Transcriptome and Metabolome Analyses Reveal New Insights into the Regulatory Mechanism of Head Milled Rice Rate
Abstract
1. Introduction
2. Results
2.1. Screening of Rice Accessions with Differential HMRR
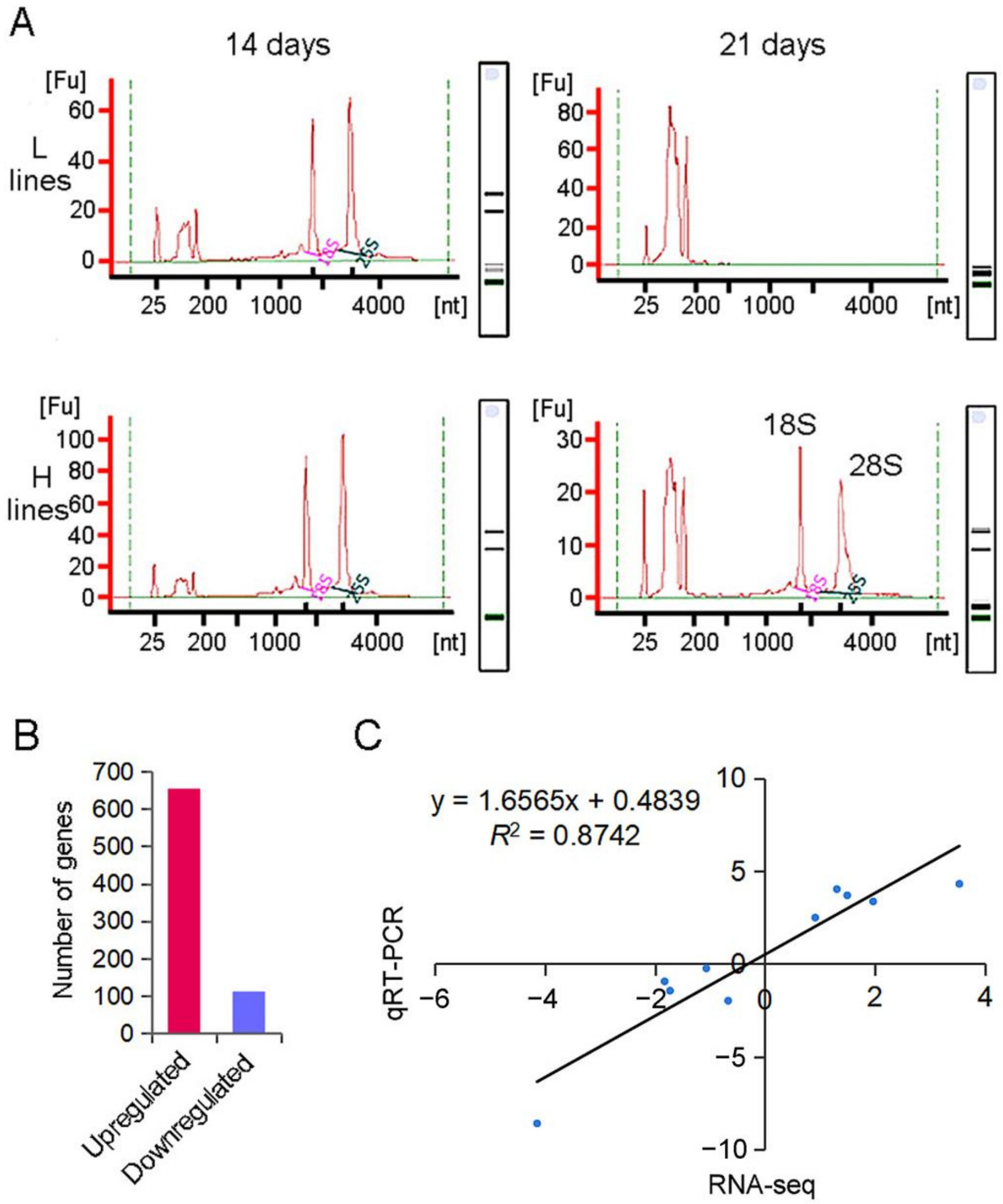
2.2. Transcriptome Profiles of Developing Seeds at Grain-Filling Stage
2.3. GO Enrichment Analyses of DEGs
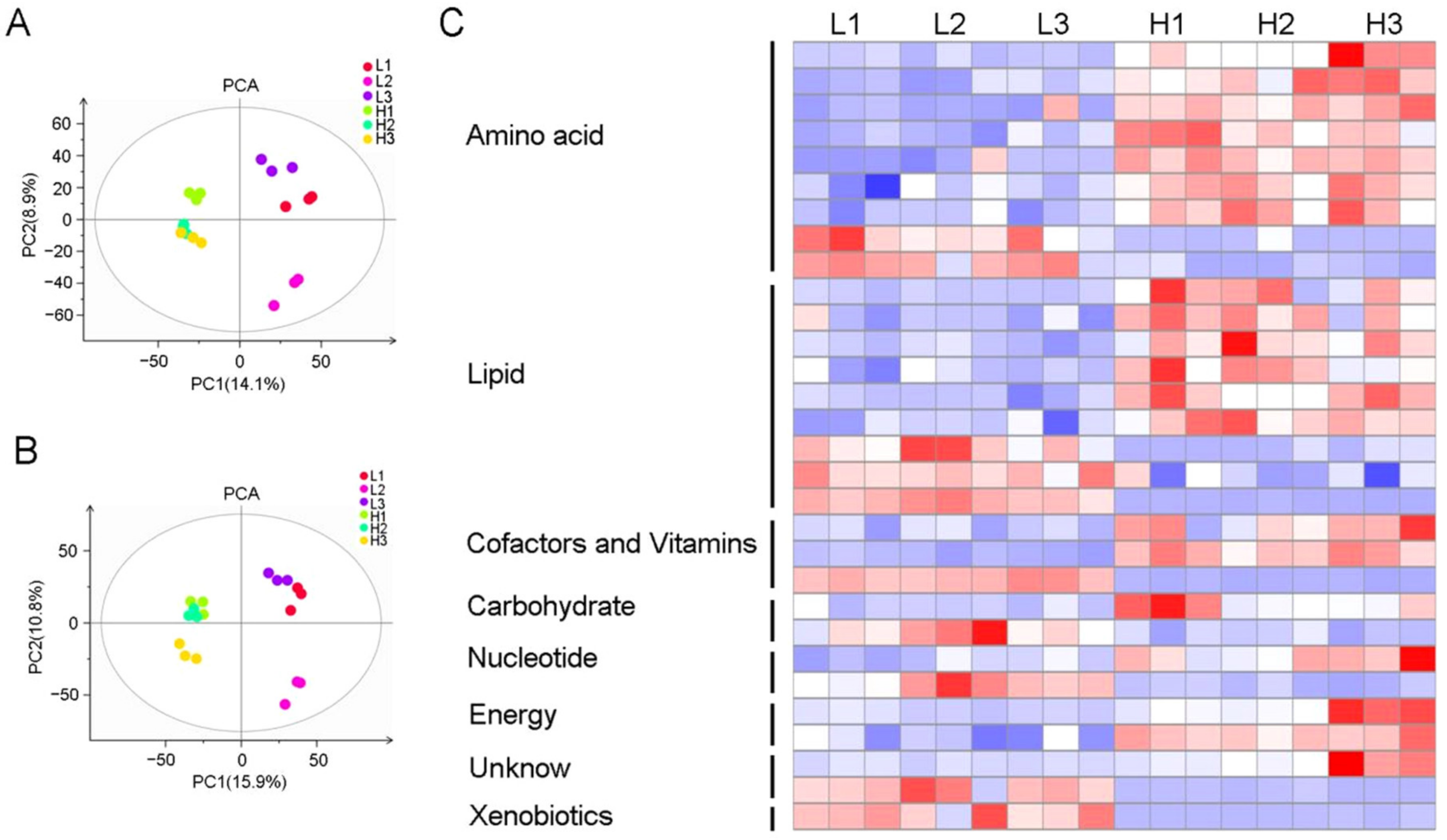
2.4. Metabolite Profiles of Brown Rice
2.5. Integrated Pathway Analysis of Differential Metabolites
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Evaluation of HMRR
4.3. RNA Extraction and Quality Detection
4.4. RNA Sequencing and GO Analyses
4.5. Real-Time PCR Analysis
4.6. Metabolite Identification and Data Analysis
4.7. Integration Analysis of Metabolite Pathways
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, H.; Xia, D.; He, Y.Q. Rice grain quality-traditional traits for high quality rice and health-plus substances. Mol. Breed. 2020, 40, 1. [Google Scholar] [CrossRef]
- Khammari, M.; Khammari, D.A.; Nia, M.R.; Khani, M.M. Parameters and QTLs for milling quality in rice: A review. Int. J. Sci. Res. Sci. Technol. 2016, 2, 145–149. [Google Scholar]
- Zhao, X.Q.; Zhou, L.J.; Ponce, K.; Ye, G.Y. The usefulness of known genes/Qtls for grain quality traits in an indica population of diverse breeding lines tested using association analysis. Rice 2015, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Fan, C.C.; Xing, Y.Z.; Yun, P.; Luo, L.J.; Yan, B.; Peng, B.; Xie, W.B.; Wang, G.W.; Li, X.H.; et al. Chalk5 encodes a vacuolar H+-translocating pyrophosphatase influencing grain chalkiness in rice. Nat. Genet. 2014, 46, 398–404. [Google Scholar] [CrossRef]
- Nelson, J.C.; McClung, A.M.; Fjellstrom, R.G.; Moldenhauer, K.A.K.; Jodari, E.B.F.; Oard, J.H.; Linscombe, S.; Scheffler, B.E.; Yeater, M. Mapping QTL main and interaction influences on milling quality in elite US rice germplasm. Theor. Appl. Genet. 2011, 122, 291–309. [Google Scholar] [CrossRef]
- Qiu, X.J.; Pang, Y.L.; Yuan, Z.H.; Xing, D.Y.; Xu, J.L.; Dingkuhn, M.; Li, Z.K.; Ye, G.Y. Genome-wide association study of grain appearance and milling quality in a worldwide collection of indica rice germplasm. PLoS ONE 2015, 10, e0145577. [Google Scholar] [CrossRef]
- Wang, X.Q.; Pang, Y.L.; Wang, C.C.; Chen, K.; Zhu, Y.J.; Shen, C.C.; Ali, J.; Xu, J.L.; Li, Z.K. New candidate genes affecting rice grain appearance and milling quality detected by genome-wide and gene-based association analyses. Front. Plant Sci. 2017, 7, 1998. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H. Grain-filling problem in ‘super’ rice. J. Exp. Bot. 2010, 61, 1–5. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, T.T.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Involvement of cytokinins in the grain filling of rice under alternate wetting and drying irrigation. J. Exp. Bot. 2010, 61, 3719–3733. [Google Scholar] [CrossRef]
- Bai, A.N.; Lu, X.D.; Li, D.Q.; Liu, J.X.; Liu, C.M. NF-YB1-regulated expression of sucrose transporters in aleurone facilitates sugar loading to rice endosperm. Cell Res. 2016, 26, 384–388. [Google Scholar] [CrossRef]
- Xue, J.; Lu, D.B.; Wang, S.G.; Lu, Z.H.; Liu, W.; Wang, X.F.; Fang, Z.Q.; He, X.Y. Integrated transcriptomic and metabolomic analysis provides insight into the regulation of leaf senescence in rice. Sci. Rep. 2021, 11, 14083. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.T.; Zhou, G.A.; Liang, C.Z.; Tian, Z.X. Omics-based interdisciplinarity is accelerating plant breeding. Curr. Opin. Plant Biol. 2022, 66, 102167. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.J.; Sun, S.S.M. Profiling the expression of genes controlling rice grain quality. Plant Mol. Biol. 2005, 59, 165–178. [Google Scholar] [CrossRef]
- Venu, R.; Sreerekha, M.; Nobuta, K.; Beló, A.; Ning, Y.; An, G.; Meyers, B.C.; Wang, G.L. Deep sequencing reveals the complex and coordinated transcriptional regulation of genes related to grain quality in rice cultivars. BMC Genom. 2011, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Xu, J.; Huang, L.C.; Leng, Y.J.; Dai, L.P.; Rao, Y.C.; Chen, L.; Wang, Y.Q.; Tu, Z.J.; Hu, J.; et al. PGL, encoding chlorophyllide a oxygenase 1, impacts leaf senescence and indirectly affects grain yield and quality in rice. J. Exp. Bot. 2016, 67, 1297–1310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tan, G.L.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Ethylene and ACC levels in developing grains are related to the poor appearance and milling quality of rice. Plant Growth Regul. 2009, 58, 85–96. [Google Scholar] [CrossRef]
- Chen, T.T.; Xu, Y.J.; Wang, J.C.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Polyamines and ethylene interact in rice grains in response to soil drying during grain filling. J. Exp. Bot. 2013, 64, 2523–2538. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.H.; Lu, J.; Zhang, C.Q.; Liu, Q.Q.; Li, Q.F. Genes and their molecular functions determining seed structure, components, and quality of rice. Rice 2022, 15, 18. [Google Scholar] [CrossRef]
- Yang, W.; Chen, L.; Zhao, J.L.; Wang, J.; Li, W.H.; Yang, T.F.; Dong, J.F.; Ma, Y.M.; Zhou, L.; Chen, J.S.; et al. Genome-wide association study of pericarp color in rice using different germplasm and phenotyping methods reveals different genetic architectures. Front. Plant Sci. 2022, 13, 841191. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, J.L.; Zhang, S.H.; Chen, L.; Yang, T.F.; Dong, J.F.; Fu, H.; Ma, Y.M.; Zhou, L.; Wang, J.; et al. Genome-wide association mapping and gene expression analysis reveal the negative role of OsMYB21 in regulating bacterial blight resistance in rice. Rice 2021, 14, 58. [Google Scholar] [CrossRef]
- Sangster, T.; Major, H.; Plumb, R.; Wilson, A.J.; Wilson, I.D. A pragmatic and readily implemented quality control strategy for HPLC-MS and GC-MS-based metabonomic analysis. Analyst 2006, 131, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Vos, R.C.H.D.; Moco, S.; Lommen, A.; Keurentjes, J.J.B.; Bino, R.J.; Hall, R.D. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Wilson, I.D.; Gika, H.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Holmes, E.; Nicholson, J.K. Global metabolic profiling procedures for urine using UPLC-MS. Nat. Protoc. 2010, 5, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
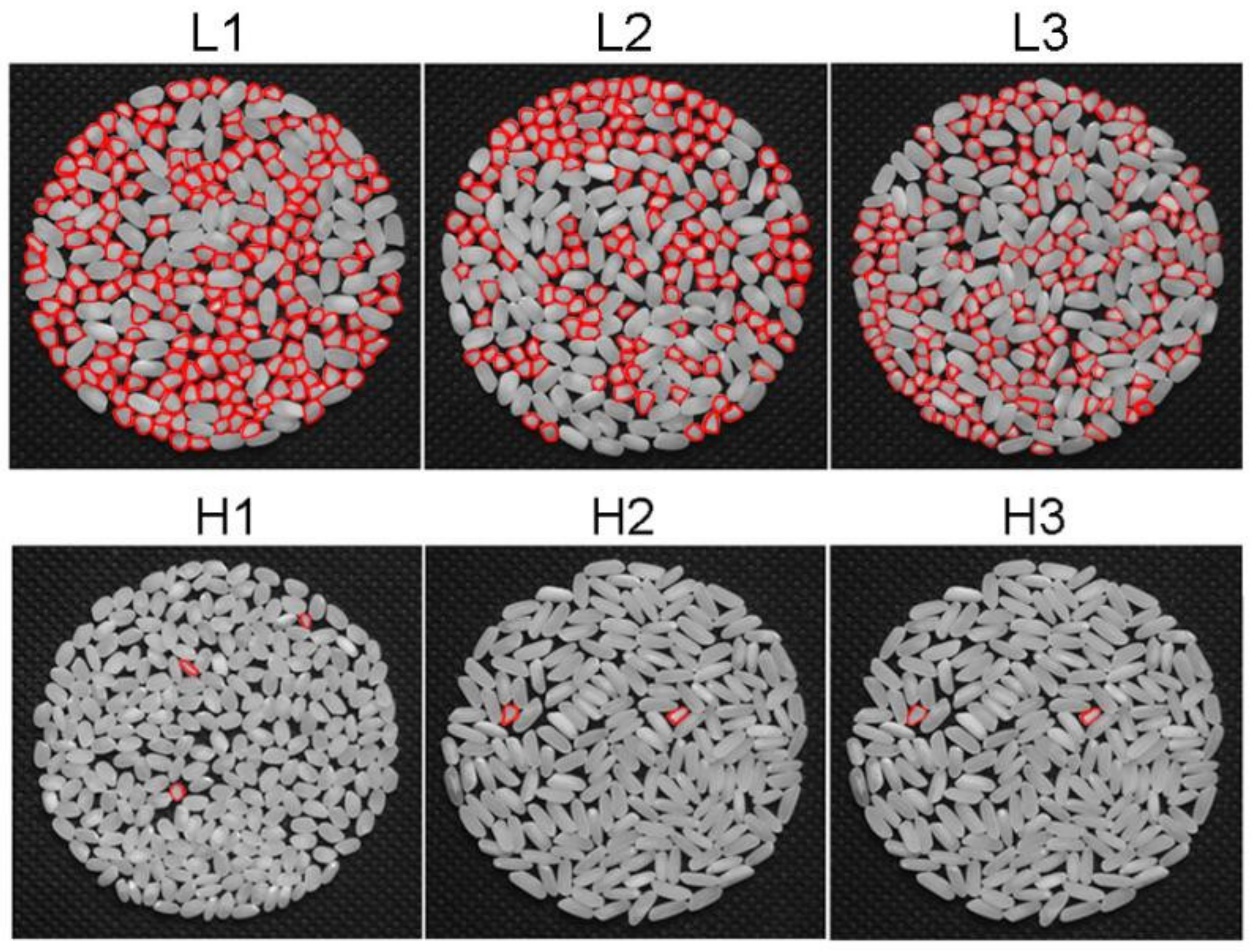


| No. | Country of Origin | HMRR (%) | Heading Date (d) | Grain Length (mm) | Grain Width (mm) | Grain Length–Width Ratio |
|---|---|---|---|---|---|---|
| L1 | Bangladesh | 24.70 ± 4.67 | 67.83 ± 3.06 | 7.99 ± 0.16 | 3.85 ± 0.05 | 2.07 ± 0.01 |
| L2 | Bangladesh | 24.40 ± 5.75 | 64.42 ± 1.53 | 7.84 ± 0.15 | 3.46 ± 0.05 | 2.27 ± 0.01 |
| L3 | India | 26.80 ± 6.64 | 72.92 ± 3.65 | 8.49 ± 0.16 | 3.90 ± 0.06 | 2.18 ± 0.01 |
| H1 | Bangladesh | 67.10 ± 1.20 | 70.33 ± 3.77 | 6.06 ± 0.11 | 3.46 ± 0.05 | 1.75 ± 0.01 |
| H2 | Pakistan | 66.98 ± 1.55 | 71.67 ± 1.89 | 9.04 ± 0.17 | 3.40 ± 0.05 | 2.66 ± 0.01 |
| H3 | Pakistan | 64.90 ± 2.83 | 70.42 ± 1.53 | 9.70 ± 0.18 | 2.60 ± 0.04 | 3.73 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Jiang, X.; Xie, Y.; Chen, L.; Zhao, J.; Liu, B.; Zhang, S.; Liu, D. Transcriptome and Metabolome Analyses Reveal New Insights into the Regulatory Mechanism of Head Milled Rice Rate. Plants 2022, 11, 2838. https://doi.org/10.3390/plants11212838
Yang W, Jiang X, Xie Y, Chen L, Zhao J, Liu B, Zhang S, Liu D. Transcriptome and Metabolome Analyses Reveal New Insights into the Regulatory Mechanism of Head Milled Rice Rate. Plants. 2022; 11(21):2838. https://doi.org/10.3390/plants11212838
Chicago/Turabian StyleYang, Wu, Xianya Jiang, Yuelan Xie, Luo Chen, Junliang Zhao, Bin Liu, Shaohong Zhang, and Dilin Liu. 2022. "Transcriptome and Metabolome Analyses Reveal New Insights into the Regulatory Mechanism of Head Milled Rice Rate" Plants 11, no. 21: 2838. https://doi.org/10.3390/plants11212838
APA StyleYang, W., Jiang, X., Xie, Y., Chen, L., Zhao, J., Liu, B., Zhang, S., & Liu, D. (2022). Transcriptome and Metabolome Analyses Reveal New Insights into the Regulatory Mechanism of Head Milled Rice Rate. Plants, 11(21), 2838. https://doi.org/10.3390/plants11212838





