New Insights into the Phosphorus Acquisition Capacity of Chilean Lowland Quinoa Roots Grown under Low Phosphorus Availability
Abstract
1. Introduction
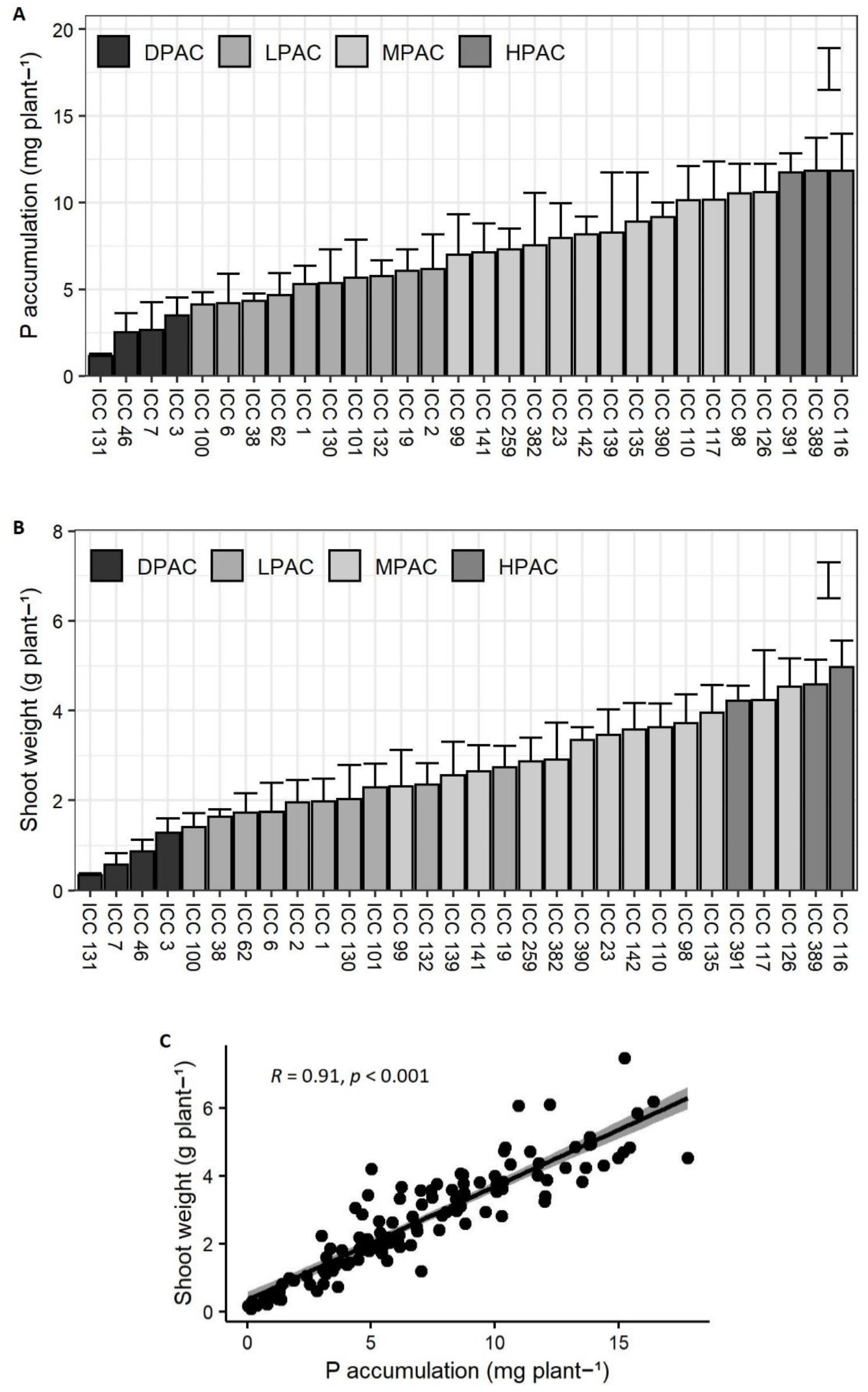
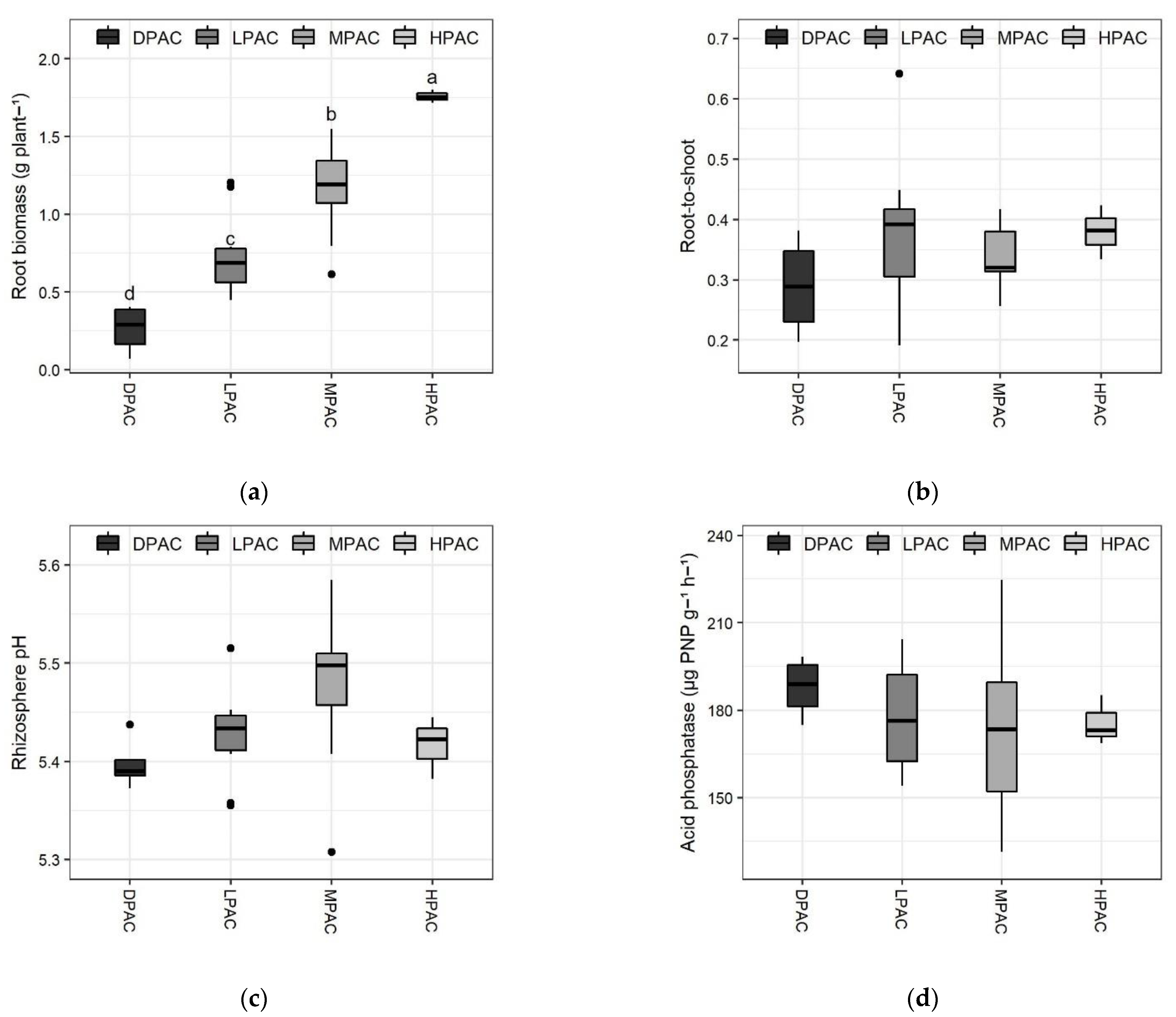
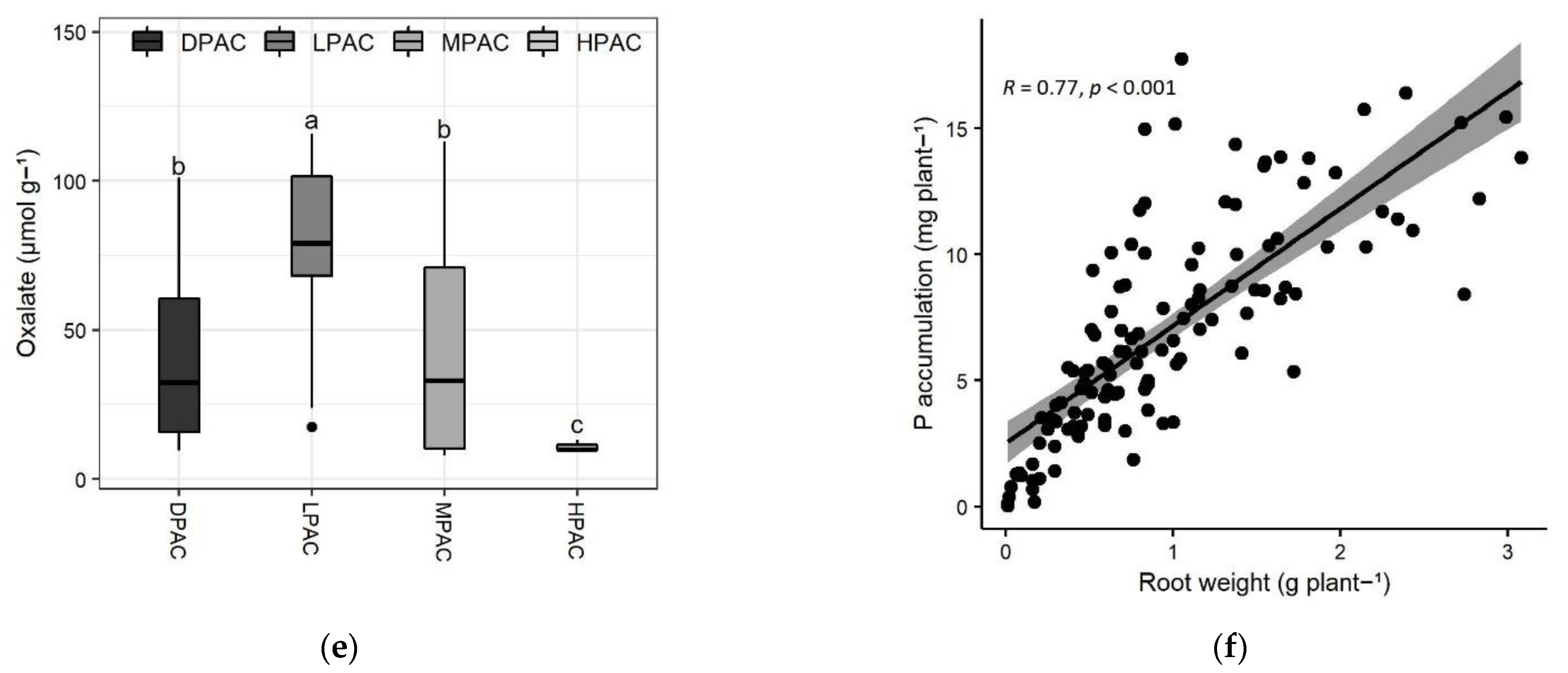
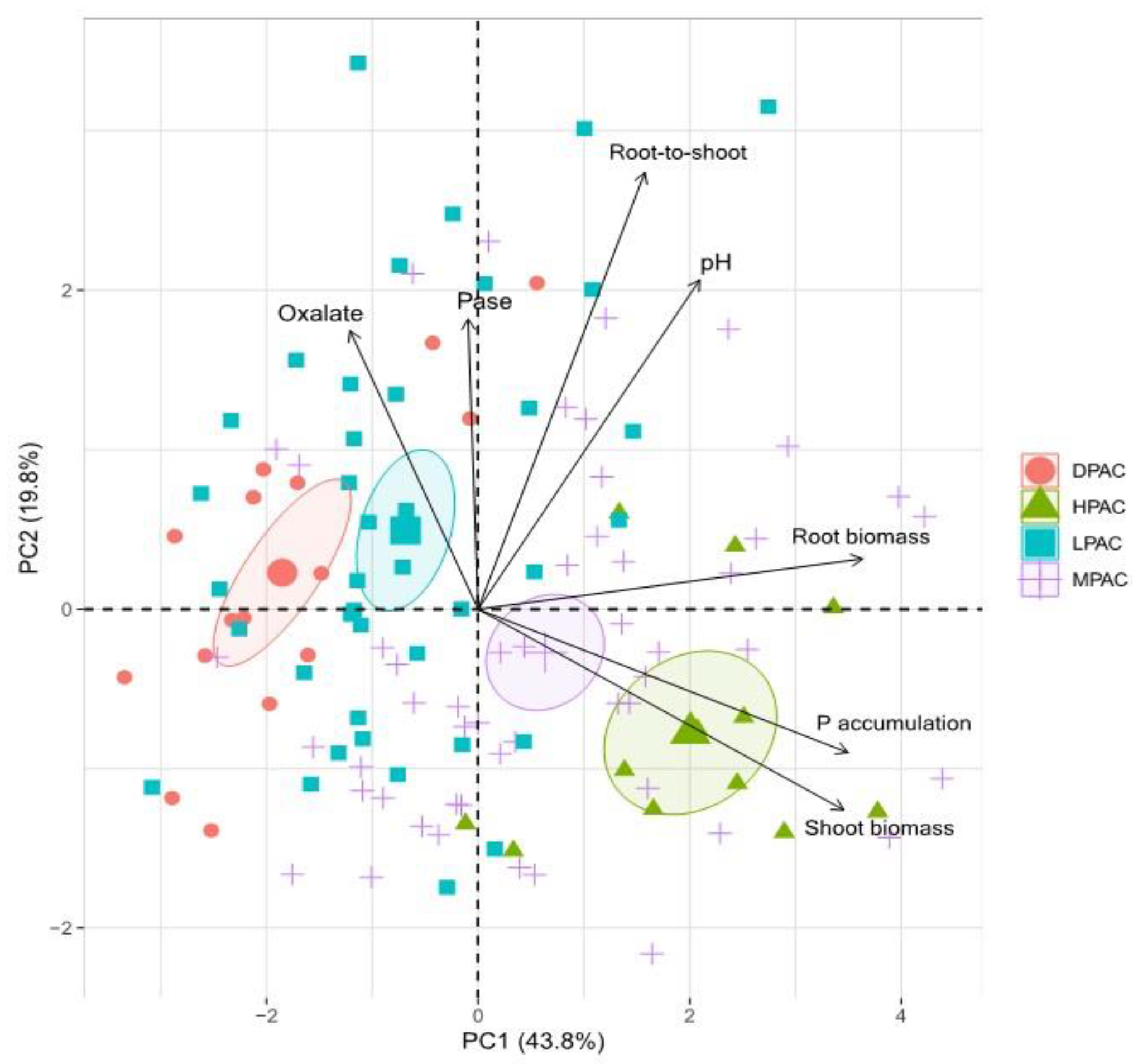
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, R.A.; Connor, D.J. Issues for Cropping and Agricultural Science in the next 20 Years. Field Crops Res. 2018, 222, 121–142. [Google Scholar] [CrossRef]
- Mogollón, J.M.; Beusen, A.H.W.; van Grinsven, H.J.M.; Westhoek, H.; Bouwman, A.F. Future Agricultural Phosphorus Demand According to the Shared Socioeconomic Pathways. Glob. Environ. Chang. 2018, 50, 149–163. [Google Scholar] [CrossRef]
- Fixen, P.E.; Johnston, A.M. World Fertilizer Nutrient Reserves: A View to the Future. J. Sci. Food Agric. 2012, 92, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.E.; Poulton, P.R.; Fixen, P.E.; Curtin, D. Phosphorus: Its Efficient Use in Agriculture. Adv. Agron. 2014, 123, 177–228. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving Nutrition through Biofortification: A Review of Evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Choukr-Allah, R.; Rao, N.K.; Hirich, A.; Shahid, M.; Alshankiti, A.; Toderich, K.; Gill, S.; Butt, K.U.R. Quinoa for Marginal Environments: Toward Future Food and Nutritional Security in MENA and Central Asia Regions. Front. Plant Sci. 2016, 7, 346. [Google Scholar] [CrossRef]
- Masson Salaue, L. 12th IFDC 2017 Special Issue—Foods from Latin America and Their Nutritional Contribution: A Global Perspective. J. Food Compos. Anal. 2019, 83, 103291. [Google Scholar] [CrossRef]
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M.; et al. Worldwide Evaluations of Quinoa: Preliminary Results from Post International Year of Quinoa FAO Projects in Nine Countries. Front. Plant Sci. 2016, 7, 850. [Google Scholar] [CrossRef]
- Zurita-Silva, A.; Fuentes, F.; Zamora, P.; Jacobsen, S.E.; Schwember, A.R. Breeding Quinoa (Chenopodium Quinoa Willd.): Potential and Perspectives. Mol. Breed. 2014, 34, 13–30. [Google Scholar] [CrossRef]
- Wang, N.; Wang, F.; Shock, C.C.; Meng, C.; Qiao, L. Effects of Management Practices on Quinoa Growth, Seed Yield, and Quality. Agronomy 2020, 10, 445. [Google Scholar] [CrossRef]
- Jacobsen, S.E. The Scope for Adaptation of Quinoa in Northern Latitudes of Europe. J. Agron. Crop Sci. 2017, 203, 603–613. [Google Scholar] [CrossRef]
- Díaz, J.S.; Mera, M.K.; Galdames, R.G.; López-Olivari, R.; Seguel, I.B.; Morales, A.M.; Navarro, P.D.; Campllo, R.R.; Torralbo, L.B.; Valenzuela, S.A.; et al. Quínoa del Sur de Chile: Alternativa Productiva y Agroindustrial de Alto Valor; Díaz, J., Ed.; INIA: Temuco, Chile, 2019. [Google Scholar]
- Erktan, A.; McCormack, M.L.; Roumet, C. Frontiers in Root Ecology: Recent Advances and Future Challenges. Plant Soil 2018, 424, 1–9. [Google Scholar] [CrossRef]
- Preece, C.; Peñuelas, J. A Return to the Wild: Root Exudates and Food Security. Trends Plant Sci. 2020, 25, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Turner Review No. 14. Roots of the Second Green Revolution. Aust. J. Bot. 2007, 55, 493–512. [Google Scholar] [CrossRef]
- Wissuwa, M.; Kretzschmar, T.; Rose, T.J. From Promise to Application: Root Traits for Enhanced Nutrient Capture in Rice Breeding. J. Exp. Bot. 2016, 67, 3605–3615. [Google Scholar] [CrossRef]
- Tracy, S.R.; Nagel, K.A.; Postma, J.A.; Fassbender, H.; Wasson, A.; Watt, M. Crop Improvement from Phenotyping Roots: Highlights Reveal Expanding Opportunities. Trends Plant Sci. 2020, 25, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H. Annual Review of Plant Biology Phosphorus Acquisition and Utilization in Plants. Artic. Annu. Rev. Plant Biol. 2022, 73, 11–26. [Google Scholar] [CrossRef]
- Lynch, J.P.; Ho, M.D. Rhizoeconomics: Carbon Costs of Phosphorus Acquisition. Plant Soil 2005, 269, 45–56. [Google Scholar] [CrossRef]
- Lan, P.; Li, W.; Schmidt, W. ‘Omics’ Approaches Towards Understanding Plant Phosphorus Acquisition and Use. In Annual Plant Reviews Online; John Wiley & Sons, Ltd.: Chichester, UK, 2017; Volume 48, pp. 65–97. ISBN 9781119312994. [Google Scholar]
- Campos, P.; Borie, F.; Cornejo, P.; López-Ráez, J.A.; López-García, Á.; Seguel, A. Phosphorus Acquisition Efficiency Related to Root Traits: Is Mycorrhizal Symbiosis a Key Factor to Wheat and Barley Cropping? Front Plant Sci. 2018, 9, 752. [Google Scholar] [CrossRef]
- Cong, W.F.; Suriyagoda, L.D.B.; Lambers, H. Tightening the Phosphorus Cycle through Phosphorus-Efficient Crop Genotypes. Trends Plant Sci. 2020, 25, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root Structure and Functioning for Efficient Acquisition of Phosphorus: Matching Morphological and Physiological Traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Camenzind, T.; Cavagnaro, T.R.; Degrune, F.; Hohmann, P.; Lammel, D.R.; Mansour, I.; Roy, J.; van der Heijden, M.G.A.; et al. Why Farmers Should Manage the Arbuscular Mycorrhizal Symbiosis. New Phytol. 2019, 222, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, J.A.; Reganold, J.P.; Murphy, K.M.; Carpenter-Boggs, L.A. A Plant-Fungus Bioassay Supports the Classification of Quinoa (Chenopodium Quinoa Willd.) as Inconsistently Mycorrhizal. Microb. Ecol. 2021, 82, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, F.F.; Bazile, D.; Bhargava, A.; Martínez, E.A. Implications of Farmers’ Seed Exchanges for on-Farm Conservation of Quinoa, as Revealed by Its Genetic Diversity in Chile. J. Agric. Sci. 2012, 150, 702–716. [Google Scholar] [CrossRef]
- Meier, S.; Moore, F.; Morales, A.; Jobet, C.; López-Olivari, R.; Aponte, H.; Cartes, P.; Campos, P.; Khan, N. Interactive Role between Phosphorus Utilization Efficiency and Water Use Efficiency. A Tool to Categorize Wheats Co-Adapted to Water and Phosphorus Limiting Conditions. Agric. Water Manag. 2021, 248, 106765. [Google Scholar] [CrossRef]
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; López-Arredondo, D.; Wissuwa, M.; Delhaize, E.; Rouached, H. Improving Phosphorus Use Efficiency: A Complex Trait with Emerging Opportunities. Plant J. 2017, 90, 868–885. [Google Scholar] [CrossRef]
- Kirk, G.J.D.; Bajita, J.B. Root-Induced Iron Oxidation, PH Changes and Zinc Solubilization in the Rhizosphere of Lowland Rice. New Phytol. 1995, 131, 129–137. [Google Scholar] [CrossRef]
- George, T.S.; Gregory, P.J.; Robinson, J.S.; Buresh, R.J. Changes in Phosphorus Concentrations and PH in the Rhizosphere of Some Agroforestry and Crop Species. Plant Soil 2002, 246, 65–73. [Google Scholar] [CrossRef]
- Sun, B.; Gao, Y.; Wu, X.; Ma, H.; Zheng, C.; Wang, X.; Zhang, H.; Li, Z.; Yang, H. The Relative Contributions of PH, Organic Anions, and Phosphatase to Rhizosphere Soil Phosphorus Mobilization and Crop Phosphorus Uptake in Maize/Alfalfa Polyculture. Plant Soil 2020, 447, 117–133. [Google Scholar] [CrossRef]
- Marschner, P.; Rengel, Z. Nutrient Availability in Soils. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 315–330. ISBN 9780123849052. [Google Scholar]
- Borie, F.; Aguilera, P.; Castillo, C.; Valentine, A.; Seguel, A.; Barea, J.M.; Cornejo, P. Revisiting the Nature of Phosphorus Pools in Chilean Volcanic Soils as a Basis for Arbuscular Mycorrhizal Management in Plant P Acquisition. J. Soil Sci. Plant Nutr. 2019, 19, 390–401. [Google Scholar] [CrossRef]
- George, T.S.; Giles, C.D.; Menezes-Blackburn, D.; Condron, L.M.; Gama-Rodrigues, A.C.; Jaisi, D.; Lang, F.; Neal, A.L.; Stutter, M.I.; Almeida, D.S.; et al. Organic Phosphorus in the Terrestrial Environment: A Perspective on the State of the Art and Future Priorities. Plant Soil 2017, 427, 191–208. [Google Scholar] [CrossRef]
- Jones, D.L.; Oburger, E. Solubilization of Phosphorus by Soil Microorganisms. In Phosphorus in Action; Springer: Berlin/Heidelberg, Germany, 2011; pp. 169–198. [Google Scholar]
- Lambers, H.; Martinoia, E.; Renton, M. Plant Adaptations to Severely Phosphorus-Impoverished Soils. Curr. Opin. Plant Biol. 2015, 25, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.R.; James, R.A.; Weligama, C.; Delhaize, E.; Rattey, A.; Lewis, D.C.; Bovill, W.D.; Mcdonald, G.; Rathjen, T.M.; Wang, E.; et al. Can Citrate Efflux from Roots Improve Phosphorus Uptake by Plants? Testing the Hypothesis with near-Isogenic Lines of Wheat. Physiol. Plant 2014, 151, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Pearse, S.J.; Veneklaas, E.J.; Cawthray, G.; Bolland, M.D.A.; Lambers, H. Carboxylate Composition of Root Exudates Does Not Relate Consistently to a Crop Species’ Ability to Use Phosphorus from Aluminium, Iron or Calcium Phosphate Sources. New Phytol. 2007, 173, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Meena, S.K.; Krishnapriya, V.; Ahmad, A.; Kishora, N. Root Carboxylate Exudation Capacity under Phosphorus Stress Does Not Improve Grain Yield in Green Gram. Plant Cell Rep. 2014, 33, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lambers, H. Root-Released Organic Anions in Response to Low Phosphorus Availability: Recent Progress, Challenges and Future Perspectives. Plant Soil 2020, 447, 135–156. [Google Scholar] [CrossRef]
- Sadzawka, A.; Carrasco, M.A.; Gez, R.; Mora, M.M.D.L.L.; Flores, H.; Neaman, A.; Grez, R.; Mora, M.M.D.L.L.; Flores, H.; Neaman, A.; et al. Métodos de Análisis Recomendados Para Los Suelos de Chile; Revisión 2; Instituto de Investigaciones Agropecuarias (INIA): Santiago de Chile, Chile, 2006; Volume 163, ISBN 0717-4810. [Google Scholar]
- Horneck, D.A.; Miller, R.O. Determination of Total Nitrogen in Plant Tissue. In Handbook of Reference Methods for Plant Analysis; CRC Press: Boca Raton, FL, USA, 2019; pp. 75–83. ISBN 0367448009. [Google Scholar]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Metson, A.J.; Blakemore, L.C.; Rhoades, D.A. Methods for the Determination of Soil Organic Carbon: A Review, and Application to New Zealand Soils. N. Z. J. Sci. 1979, 22, 205–228. [Google Scholar]
- Hanson, W.C. The Photometric Determination of Phosphorus in Fertilizers Using the Phosphovanado-molybdate Complex. J. Sci. Food Agric. 1950, 1, 172–173. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of P-Nitrophenyl Phosphate for Assay of Soil Phosphatase Activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- de Souza Campos, P.M.; Borie, F.; Cornejo, P.; Meier, S.; López-Ráez, J.A.; López-Garcia, Á.; Seguel, A. Wheat Root Trait Plasticity, Nutrient Acquisition and Growth Responses Are Dependent on Specific Arbuscular Mycorrhizal Fungus and Plant Genotype Interactions. J. Plant Physiol. 2021, 256, 153297. [Google Scholar] [CrossRef] [PubMed]
- Parada, J.; Valenzuela, T.; Gómez, F.; Tereucán, G.; García, S.; Cornejo, P.; Winterhalter, P.; Ruiz, A. Effect of Fertilization and Arbuscular Mycorrhizal Fungal Inoculation on Antioxidant Profiles and Activities in Fragaria Ananassa Fruit. J. Sci. Food Agric. 2019, 99, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Value |
|---|---|
| N (mg kg−1) a | 40.7 |
| P (mg kg−1) b | 7.0 |
| K (mg kg−1) c | 176.1 |
| pH d | 5.58 |
| Organic matter (%) e | 21.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza Campos, P.M.; Meier, S.; Morales, A.; Lavanderos, L.; Nahuelcura, J.; Ruiz, A.; López-García, Á.; Seguel, A. New Insights into the Phosphorus Acquisition Capacity of Chilean Lowland Quinoa Roots Grown under Low Phosphorus Availability. Plants 2022, 11, 3043. https://doi.org/10.3390/plants11223043
de Souza Campos PM, Meier S, Morales A, Lavanderos L, Nahuelcura J, Ruiz A, López-García Á, Seguel A. New Insights into the Phosphorus Acquisition Capacity of Chilean Lowland Quinoa Roots Grown under Low Phosphorus Availability. Plants. 2022; 11(22):3043. https://doi.org/10.3390/plants11223043
Chicago/Turabian Stylede Souza Campos, Pedro M., Sebastián Meier, Arturo Morales, Laura Lavanderos, Javiera Nahuelcura, Antonieta Ruiz, Álvaro López-García, and Alex Seguel. 2022. "New Insights into the Phosphorus Acquisition Capacity of Chilean Lowland Quinoa Roots Grown under Low Phosphorus Availability" Plants 11, no. 22: 3043. https://doi.org/10.3390/plants11223043
APA Stylede Souza Campos, P. M., Meier, S., Morales, A., Lavanderos, L., Nahuelcura, J., Ruiz, A., López-García, Á., & Seguel, A. (2022). New Insights into the Phosphorus Acquisition Capacity of Chilean Lowland Quinoa Roots Grown under Low Phosphorus Availability. Plants, 11(22), 3043. https://doi.org/10.3390/plants11223043








