afr. J. Biotechnol. 2012, 11, 7855–7863. [Google Scholar]
- Zhu, W.D.; Cao, A.C.; Yan, D.D.; Li, L.; Liu, X.Y.; Guo, Z.B. Vegetative and reproductive growth of Eupatorium adenophorum Spreng in different forest communities. Ecol. Environ. Sci. 2013, 22, 1790–1794. [Google Scholar]
- Leishman, M.R.; Cooke, J.; Richardson, D.M. Evidence for shifts to faster growth strategies in the new ranges of invasive alien plants. J. Ecol. 2014, 102, 1451–1461. [Google Scholar] [CrossRef]
- Bush, A.; Mokany, K.; Catullo, R.; Hoffmann, A.; Kellermann, V.; Sgro, C.; McEvey, S.; Ferrier, S. Incorporating evolutionary adaptation in species distribution modelling reduces projected vulnerability to climate change. Ecol. Lett. 2016, 19, 1468–1478. [Google Scholar] [CrossRef]
- Caplat, P.; Coutts, S.; Buckley, Y.M. Modeling population dynamics, landscape structure, and management decisions for controlling the spread of invasive plants. Ann. N. Y. Acad. Sci. 2012, 1249, 72–83. [Google Scholar] [CrossRef]
- Hulme, P.E. Beyond control: Wider implications for the management of biological invasions. J. Appl. Ecol. 2006, 43, 835–847. [Google Scholar] [CrossRef]
- Rejmánek, M.; Pitcairn, M. When is eradication of exotic pest plants a realistic goal. In Turning the Tide: The Eradication of Invasive Species; Veitch, C.R., Clout, M.N., Eds.; The World Conservation Union: Gland, Switzerland; Cambridge, UK, 2002; pp. 249–253. [Google Scholar]
- Meier, E.S.; Dullinger, S.; Zimmermann, N.E.; Baumgartner, D.; Gattringer, A.; Hülber, K. Space matters when defining effective management for invasive plants. Divers. Distrib. 2014, 20, 1029–1043. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Baldwin, R.A. Use of Maximum Entropy Modeling in Wildlife Research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Cruz-Cardenas, G.; Lopez-Mata, L.; Luis Villasenor, J.; Ortiz, E. Potential species distribution modeling and the use of principal component analysis as predictor variables. Rev. Mex. Biodivers. 2014, 85, 189–199. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carre, G.; Garcia Marquez, J.R.; Gruber, B.; Lafourcade, B.; Leitao, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Naimi, B. Usdm: Uncertainty Analysis for Species Distribution Models. R Package Version 1.1–18. 2017. Available online: https://CRAN.R-project.org/package=usdm (accessed on 25 June 2022).
- Liao, Z.Y.; Chen, Y.H.; Pan, K.W.; Dakhil, M.A.; Lin, K.X.; Tian, X.L.; Zhang, F.Y.; Wu, X.G.; Pandey, B.K.; Wang, B.; et al. Current climate overrides past climate change in explaining multi-site beta diversity of Lauraceae species in China. For. Ecosyst. 2022, 9, 100018. [Google Scholar] [CrossRef]
- Zhu, G.P.; Illan, J.G.; Looney, C.; Crowder, D.W. Assessing the ecological niche and invasion potential of the Asian giant hornet. Proc. Natl. Acad. Sci. USA 2020, 117, 24646–24648. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudik, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Lamsal, P.; Kumar, L.; Aryal, A.; Atreya, K. Invasive alien plant species dynamics in the Himalayan region under climate change. Ambio 2018, 47, 697–710. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]



| Code | Name | Unit | Importance | VIF | ||||
|---|---|---|---|---|---|---|---|---|
| GLM | GBM | RF | MaxEnt | Ensemble | ||||
| ISO | Isothermality | (*100) - | 0.04 | 0.01 | 0.20 | 0.07 | 0.08 | 3.1 |
| PDM | Precipitation of driest month | mm | 0.41 | 0.19 | 0.04 | 0.31 | 0.24 | 4.1 |
| HW | Highway density | 1 | 0.04 | 0.07 | 0.18 | 0.25 | 0.13 | 1.1 |
| TAR | Temperature annual range (bio05-bio06) | °C | 0.54 | 0.65 | 0.17 | 0.53 | 0.47 | 5.0 |
| LULC | The land use/land cover | - | 0.01 | 0.00 | 0.05 | 0.09 | 0.04 | 1.5 |
| PS | Precipitation seasonality (coefficient of variation) | 1 | 0.13 | 0.00 | 0.05 | 0.10 | 0.07 | 3.8 |
| ST | Soil type | - | 0.01 | 0.00 | 0.01 | 0.04 | 0.02 | 1.6 |
| SR | Solar radiation | kJ m−2 day−1 | 0.02 | 0.00 | 0.04 | 0.03 | 0.02 | 2.5 |
| PD | Population_density | 1 | 0.02 | 0.05 | 0.09 | 0.31 | 0.12 | 1.3 |
| WS | Wind speed | m/s | 0.27 | 0.12 | 0.02 | 0.13 | 0.13 | 4.1 |
| PWQ | Precipitation of warmest quarter | mm | 0.02 | 0.01 | 0.03 | 0.13 | 0.05 | 4.6 |
| RD | River_density | 1 | 0.01 | 0.00 | 0.00 | 0.02 | 0.01 | 1.0 |
| AI | Aspect_index | 1 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 1.0 |
| SLO | Slope | ° | 0.01 | 0.02 | 0.01 | 0.05 | 0.02 | 1.3 |
| HII | Human interference index | 1 | 0.01 | 0.00 | 0.10 | 0.28 | 0.10 | 2.1 |
| RAD | Railway density | 1 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 1.0 |
| Parameter | Value or Setting | Meaning | Ref. |
|---|---|---|---|
| rcThreshold | 828 | MaxTSS | Simulated in this study |
| envChgSteps | 1 | Without environmental change | [19] |
| dispSteps | 70 | Update times of the habitat suitability layer | From 1940s to current, ca. 70 years |
| dispKernel | c(1,0.607,0.368,0.223,0.135,0.082,0.05,0.03,0.018,0.011,0.007,0.004,0.002,0.002) | Probability of a source cell to disperse A. adenophora as a function of distance | 14 km/yr for short-distance events [13] |
| iniMatAge | 1 | Newly colonized cells are ready to produce propagules after one year | [15] |
| propaguleProd | c(0.02,0.25, 0.90,0.99) | Probability of an occupied cell to produce A. adenophora as a function of time | [20,21,22] |
| lddFreq | 0.96 | High occurrence probability of long-distance dispersal events | [15] |
| lddMinDist | 15 | The minimum distance of long-distance dispersal events | [13,15] |
| lddMaxDist | 88 | The max distance of long-distance dispersal events | [15] |
| replicateNb | 10 | The spread simulation is replicated 10 times | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liao, Z.; Jiang, H.; Tu, W.; Wu, N.; Qiu, X.; Zhang, Y. Climatic Variability Caused by Topographic Barrier Prevents the Northward Spread of Invasive Ageratina adenophora. Plants 2022, 11, 3108. https://doi.org/10.3390/plants11223108
Zhang Y, Liao Z, Jiang H, Tu W, Wu N, Qiu X, Zhang Y. Climatic Variability Caused by Topographic Barrier Prevents the Northward Spread of Invasive Ageratina adenophora. Plants. 2022; 11(22):3108. https://doi.org/10.3390/plants11223108
Chicago/Turabian StyleZhang, Yi, Ziyan Liao, Han Jiang, Wenqin Tu, Ning Wu, Xiaoping Qiu, and Yongmei Zhang. 2022. "Climatic Variability Caused by Topographic Barrier Prevents the Northward Spread of Invasive Ageratina adenophora" Plants 11, no. 22: 3108. https://doi.org/10.3390/plants11223108
APA StyleZhang, Y., Liao, Z., Jiang, H., Tu, W., Wu, N., Qiu, X., & Zhang, Y. (2022). Climatic Variability Caused by Topographic Barrier Prevents the Northward Spread of Invasive Ageratina adenophora. Plants, 11(22), 3108. https://doi.org/10.3390/plants11223108





