Ecological Niche and Interspecific Association of Plant Communities in Alpine Desertification Grasslands: A Case Study of Qinghai Lake Basin
Abstract
1. Introduction
2. Results
2.1. Niche Width
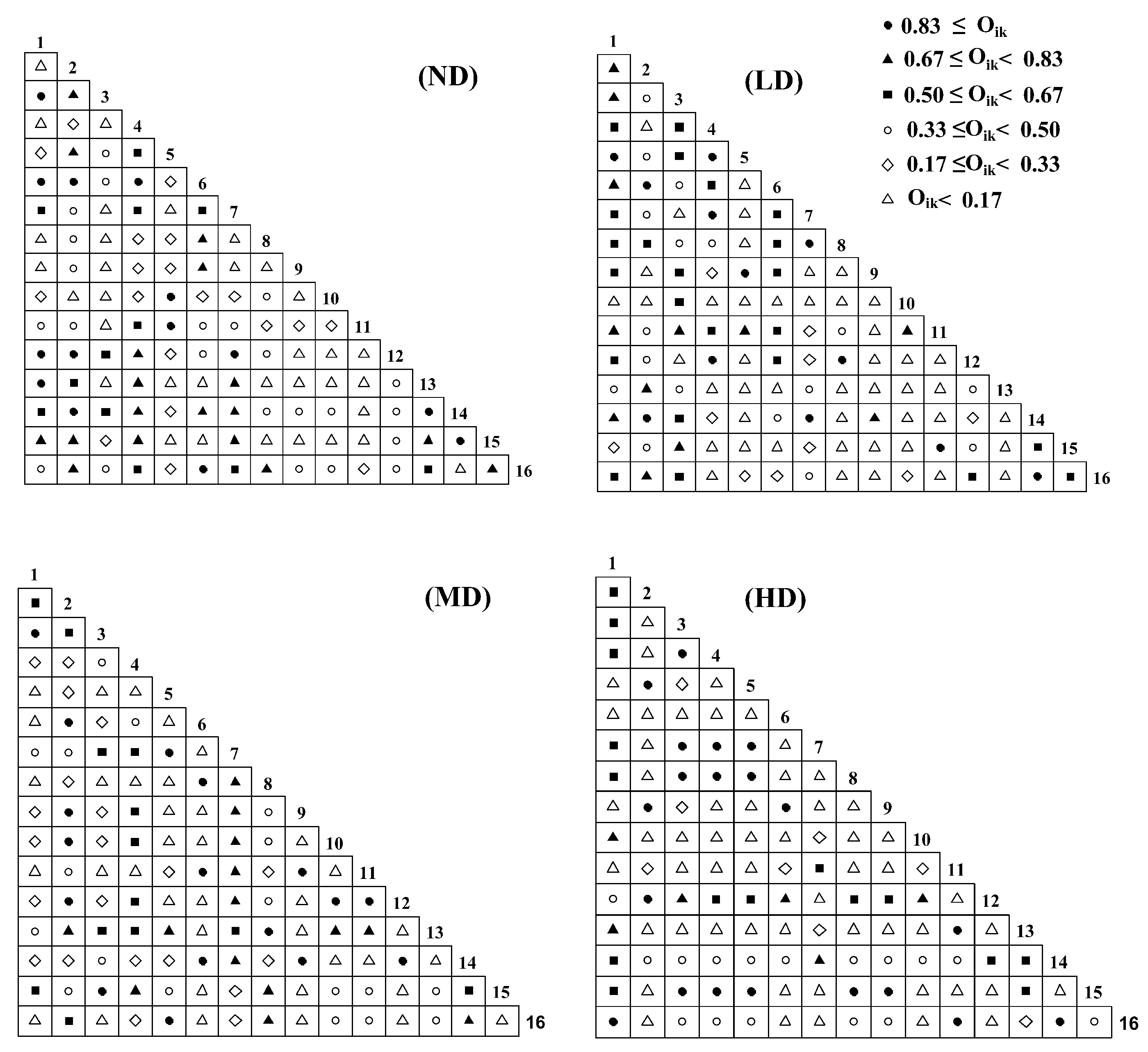
2.2. Niche Overlap
2.3. Analysis of Overall Association between Multiple Species
2.3.1. Overall Connectedness
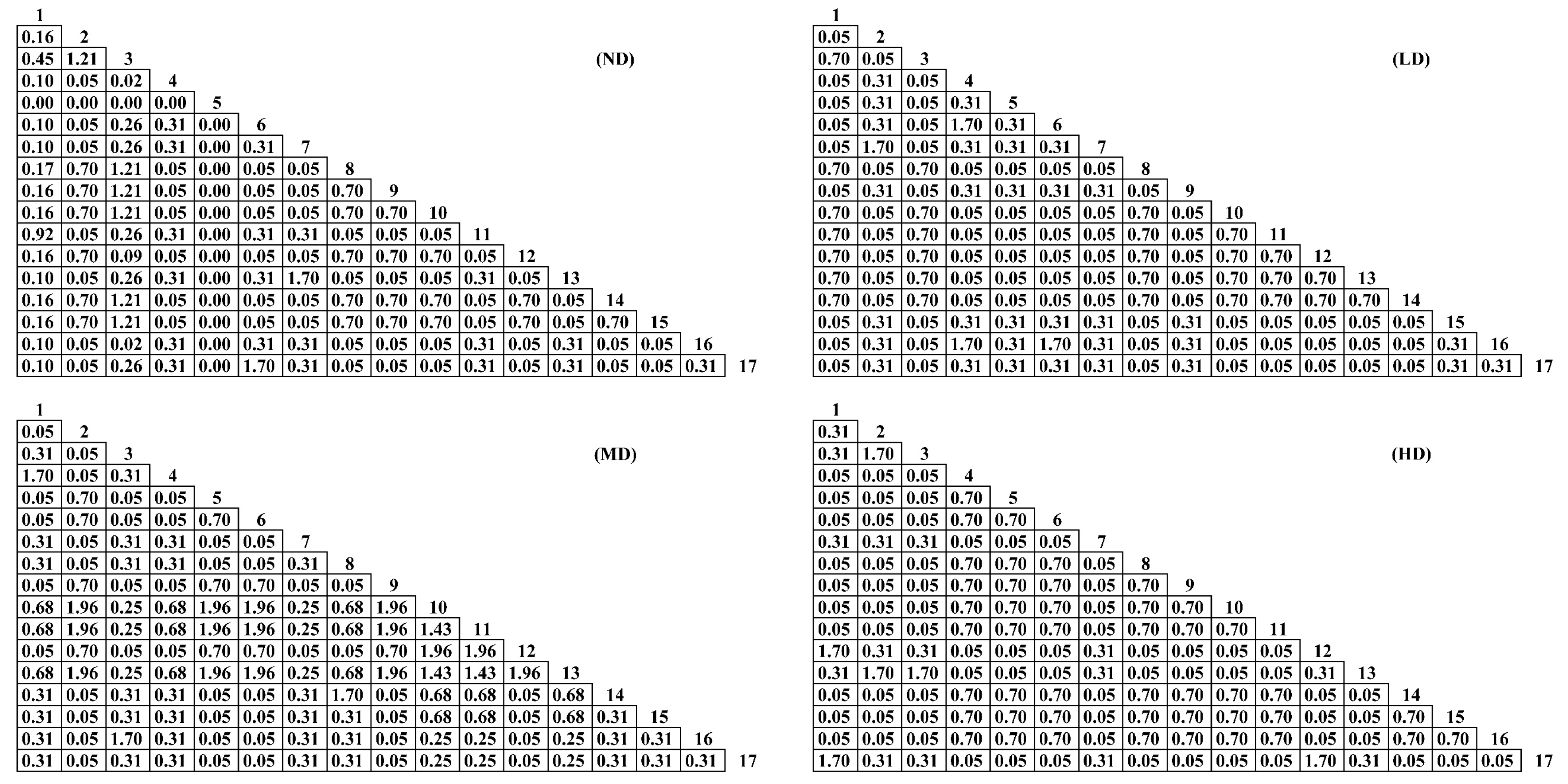
2.3.2. Chi-Square Statistic Test
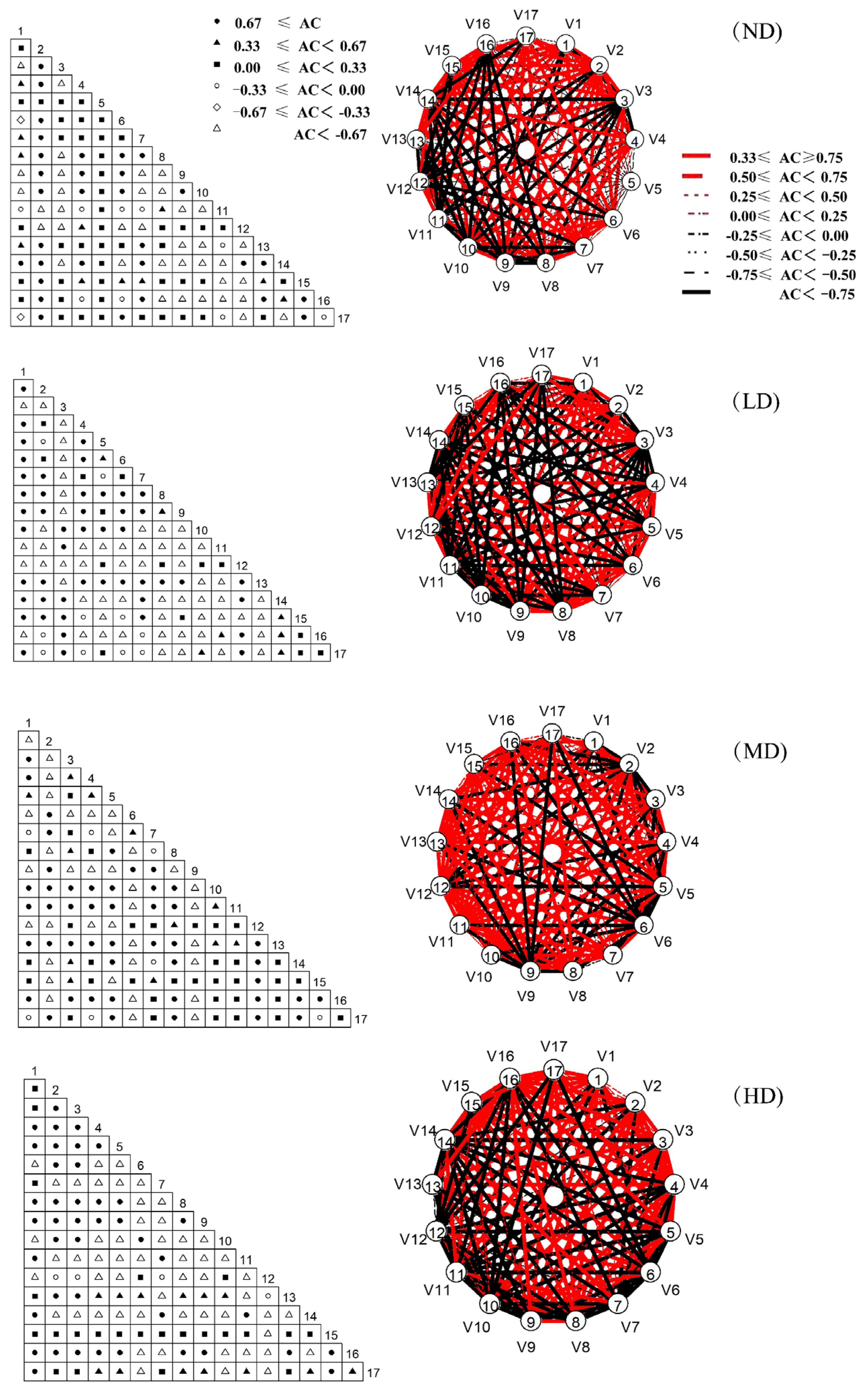
2.3.3. Association Coefficient
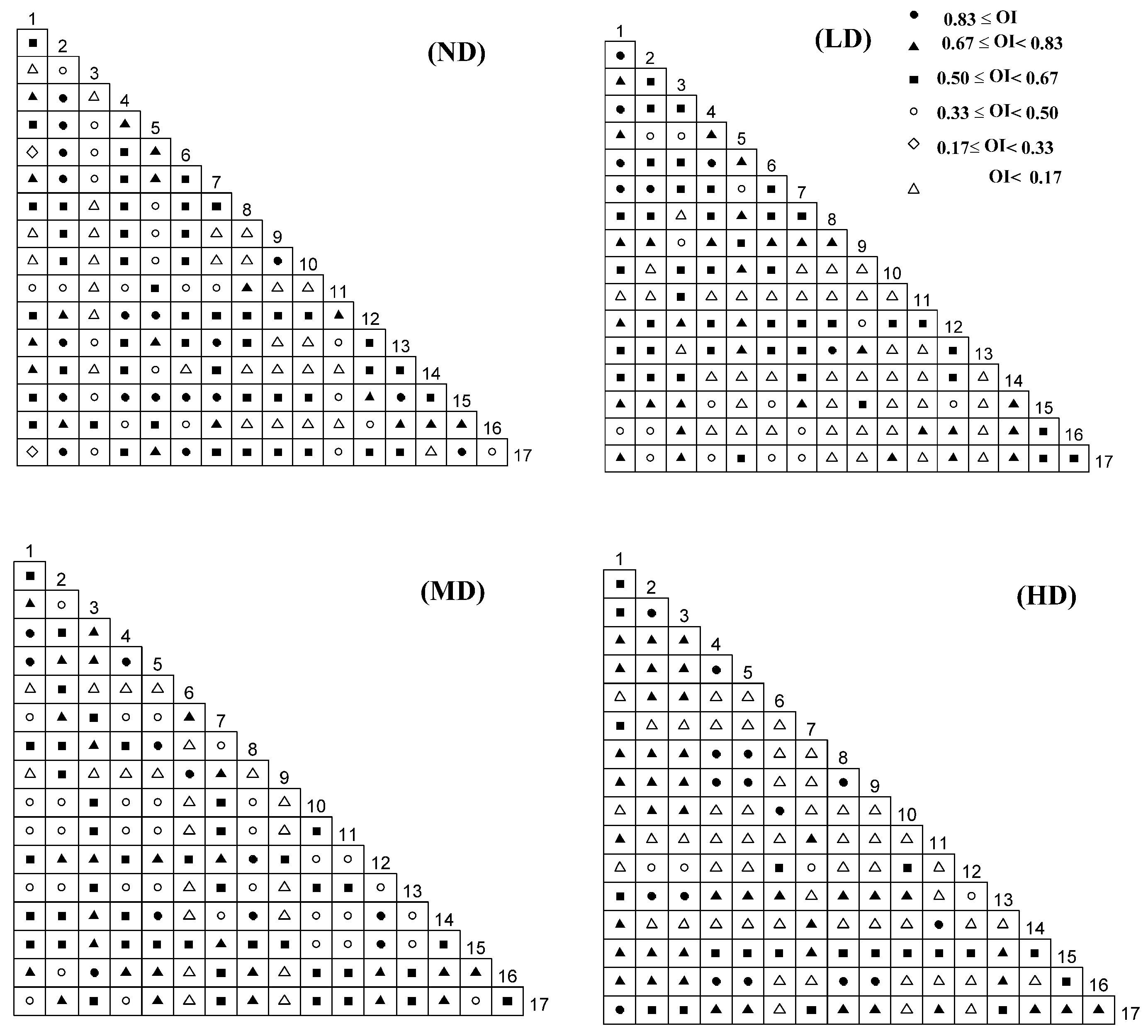
2.3.4. OI Semi-Exponential Matrix
2.3.5. Correlation Analysis of Different Desertification Plots
3. Discussion
4. Materials and Methods
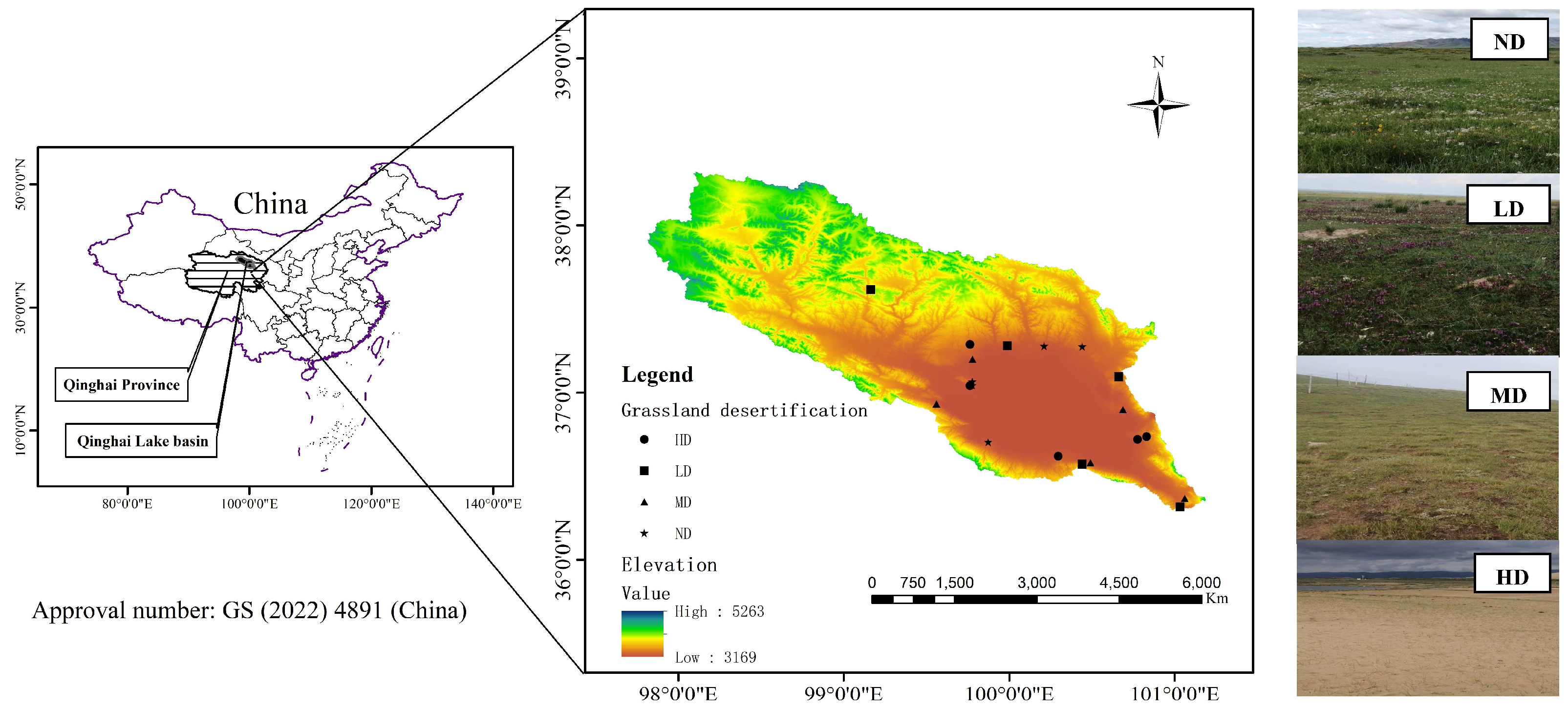
4.1. Overview of the Study Area
4.2. Meths
4.2.1. Sample Set
4.2.2. Computational Formula
- (1)
- Niche breadth and niche overlap
- (2)
- Overall connectedness
- (3)
- Interspecific association
- (1)
- Statistical magnitude
- (2)
- Connection strength
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holt, R.D. On the Relation between Niche Overlap and Competition: The Effect of Incommensurable Niche Dimensions. Oikos. 1987, 48, 110–114. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, Z.; Bi, S.; Wang, X.; Ye, Y.; Svenning, J.C. Macrofungal species distributions depend on habitat partitioning of topography, light, and vegetation in a temperate mountain forest. Sci. Rep. 2018, 8, 13589. [Google Scholar] [CrossRef]
- Martínez, I.; Wiegand, T.; González-Taboada, F.; Obeso, J.R. Spatial associations among tree species in a temperate forest community in North-western Spain. For. Ecol. Manag. 2010, 260, 456–465. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Qu, Q.; Yang, Z.; Wang, M.; Liu, G.; Xue, S. Variations and factors characterizing ecological niches of species in a stable grassland plant community. Ecol. Indic. 2021, 128, 1–9. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; Serna-Chavez, H.M.; Villalobos-Arambula, A.R.; Pérez de la Rosa, J.A.; Raes, N.; Franklin, J. Similar but not equivalent: Ecological niche comparison across closely–related Mexican white pines. Divers. Distrib. 2014, 21, 245–257. [Google Scholar] [CrossRef]
- Dowell, S.A.; Hekkala, E.R. Divergent lineages and conserved niches: Using ecological niche modeling to examine the evolutionary patterns of the Nile monitor (Varanus niloticus). Evol. Ecol. 2016, 30, 471–485. [Google Scholar] [CrossRef]
- FitzGerald, A.M. Division within the North American boreal forest: Ecological niche divergence between the Bicknell’s Thrush (Catharus bicknelli) and Gray-cheeked Thrush (C. minimus). Ecol. Evol. 2017, 7, 5285–5295. [Google Scholar] [CrossRef] [PubMed]
- Hending, D. Niche-separation and conservation biogeography of Madagascar’s fork-marked lemurs (Cheirogaleidae: Phaner): Evidence of a new cryptic species? Glob. Ecol. Conserv. 2021, 29, 1–17. [Google Scholar] [CrossRef]
- Wu, S.; Wen, L.; Dong, S.; Gao, X.; Xu, Y.; Li, S.; Dong, Q.; Wessell, K. The Plant Interspecific Association in the Revegetated Alpine Grasslands Determines the Productivity Stability of Plant Community Across Restoration Time on Qinghai-Tibetan Plateau. Front. Plant Sci. 2022, 13, 850854. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, J.; Zhong, J.J.; Ji, L.T.; Kang, B. [Interspecific association and functional group classification of the dominant populations in shrub layer in secondary forest of Pinus tabuliformis in Qinling Mountain, China.]. Ying Yong Sheng Tai Xue Bao 2018, 29, 1736–1744. [Google Scholar] [CrossRef]
- Tu, H.R.; Li, J.F.; Yang, L.T.; Bai, J.L.; Lu, G.Q.; Li, H.C.; Liang, S.C.; Jiang, Y. [Interspecific associations of the main tree populations of the Cyclobalanopsis glauca community in Karst hills of Guilin, Southwest China]. Ying Yong Sheng Tai Xue Bao 2019, 30, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.A.; Zhang, Y.; Wang, X.; Shrestha, N.; Malik, K.; Khan, I.; Ma, W.; Sun, Y.; Li, F.; Ran, J.; et al. Phylogenetic independence in the variations in leaf functional traits among different plant life forms in an arid environment. J. Plant Physiol. 2022, 272, 153671. [Google Scholar] [CrossRef]
- Salunkhe, S.S.; Bera, A.K.; Rao, S.S.; Venkataraman, V.R.; Raj, U.; Murthy, Y.V.N.K. Evaluation of indicators for desertification risk assessment in part of Thar Desert Region of Rajasthan using geospatial techniques. J. Earth Syst. Sci. 2018, 127, 1–24. [Google Scholar] [CrossRef]
- Yu, W.; Cui, J.; Gao, Y.; Zhu, M.; Shao, L.; Shen, Y.; Zhang, X.; Guo, C.; Zhang, H. Evolution of Desertification Types on the North Shore of Qinghai Lake. Comput. Mater. Contin. 2022, 71, 3635–3646. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, X. Multi-scenario simulation of desertification in North China for 2030. Land Degrad. Dev. 2020, 32, 1060–1074. [Google Scholar] [CrossRef]
- Feng, L.; Jia, Z.; Li, Q. The dynamic monitoring of aeolian desertification land distribution and its response to climate change in northern China. Sci. Rep. 2016, 6, 39563. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cuo, L.; Zhang, Y.; Wu, Y.; Hou, M. Desertification affecting the Tibetan Plateau between 1971–2015: Viewed from a climate perspective. Land Degrad. Dev. 2020, 31, 1956–1968. [Google Scholar] [CrossRef]
- Lu, R.; Jia, F.; Gao, S.; Shang, Y.; Li, J.; Zhao, C. Holocene aeolian activity and climatic change in Qinghai Lake basin, northeastern Qinghai–Tibetan Plateau. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 430, 1–10. [Google Scholar] [CrossRef]
- Wang, Q.; Sha, Z.; Wang, J.; Du, J.; Hu, J.; Ma, Y. Historical changes in the major and trace elements in the sedimentary records of Lake Qinghai, Qinghai-Tibet Plateau: Implications for anthropogenic activities. Env. Geochem Health 2019, 41, 2093–2111. [Google Scholar] [CrossRef]
- Wang, H.; Long, H.; Li, X.; Yu, F. Evaluation of changes in ecological security in China’s Qinghai Lake Basin from 2000 to 2013 and the relationship to land use and climate change. Environ. Earth Sci. 2013, 72, 341–354. [Google Scholar] [CrossRef]
- Gao, X.; Xire, L.; Zhang, Z.; Quan, C.; Zhou, S.; Li, K.; Song, R.; Zhao, S.; Kong, X.; Naori, C.; et al. Seroprevalence of Cystic Echinococcosis in Yaks and Sheep During 2017 on the Qinghai-Tibet Plateau, China. Front. Vet. Sci. 2022, 9, 849500. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, J.; Storozum, M.; Liu, S.; Gill, J.L.; Xiang, L.; Ren, X.; Wang, J.; Qiang, M.; Chen, F.; et al. Long-term herbivore population dynamics in the northeastern Qinghai-Tibetan Plateau and its implications for early human impacts. Rev. Palaeobot. Palynol. 2020, 275, 1–43. [Google Scholar] [CrossRef]
- Tong, C.; Li, M. Genomic signature of accelerated evolution in a saline-alkaline lake-dwelling Schizothoracine fish. Int. J. Biol. Macromol. 2020, 149, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, M.; Geng, L. Monitoring the recent trend of aeolian desertification using Landsat TM and Landsat 8 imagery on the north-east Qinghai–Tibet Plateau in the Qinghai Lake basin. Nat. Hazards 2015, 79, 1753–1772. [Google Scholar] [CrossRef]
- Yu, W.; Yao, X.; Shao, L.; Liu, J.; Shen, Y.; Zhang, H. Classification of Desertification on the North Bank of Qinghai Lake. Comput. Mater. Contin. 2022, 72, 695–711. [Google Scholar] [CrossRef]
- Jiang, B.-F.; Cui, B.-l.; Wang, Y.; Wang, Y.-X.; Li, D.-s.; Wang, L.-s.; Li, X.-Y. Stable-isotope tracing of vadose-zone water transport in Achnatherum splendens grassland of the Qinghai Lake Basin, NE Qinghai–Tibet Plateau, China. Catena 2021, 200, 1–9. [Google Scholar] [CrossRef]
- Ding, X.; Su, P.; Zhou, Z.; Shi, R.; Yang, J. Responses of Plant Bud Bank Characteristics to the Enclosure in Different Desertified Grasslands on the Tibetan Plateau. Plants 2021, 10, 141. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Qu, M.; Feng, Y.; Wu, B.; Lu, Q.; He, N.; Li, J. Testing the Functional and Phylogenetic Assembly of Plant Communities in Gobi Deserts of Northern Qinghai-Tibet Plateau. Front. Plant Sci. 2022, 13, 952074. [Google Scholar] [CrossRef]
- Ilunga wa Ilunga, E.; Séleck, M.; Colinet, G.; Faucon, M.-P.; Meerts, P.; Mahy, G. Small-scale diversity of plant communities and distribution of species niches on a copper rock outcrop in Upper Katanga, D.R.Congo. Plant Ecol. Evol. 2013, 146, 173–182. [Google Scholar] [CrossRef][Green Version]
- Rodriguez-Robles, U.; Arredondo, J.T.; Huber-Sannwald, E.; Yepez, E.A.; Ramos-Leal, J.A. Coupled plant traits adapted to wetting/drying cycles of substrates co-define niche multidimensionality. Plant Cell Environ. 2020, 43, 2394–2408. [Google Scholar] [CrossRef]
- Levine, J.I.; Levine, J.M.; Gibbs, T.; Pacala, S.W. Competition for water and species coexistence in phenologically structured annual plant communities. Ecol. Lett. 2022, 25, 1110–1125. [Google Scholar] [CrossRef] [PubMed]
- Silvertown, J.; Araya, Y.; Gowing, D.; Cornwell, W. Hydrological niches in terrestrial plant communities: A review. J. Ecol. 2015, 103, 93–108. [Google Scholar] [CrossRef]
- Tiusanen, M.; Kankaanpaa, T.; Schmidt, N.M.; Roslin, T. Heated rivalries: Phenological variation modifies competition for pollinators among arctic plants. Glob. Chang. Biol. 2020, 26, 6313–6325. [Google Scholar] [CrossRef]
- Xi, N.; Bloor, J.M.G.; Chu, C. Soil microbes alter seedling performance and biotic interactions under plant competition and contrasting light conditions. Ann. Bot. 2020, 126, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, A.S.; Gilbert, B.; Levine, J.M. Plant invasions and the niche. J. Ecol. 2009, 97, 609–615. [Google Scholar] [CrossRef]
- Silvertown, J.; Dodd, M.; Gowing, D. Phylogeny and the niche structure of meadow plant communities. J. Ecol. 2001, 89, 428–435. [Google Scholar] [CrossRef]
- Sun, J.; Zhan, T.; Liu, M.; Zhang, Z.; Wang, Y.; Liu, S.; Wu, G.-l.; Liu, G.; Tsunekawa, A. Verification of the biomass transfer hypothesis under moderate grazing across the Tibetan plateau: A meta-analysis. Plant Soil 2019, 458, 139–150. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Wen, Q.; Jia, Q.; Liu, Q. Interspecific associations of plant populations in rare earth mining wasteland in southern China. Int. Biodeterior. Biodegrad. 2017, 118, 82–88. [Google Scholar] [CrossRef]
- He, D.; Chen, Y.; Zhao, K.; Cornelissen, J.H.C.; Chu, C. Intra- and interspecific trait variations reveal functional relationships between specific leaf area and soil niche within a subtropical forest. Ann. Bot. 2018, 121, 1173–1182. [Google Scholar] [CrossRef]
- Yuan, Z.; Wei, B.; Chen, Y.; Jia, H.; Wei, Q.; Ye, Y. How do similarities in spatial distributions and interspecific associations affect the coexistence of Quercus species in the Baotianman National Nature Reserve, Henan, China. Ecol. Evol. 2018, 8, 2580–2593. [Google Scholar] [CrossRef]
- Gu, L.; O’Hara, K.L.; Li, W.Z.; Gong, Z.W. Spatial patterns and interspecific associations among trees at different stand development stages in the natural secondary forests on the Loess Plateau, China. Ecol. Evol. 2019, 9, 6410–6421. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-j.; Liu, J.-f.; He, Z.-s.; Zheng, S.-q.; Hong, W.; Xu, D.-w. Ecological species groups and interspecific association of dominant tree species in Daiyun Mountain National Nature Reserve. J. Mt. Sci. 2015, 12, 637–646. [Google Scholar] [CrossRef]
- Wang, X.; Liang, T.; Xie, H.; Huang, X.; Lin, H. Climate-driven changes in grassland vegetation, snow cover, and lake water of the Qinghai Lake basin. J. Appl. Remote Sens. 2016, 10, 1–18. [Google Scholar] [CrossRef]
- Chang, B.; He, K.-N.; Li, R.-J.; Sheng, Z.-P.; Wang, H. Linkage of Climatic Factors and Human Activities with Water Level Fluctuations in Qinghai Lake in the Northeastern Tibetan Plateau, China. Water 2017, 9, 552. [Google Scholar] [CrossRef]
- Dong, Z.; Hu, G.; Qian, G.; Lu, J.; Zhang, Z.; Luo, W.; Lyu, P. High-Altitude Aeolian Research on the Tibetan Plateau. Rev. Geophys. 2017, 55, 864–901. [Google Scholar] [CrossRef]
- Yang, L.; Long, H.; Cheng, H.; He, Z.; Hu, G. OSL dating of a mega-dune in the eastern Lake Qinghai basin (northeastern Tibetan Plateau) and its implications for Holocene aeolian activities. Quat. Geochronol. 2019, 49, 165–171. [Google Scholar] [CrossRef]
- Su Daxue, Z.Z.; Chen, Z.; Hu, X. Parameters for Degradation, Sandification and Salification of Rangelands. Available online: http://www.biaozhun8.cn/so.asp?k=GB+19377-2003 (accessed on 20 September 2022).
- Hu, W.; Ran, J.; Dong, L.; Du, Q.; Ji, M.; Yao, S.; Sun, Y.; Gong, C.; Hou, Q.; Gong, H.; et al. Aridity-driven shift in biodiversity-soil multifunctionality relationships. Nat. Commun. 2021, 12, 5350. [Google Scholar] [CrossRef]
- Gu, L.; Gong, Z.W.; Li, W.Z. Niches and Interspecific Associations of Dominant Populations in Three Changed Stages of Natural Secondary Forests on Loess Plateau, P.R. China. Sci. Rep. 2017, 7, 6604. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Q.; Pan, S.; Liu, C.; Han, M.; Brancelj, A. Niche and interspecific associations of Pseudoanabaena limnetica—Exploring the influencing factors of its succession stage. Ecol. Indic. 2022, 138, 1–11. [Google Scholar] [CrossRef]
- Chai, Z.; Sun, C.; Wang, D.; Liu, W. Interspecific associations of dominant tree populations in a virgin old-growth oak forest in the Qinling Mountains, China. Bot. Stud. 2016, 57, 23. [Google Scholar] [CrossRef]
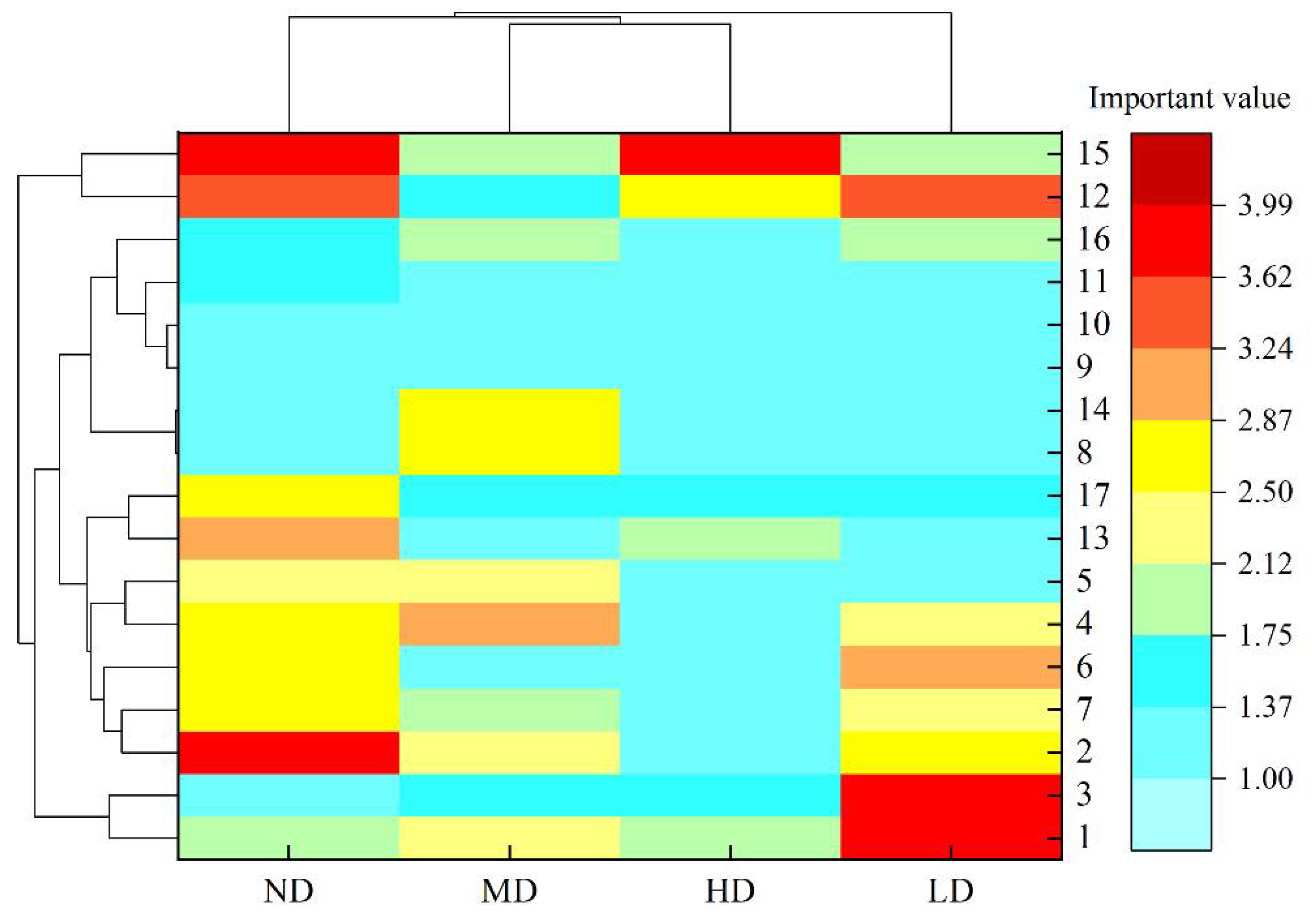
| Number | Species | Desertification Gradient | |||
|---|---|---|---|---|---|
|
Not Desertification |
Light Desertification |
Moderate
Desertification |
Heavy
Desertification | ||
| 1 | Aster altaicus | 1.91 | 3.99 | 2.37 | 1.99 |
| 2 | Kobresia humilis | 3.88 | 2.66 | 2.26 | 1.24 |
| 3 | Dracocephalum heterophyllum | 1.00 | 3.83 | 1.56 | 1.40 |
| 4 | Poa pratensis | 2.67 | 2.19 | 2.97 | 1.00 |
| 5 | Elymus nutans | 2.41 | 1.31 | 2.47 | 1.00 |
| 6 | Allium przewalskianum | 2.73 | 2.99 | 1.00 | 1.00 |
| 7 | Oxytropis kansuensis | 2.81 | 2.49 | 2.00 | 1.35 |
| 8 | Pedicularis kansuensis | 1.00 | 1.00 | 2.76 | 1.00 |
| 9 | Achnatherum splendens | 1.00 | 1.22 | 1.00 | 1.00 |
| 10 | Silene jenisseensis | 1.00 | 1.00 | 1.00 | 1.00 |
| 11 | Aconitum gymnandrum | 1.63 | 1.00 | 1.00 | 1.00 |
| 12 | Thermopsis lanceolata | 3.35 | 3.52 | 1.67 | 2.73 |
| 13 | Gentiana macrophylla | 2.92 | 1.00 | 1.00 | 1.99 |
| 14 | Lancea tibetica | 1.00 | 1.00 | 2.81 | 1.00 |
| 15 | Stellera chamaejasme | 3.90 | 2.00 | 1.89 | 3.72 |
| 16 | Taraxacum scariosum | 1.41 | 1.92 | 1.78 | 1.00 |
| 17 | Medicago sativa | 2.81 | 1.57 | 1.72 | 1.65 |
| Desertification Gradient | δr2 | Sr2 | VR | W |
Chi Squared Critical [N0.95, N0.05] | p | Result |
|---|---|---|---|---|---|---|---|
| ND | 3.15 | 8.56 | 2.72 | 13.59 | [3.00, 10.00] | p < 0.05 | Significant positive correlation |
| LD | 3.44 | 4.16 | 1.21 | 6.05 | [6.00, 10.00] | p > 0.05 | No significant positive correlation |
| MD | 3.25 | 11.29 | 3.47 | 17.36 | [4.00, 13.64] | p < 0.05 | Significant positive correlation |
| HD | 3.28 | 11.84 | 3.61 | 18.05 | [3.00, 20.00] | p > 0.05 | No significant positive correlation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Wang, H.; Jia, H.; Pen, M.; Liu, N.; Wei, J.; Zhou, B. Ecological Niche and Interspecific Association of Plant Communities in Alpine Desertification Grasslands: A Case Study of Qinghai Lake Basin. Plants 2022, 11, 2724. https://doi.org/10.3390/plants11202724
Hu Y, Wang H, Jia H, Pen M, Liu N, Wei J, Zhou B. Ecological Niche and Interspecific Association of Plant Communities in Alpine Desertification Grasslands: A Case Study of Qinghai Lake Basin. Plants. 2022; 11(20):2724. https://doi.org/10.3390/plants11202724
Chicago/Turabian StyleHu, Ying, Huichun Wang, Huiping Jia, Maodeji Pen, Nian Liu, Jingjing Wei, and Biyao Zhou. 2022. "Ecological Niche and Interspecific Association of Plant Communities in Alpine Desertification Grasslands: A Case Study of Qinghai Lake Basin" Plants 11, no. 20: 2724. https://doi.org/10.3390/plants11202724
APA StyleHu, Y., Wang, H., Jia, H., Pen, M., Liu, N., Wei, J., & Zhou, B. (2022). Ecological Niche and Interspecific Association of Plant Communities in Alpine Desertification Grasslands: A Case Study of Qinghai Lake Basin. Plants, 11(20), 2724. https://doi.org/10.3390/plants11202724





