Root Electrical Capacitance Can Be a Promising Plant Phenotyping Parameter in Wheat
Abstract
1. Introduction
2. Results
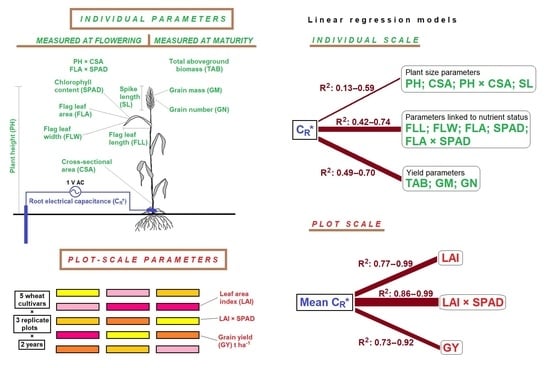
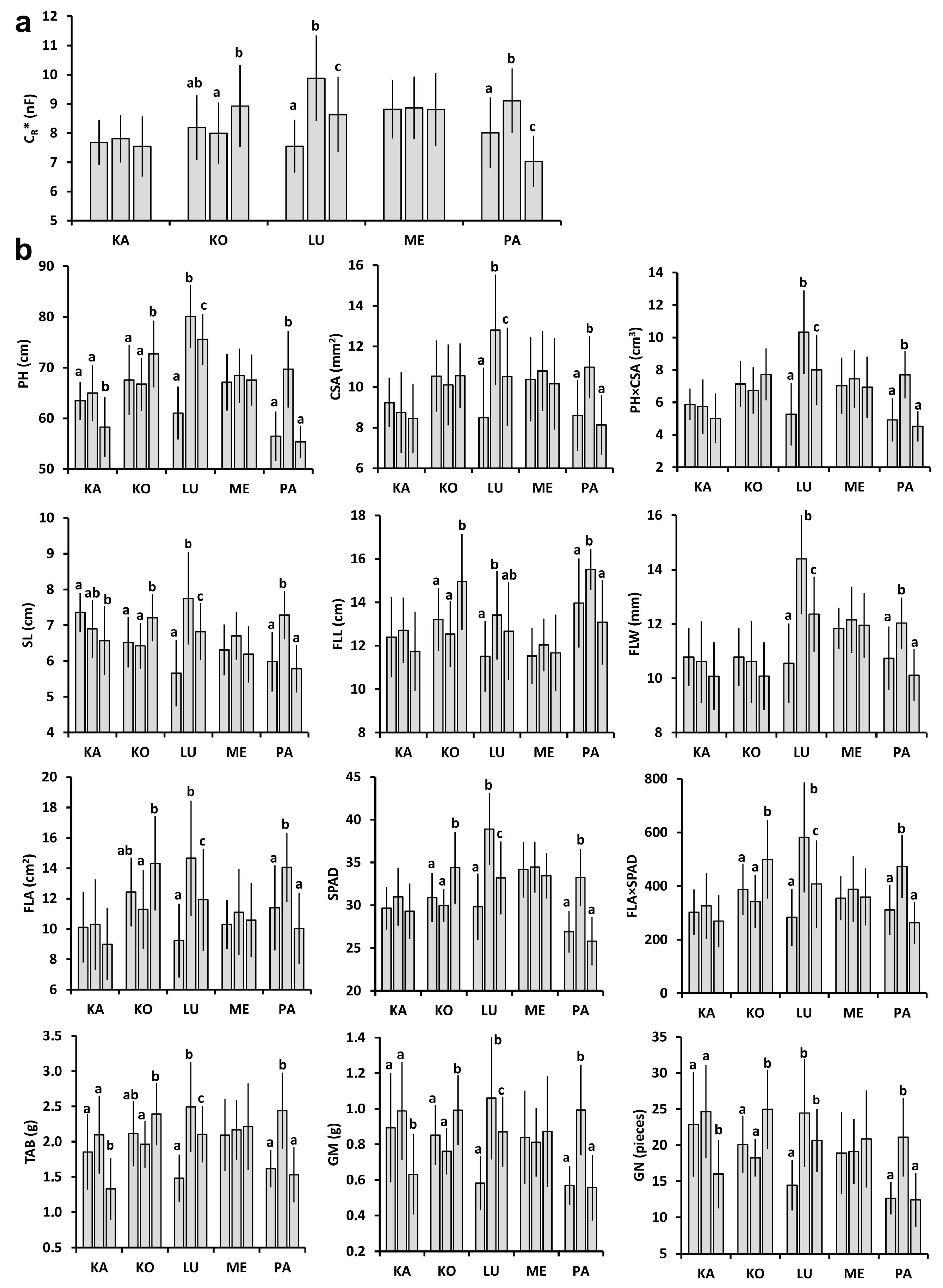
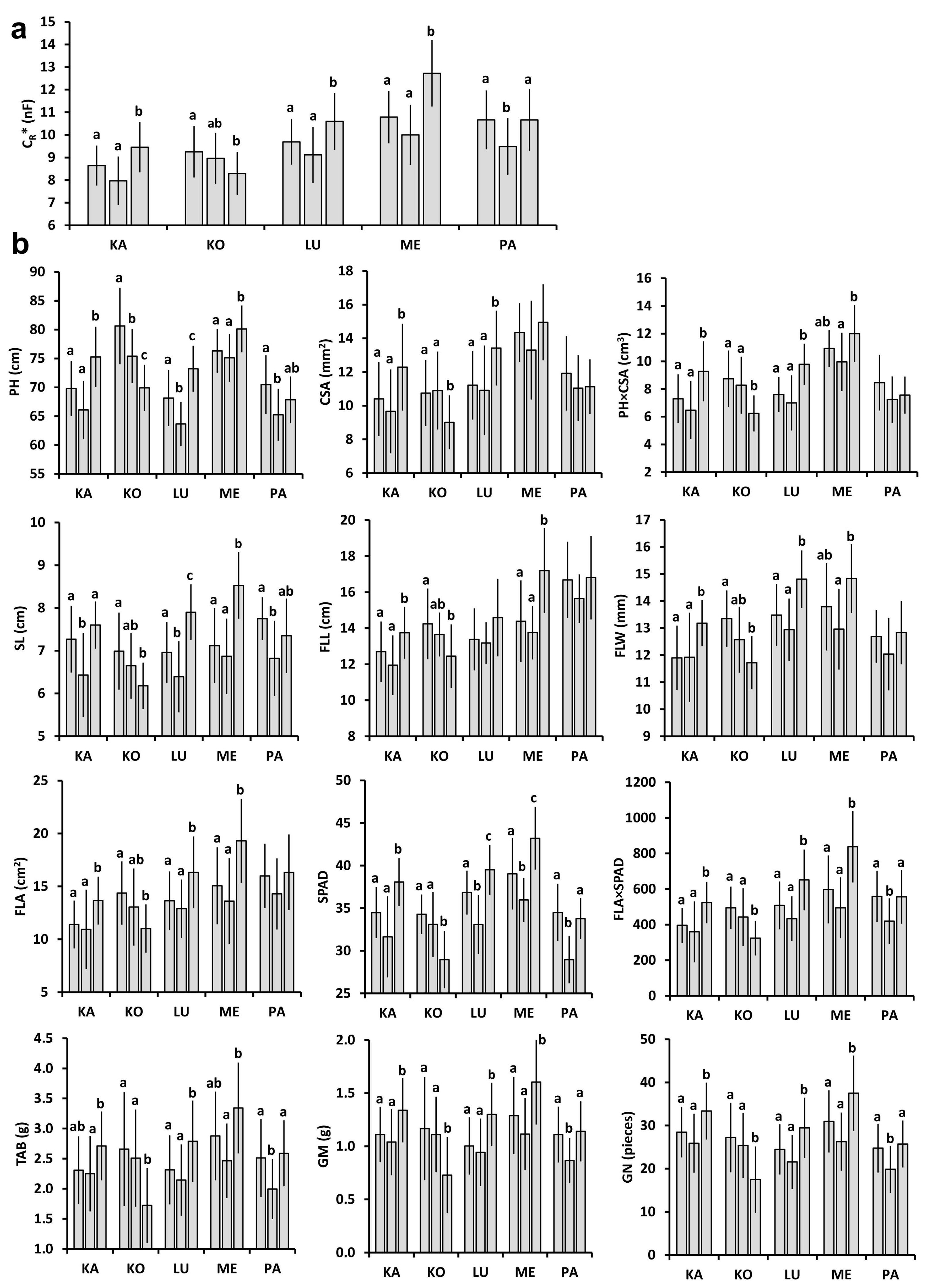
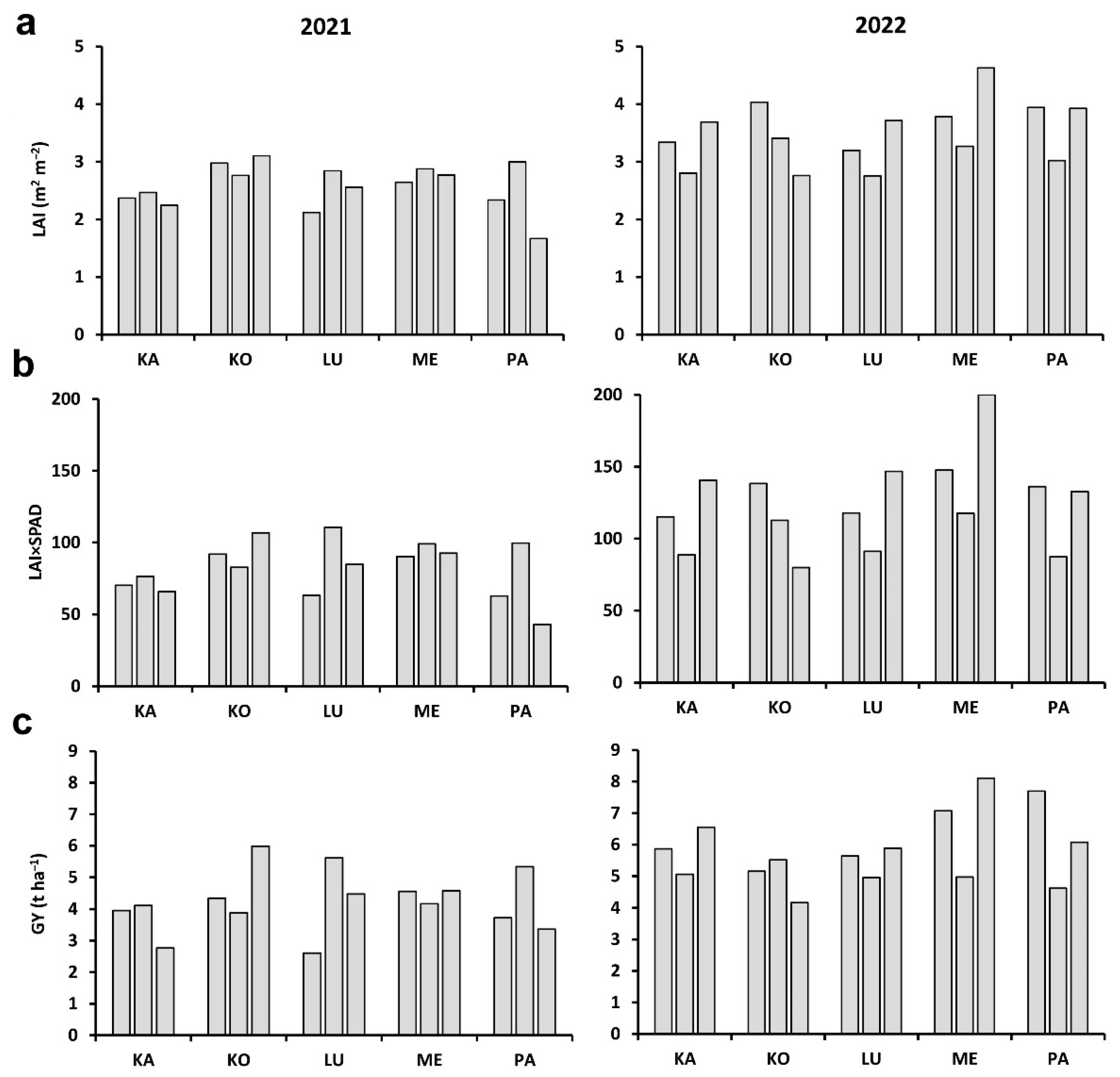
2.1. Plant and Plot-Scale Parameters
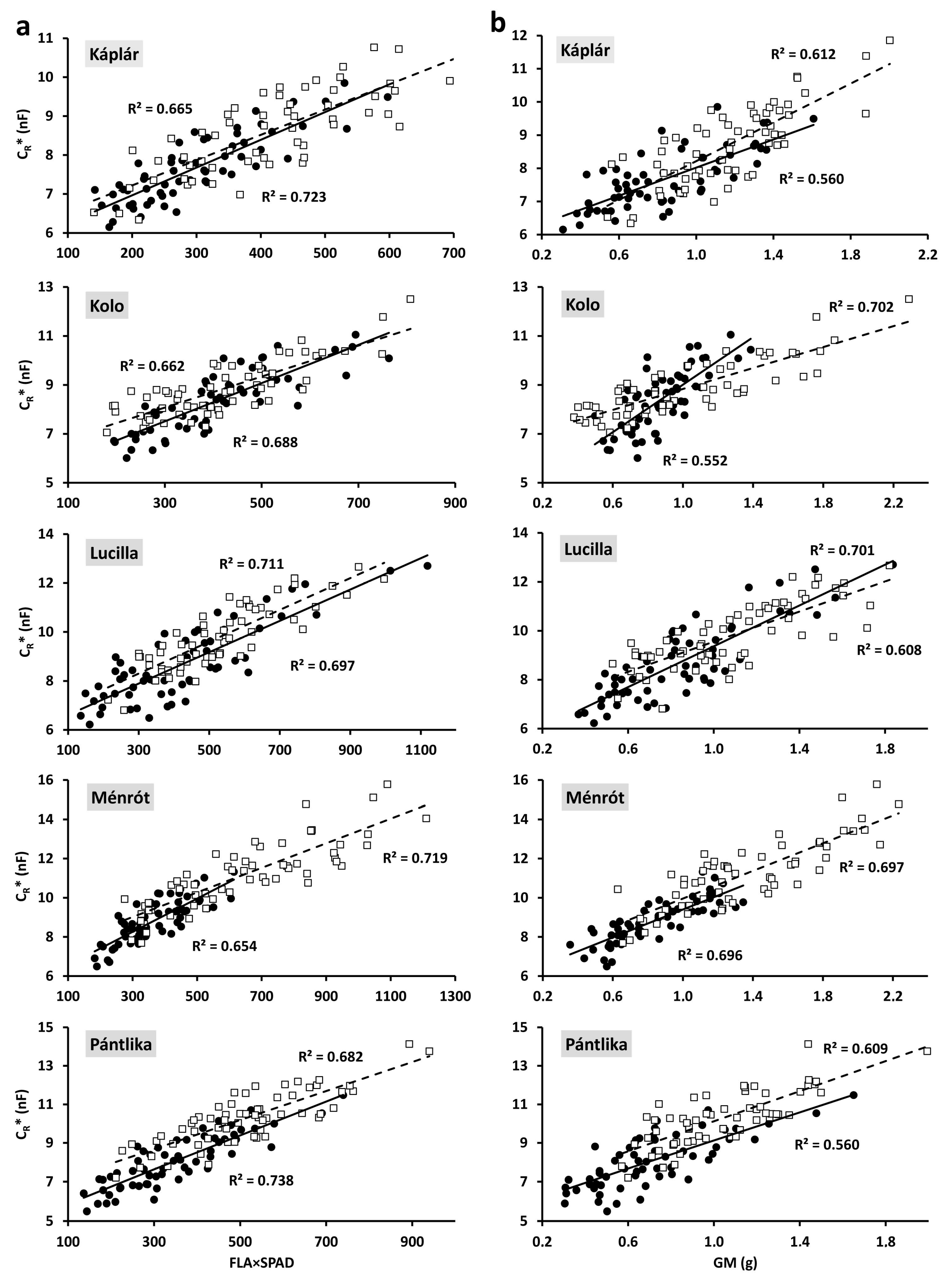
2.2. Relationships between Individual Plant Parameters
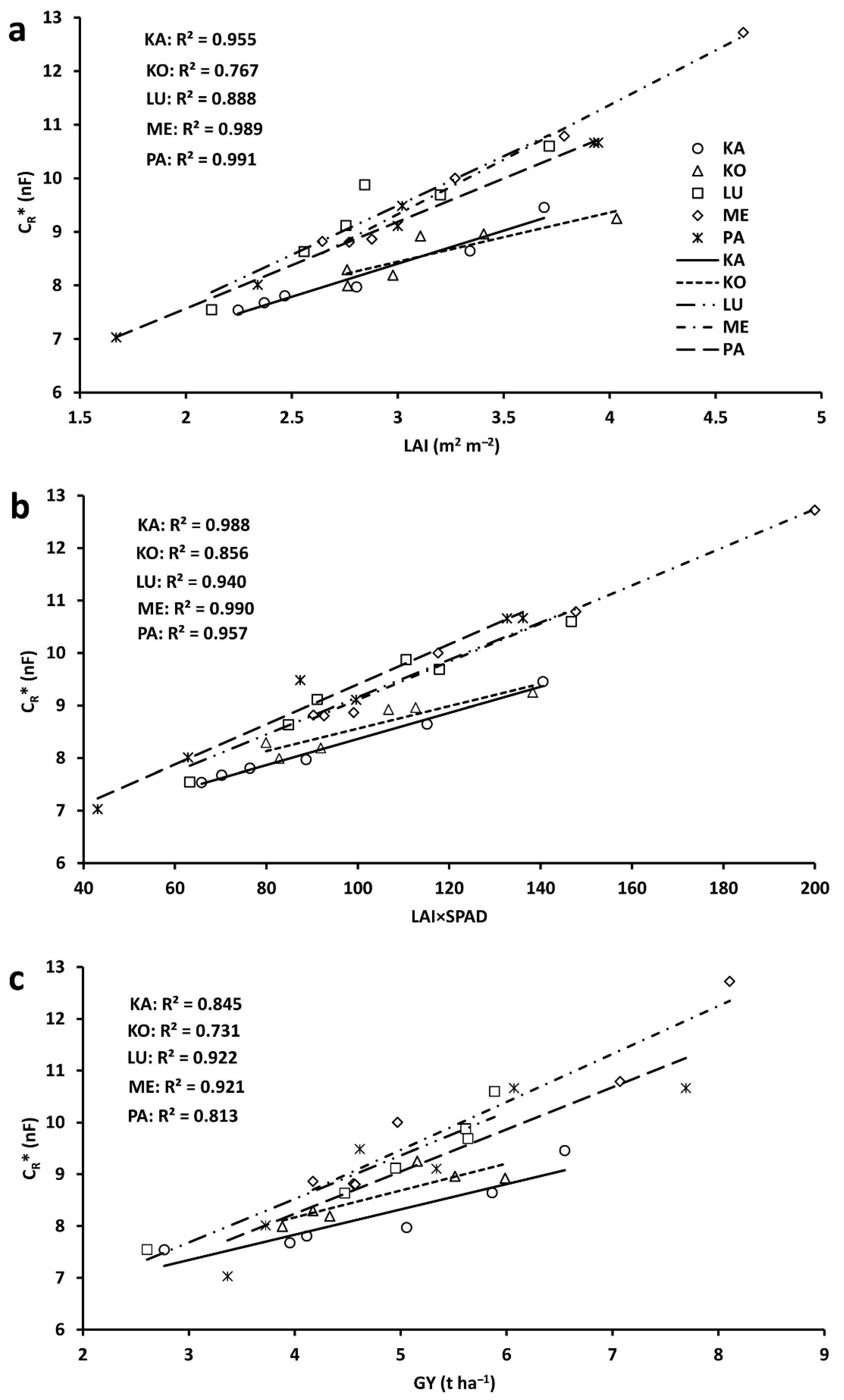
2.3. Correlations between Plot-Scale Parameters
3. Discussion
3.1. Linear Regression
3.2. Indication of Root Functions
4. Materials and Methods
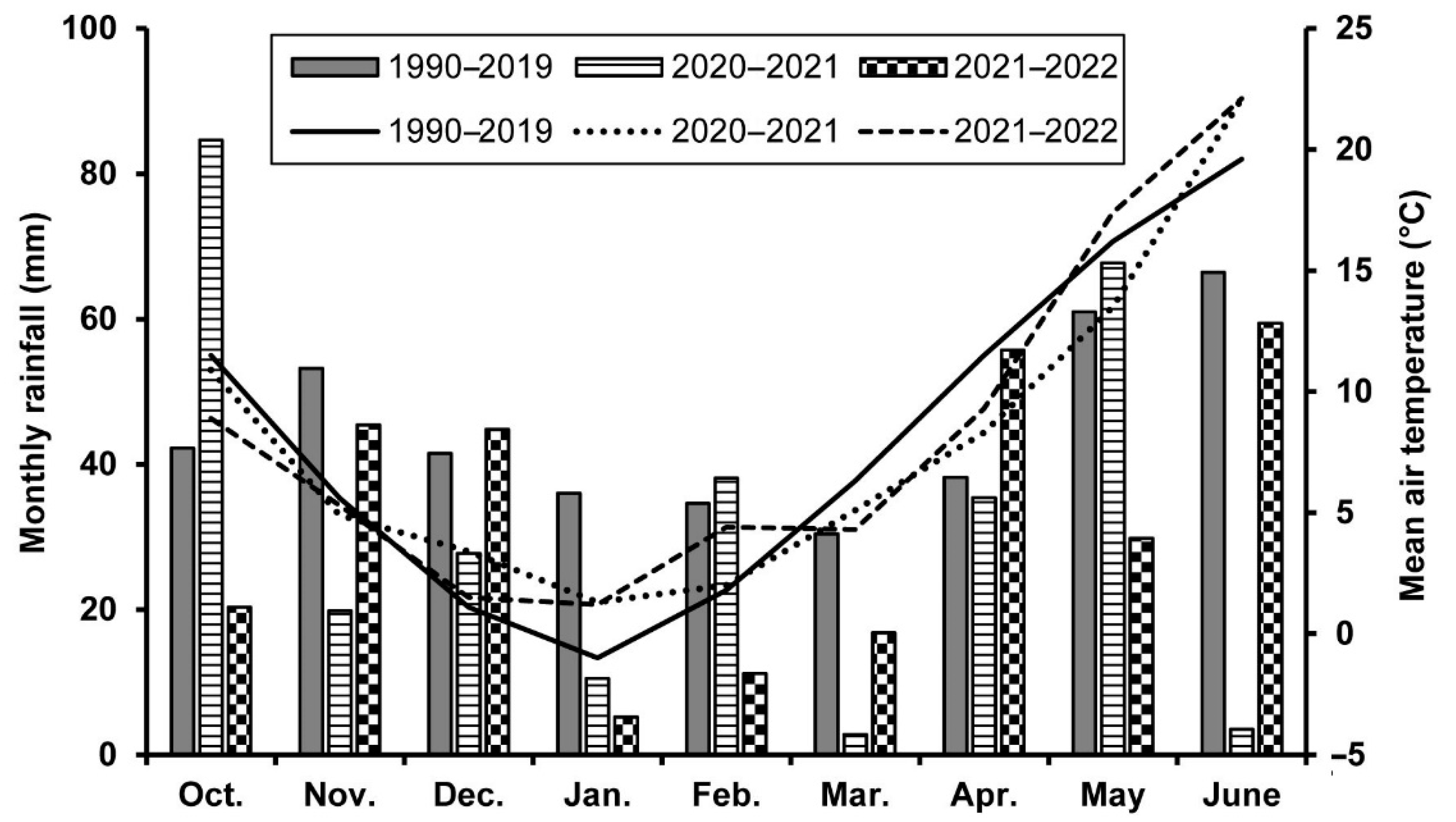
4.1. Study Site and Wheat Cultivation
4.2. Electrical Capacitance Measurement
4.3. Measurement of Individual Plant and Plot-Scale Parameters
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fageria, N.K. The Role of Plant Roots in Crop Production; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Severini, A.D.; Wasson, A.P.; Evans, J.R.; Richards, R.A.; Watt, M. Root phenotypes at maturity in diverse wheat and triticale genotypes grown in three field experiments: Relationship to shoot selection, biomass, grain yield, flowering time, and environment. Field Crops Res. 2020, 255, 107870. [Google Scholar] [CrossRef]
- Bektas, H.; Hohn, C.E.; Waines, J.G. Root and shoot traits of bread wheat (Triticum aestivum L.) landraces and cultivars. Euphytica 2016, 212, 297–311. [Google Scholar] [CrossRef]
- Milchunas, D.G. Biases and errors associated with different root production methods and their effects on field estimates of belowground net primary production. In Measuring Roots; Mancuso, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 303–339. [Google Scholar] [CrossRef]
- Chloupek, O. The relationship between electric capacitance and some other parameters of plant roots. Biol. Plant. 1972, 14, 227–230. [Google Scholar] [CrossRef]
- Dalton, F.N. In-situ root extent measurements by electrical capacitance methods. Plant Soil 1995, 173, 157–165. [Google Scholar] [CrossRef]
- Ozier-Lafontaine, H.; Bajazet, T. Analysis of root growth by impedance spectroscopy (EIS). Plant Soil 2005, 277, 299–313. [Google Scholar] [CrossRef]
- Ellis, T.; Murray, W.; Paul, K.; Kavalieris, L.; Brophy, J.; Williams, C.; Maass, M. Electrical capacitance as a rapid and non-invasive indicator of root length. Tree Physiol. 2013, 33, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Bequet, R.; Mainiero, R. Assessing the applicability of the earth impedance method for in situ studies of tree root systems. J. Exp. Bot. 2011, 62, 1857–1869. [Google Scholar] [CrossRef]
- Peruzzo, L.; Chou, C.; Wu, Y.; Schmutz, M.; Mary, B.; Wagner, F.M.; Petrov, P.; Newman, G.; Blancaflor, E.B.; Liu, X.; et al. Imaging of plant current pathways for non-invasive root phenotyping using a newly developed electrical current source density approach. Plant Soil 2020, 450, 567–584. [Google Scholar] [CrossRef]
- Dietrich, R.C.; Bengough, A.G.; Jones, H.G.; White, P.J. A new physical interpretation of plant root capacitance. J. Exp. Bot. 2012, 63, 6149–6159. [Google Scholar] [CrossRef]
- Cseresnyés, I.; Vozáry, E.; Rajkai, K. Does electrical capacitance represent roots in the soil? Acta Physiol. Plant. 2020, 42, 71. [Google Scholar] [CrossRef]
- Gu, H.; Liu, L.; Butnor, J.R.; Sun, H.; Zhang, X.; Li, C.; Liu, X. Electrical capacitance estimates crop root traits best under dry conditions—A case study in cotton (Gossypium hirsutum L.). Plant Soil 2021, 467, 549–567. [Google Scholar] [CrossRef]
- Cseresnyés, I.; Szitár, K.; Rajkai, K.; Füzy, A.; Mikó, P.; Kovács, R.; Takács, T. Application of electrical capacitance method for prediction of plant root mass and activity in field-grown crops. Front. Plant Sci. 2018, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Ehosioke, S.; Nguyen, F.; Rao, S.; Kremer, T.; Placencia-Gomez, E.; Huisman, J.A.; Kemna, A.; Javaux, M.; Garré, S. Sensing the electrical properties of roots: A review. Vadose Zone J. 2020, 19, e20082. [Google Scholar] [CrossRef]
- Chloupek, O.; Forster, B.P.; Thomas, W.T.B. The effect of semi-dwarf genes on root system size in field-grown barley. Theor. Appl. Genet. 2006, 112, 779–786. [Google Scholar] [CrossRef]
- Středa, T.; Haberle, J.; Klimešová, J.; Klimek-Kopyra, A.; Středová, H.; Bodner, G.; Chloupek, O. Field phenotyping of plant roots by electrical capacitance—A standardized methodological protocol for application in plant breeding: A review. Int. Agrophys. 2020, 34, 173–184. [Google Scholar] [CrossRef]
- Chloupek, O.; Dostál, V.; Středa, T.; Psota, V.; Dvořáčková, O. Drought tolerance of barley varieties in relation to their root system size. Plant Breed. 2010, 129, 630–636. [Google Scholar] [CrossRef]
- Kormanek, M.; Głąb, T.; Klimek-Kopyra, A. Modification of the tree root electrical capacitance method under laboratory conditions. Tree Physiol. 2016, 36, 121–127. [Google Scholar] [CrossRef][Green Version]
- Svačina, P.; Středa, T.; Chloupek, O. Uncommon selection by root system size increases barley yield. Agron. Sustain. Dev. 2014, 34, 545–551. [Google Scholar] [CrossRef]
- Středa, T.; Dostál, V.; Horáková, V.; Chloupek, O. Effective use of water by wheat varieties with different root system sizes in rain-fed experiments in Central Europe. Agric. Water Manag. 2012, 104, 203–209. [Google Scholar] [CrossRef]
- Heřmanská, A.; Středa, T.; Chloupek, O. Improved wheat grain yield by a new method of root selection. Agron. Sustain. Dev. 2015, 35, 195–202. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B.-L. A new method for assessing plant lodging and the impact of management options on lodging in canola crop production. Sci. Rep. 2016, 9, 31890. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, L.; Dodd, I.C.; Binley, A.; Ashton, R.W.; White, R.P.; Watts, C.W.; Whalley, W.R. Root growth in field-grown winter wheat: Some effects of soil conditions, season and genotype. Eur. J. Agron. 2017, 91, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, P.; You, D.; Liu, W. Coupling evapotranspiration partitioning with root water uptake to identify the water consumption characteristics of winter wheat: A case study in the North China Plain. Agric. For. Meteorol. 2018, 259, 296–304. [Google Scholar] [CrossRef]
- Cseresnyés, I.; Kelemen, B.; Takács, T.; Füzy, A.; Kovács, R.; Megyeri, M.; Parádi, I.; Mikó, P. Electrical capacitance versus minirhizotron technique: A study of root dynamics in wheat–pea intercrops. Plants 2021, 10, 1991. [Google Scholar] [CrossRef]
- Cseresnyés, I.; Mikó, P.; Kelemen, B.; Füzy, A.; Parádi, I.; Takács, T. Prediction of wheat grain yield by measuring root electrical capacitance at anthesis. Int. Agrophys. 2021, 35, 159–165. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Soltani, A.; Zeinali, E.; Kallate-Arabi, M. Prediction of plant height by allometric relationships in field-grown wheat. Cereal Res. Comm. 2012, 40, 413–422. [Google Scholar] [CrossRef]
- Monostori, I.; Árendás, T.; Hoffman, B.; Galiba, G.; Gierczik, K.; Szira, F.; Vágújfalvi, A. Relationship between SPAD value and grain yield can be affected by cultivar, environment and soil nitrogen content in wheat. Euphytica 2016, 211, 103–112. [Google Scholar] [CrossRef]
- Ma, J.; Tu, Y.; Zhu, J.; Luo, W.; Liu, H.; Li, C.; Li, S.; Liu, J.; Ding, P.; Habib, A.; et al. Flag leaf size and posture of bread wheat: Genetic dissection, QTL validation and their relationships with yield-related traits. Theor. Appl. Genet. 2020, 133, 297–315. [Google Scholar] [CrossRef]
- Nakano, H.; Morita, S.; Kusuda, O.; Sasaki, Y. Leaf blade dry weight and leaf area index × SPAD value at anthesis can be used to estimate nitrogen application rate at anthesis required to obtain target protein content of grain in bread wheat. Plant Prod. Sci. 2010, 13, 297–306. [Google Scholar] [CrossRef]
- Figueroa-Bustos, V.; Palta, J.A.; Chen, Y.; Siddique, K.H.M. Characterization of root and shoot traits in wheat cultivars with putative differences in root system size. Agronomy 2018, 8, 109. [Google Scholar] [CrossRef]
- Postic, F.; Beauchêne, K.; Gouache, D.; Doussan, C. Scanner-based minirhizotrons help to highlight relations between deep roots and yield in various wheat cultivars under combined water and nitrogen deficit conditions. Agronomy 2019, 9, 297. [Google Scholar] [CrossRef]
- Qin, X.-L.; Weiner, J.; Qi, L.; Xiong, Y.-C.; Li, F.-M. Allometric analysis of the effects of density on reproductive allocation and Harvest Index in 6 wheat varieties of wheat (Triticum). Field Crops Res. 2013, 144, 162–166. [Google Scholar] [CrossRef]
- Du, Y.-L.; Xi, Y.; Cui, T.; Anten, N.P.R.; Weiner, J.; Li, X.; Turner, N.C.; Zhao, Y.-M.; Li, F.-M. Yield components, reproductive allometry and the tradeoff between grain yield and yield stability in dryland spring wheat. Field Crops Res. 2020, 257, 107930. [Google Scholar] [CrossRef]
- Le Bail, M.; Jeuffroy, M.-H.; Bouchard, C.; Barbottin, A. Is it possible to forecast the grain quality and yield of different varieties of winter wheat from Minolta SPAD meter measurements? Eur. J. Agron. 2005, 23, 379–391. [Google Scholar] [CrossRef]
- Mary, B.; Peruzzo, L.; Boaga, J.; Schmutz, M.; Wu, Y.; Hubbard, S.S.; Cassiani, G. Small-scale characterization of vine plant root water uptake via 3-D electrical resistivity tomography and mise-à-la-masse method. Hydrol. Earth Syst. Sci. 2018, 22, 5427–5444. [Google Scholar] [CrossRef]
- IUSS Working Group. World Reference Base (WRB) for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps—Update 2015; World Soil Resources Reports 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Sallam, A.; Hamed, E.; Hashad, M.; Omara, M. Inheritance of stem diameter and its relationship to heat and drought tolerance in wheat (Triticum aestivum L.). J. Plant Breed. Crop Sci. 2014, 6, 11–23. [Google Scholar] [CrossRef][Green Version]
- Pokovai, K.; Fodor, N. Adjusting ceptometer data to improve leaf area index measurements. Agronomy 2019, 9, 866. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.R-project.org/ (accessed on 1 May 2022).
| Year | Parameter | Cultivar | ||||
|---|---|---|---|---|---|---|
| Káplár | Kolo | Lucilla | Ménrót | Pántlika | ||
| 2021 | PH | 0.281 *** | 0.275 *** | 0.412 *** | 0.165 ** | 0.361 *** |
| CSA | 0.497 *** | 0.368 *** | 0.405 *** | 0.355 *** | 0.533 *** | |
| PH×CSA | 0.501 *** | 0.511 *** | 0.475 *** | 0.354 *** | 0.533 *** | |
| SL | 0.564 *** | 0.338 *** | 0.451 *** | 0.385 *** | 0.572 *** | |
| FLL | 0.594 *** | 0.680 *** | 0.522 *** | 0.533 *** | 0.600 *** | |
| FLW | 0.621 *** | 0.507 *** | 0.650 *** | 0.595 *** | 0.664 *** | |
| FLA | 0.693 *** | 0.723 *** | 0.680 *** | 0.604 *** | 0.688 *** | |
| SPAD | 0.502 *** | 0.424 *** | 0.565 *** | 0.533 *** | 0.626 *** | |
| FLA×SPAD | 0.723 *** | 0.688 *** | 0.697 *** | 0.654 *** | 0.738 *** | |
| TAB | 0.524 *** | 0.545 *** | 0.645 *** | 0.633 *** | 0.551 *** | |
| GM | 0.560 *** | 0.552 *** | 0.701 *** | 0.696 *** | 0.560 *** | |
| GN | 0.489 *** | 0.508 *** | 0.662 *** | 0.628 *** | 0.548 *** | |
| 2022 | PH | 0.249 *** | 0.043 NS | 0.149 ** | 0.155 ** | 0.268 *** |
| CSA | 0.464 *** | 0.216 *** | 0.193 *** | 0.291 *** | 0.129 ** | |
| PH×CSA | 0.474 *** | 0.173 *** | 0.255 *** | 0.402 *** | 0.225 *** | |
| SL | 0.458 *** | 0.262 *** | 0.588 *** | 0.415 *** | 0.381 *** | |
| FLL | 0.474 *** | 0.602 *** | 0.611 *** | 0.621 *** | 0.484 *** | |
| FLW | 0.552 *** | 0.581 *** | 0.558 *** | 0.507 *** | 0.634 *** | |
| FLA | 0.564 *** | 0.681 *** | 0.660 *** | 0.625 *** | 0.601 *** | |
| SPAD | 0.628 *** | 0.328 *** | 0.605 *** | 0.599 *** | 0.509 *** | |
| FLA×SPAD | 0.665 *** | 0.662 *** | 0.711 *** | 0.719 *** | 0.682 *** | |
| TAB | 0.522 *** | 0.583 *** | 0.565** | 0.620 *** | 0.557 *** | |
| GM | 0.612 *** | 0.702 *** | 0.608 *** | 0.697 *** | 0.609 *** | |
| GN | 0.552 *** | 0.696 *** | 0.576 *** | 0.696 *** | 0.603 *** | |
| Parameter | Slope | y-Intercept | ||||
|---|---|---|---|---|---|---|
| CV | Y | CV×Y | CV | Y | CV×Y | |
| PH | *** | NS | NS | *** | *** | *** |
| CSA | * | NS | NS | *** | *** | ** |
| PH×CSA | ** | NS | ** | *** | *** | *** |
| SL | NS | NS | NS | *** | *** | NS |
| FLL | NS | NS | NS | *** | *** | * |
| FLW | *** | NS | NS | *** | *** | ** |
| FLA | * | NS | NS | *** | *** | * |
| SPAD | *** | NS | NS | *** | *** | *** |
| FLA×SPAD | *** | NS | NS | *** | *** | ** |
| TAB | *** | NS | ** | *** | *** | *** |
| GM | *** | * | *** | *** | *** | ** |
| GN | *** | NS | ** | *** | *** | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cseresnyés, I.; Pokovai, K.; Bányai, J.; Mikó, P. Root Electrical Capacitance Can Be a Promising Plant Phenotyping Parameter in Wheat. Plants 2022, 11, 2975. https://doi.org/10.3390/plants11212975
Cseresnyés I, Pokovai K, Bányai J, Mikó P. Root Electrical Capacitance Can Be a Promising Plant Phenotyping Parameter in Wheat. Plants. 2022; 11(21):2975. https://doi.org/10.3390/plants11212975
Chicago/Turabian StyleCseresnyés, Imre, Klára Pokovai, Judit Bányai, and Péter Mikó. 2022. "Root Electrical Capacitance Can Be a Promising Plant Phenotyping Parameter in Wheat" Plants 11, no. 21: 2975. https://doi.org/10.3390/plants11212975
APA StyleCseresnyés, I., Pokovai, K., Bányai, J., & Mikó, P. (2022). Root Electrical Capacitance Can Be a Promising Plant Phenotyping Parameter in Wheat. Plants, 11(21), 2975. https://doi.org/10.3390/plants11212975