1. Introduction
Wine grape production is a rapidly growing industry in North Carolina (NC), which ranks 10th in the United States (US) in wine grape production, 7th in wine production, and represents a
$2 billion total economic impact [
1,
2]. The dominant wine grapes produced in the western Piedmont and mountain regions of NC are
V. vinifera, which includes the European varieties and both French and American hybrids [
3]. As the NC wine industry expanded, the demand increased for research-based information to support management decisions. However, soil fertility and plant nutrition research supporting soil and plant diagnostic criteria were limited. Despite these limitations and driven by increasing demand for information, guidelines have been established based on research from other regions [
4,
5], which may not be appropriate for NC conditions.
Although soil testing traditionally is used to assess the potential for nutrient stress in annual crops, with perennial fruit crops, tissue analysis is considered a more reliable diagnostic tool to assess nutritional status [
6]. Soil tests are essential to determine soil nutrient status prior to vineyard establishment. However, plant analysis is critical to monitoring plant nutrient supply each growing season. Both forms of analysis should be used to maintain optimum nutrient availability [
7]. Most grape production regions recommend use of petiole sampling at full bloom, although if nutrient stress symptoms appear later in the growing season, veraison sampling guidelines have also been established [
8,
9]. For nitrogen (N), there are sufficient data to suggest that leaf blade analysis may provide more stable results, especially during full bloom [
10,
11], although petiole analysis is dominantly used in many regions.
Ultisols are the dominant soil order in the southeastern US and are commonly characterized by low pH and base saturation (BS) throughout the soil profile [
12]. Low BS is mostly due to soil formed from parent material high in silica and low in basic cations, but is also due to intense leaching. Due to low pH and BS, NC soils are commonly low in plant-available calcium (Ca), magnesium (Mg), and potassium (K). In addition, low phosphorus (P) availability is due to low P-containing parent materials, intense weathering, and low solubility of Al-P minerals [
13]. While adequate availability of all nutrients is essential to sustaining healthy vines and wine quality, the inherent properties of dominant soils in the V. vinifera region of NC suggest that aluminum (Al) toxicity and P, K, Ca, and Mg deficiency are important parameters to monitor.
Adequate N availability is required to support optimum grape yield and fruit quality [
14]. Application of N fertilizers to vineyard soils can be an effective management tool, particularly where soil N status is low [
3]. In N deficient soils, soil applied N may optimize yield and plant N content; however, in humid regions, growers are generally hesitant to soil apply N due to increased risk of excessive vine vigor. Under elevated N supply, canopy density (leaf area) increases, reducing airflow through the canopy and extending the duration of leaf and cluster wetness [
14]. This change in microclimate increases the potential for Botrytis and other leaf and cluster diseases [
4,
5,
15]. In addition, extensive shoot growth from full bloom to post-veraison often requires additional thinning to create an acceptable canopy microclimate for fruit and wood maturation [
16].
In the southern US where excessive plant available water encourages vine growth and soils are low in organic matter [
17], only low N rates are applied early in the growing season to avoid the potential negative consequences of excessive N fertilization [
18,
19]. Consequently, yeast assimilable N (YAN) in grape musts is frequently below the minimum threshold (140–150 mg N L
−1) required to avoid incomplete fermentation [
20,
21,
22,
23]. Grape must YAN composition represents NH
4+ and amino acids (except proline) and influences the extent of fermentation and formation of flavor compounds in the wine [
24]. Wines produced from low-YAN musts are also prone to develop a disorder called atypical aging that occurs after bottling.
The problem of low YAN is not exclusive to NC. For example, 13% of the grape musts in California, Oregon (OR), and Washington (WA) vineyards were <140 mg N L
−1 YAN [
25]; 50% of musts selected from WA, OR, and Idaho vineyards had YAN values <150 mg N L
−1 [
26]. Although mono-or diammonium phosphate are commonly added to supplement grape must N levels prior to fermentation, wine flavors are generally inferior to wines with sufficient grape must YAN levels prior to fermentation [
20,
27] Therefore, it is more desirable to enhance YAN levels through vineyard N management practices than adding N during fermentation [
28].
One potential solution to avoid excessive vine vigor associated with N applications in
V. vinifera grapes grown in NC and other southeastern states is to foliar apply N in late-season to increase YAN while causing minimal changes to vine growth and disease potential. At veraison, a large part of N uptake is translocated to the grape clusters [
29] Late-season soil applied N may not be effective in enhancing cluster N due to low surface soil moisture content [
30], reducing N absorption by roots. If N uptake were increased with soil applied N at veraison, enhanced vine vigor is not desirable due to increased disease potential. In contrast, grape leaves are able to absorb N as urea and is usually taken up rapidly by the leaf cuticle [
31].
In one of the early studies of late-season foliar N on wine quality, Lacroux et al. [
32] demonstrated soil applied N increased vigor and Botrytis incidence, whereas, foliar N improved vine N status and enhanced aroma characteristics of Sauvignon blanc without increasing vigor or Botrytis susceptibility. Other recent studies confirm the positive effects of foliar N on increased YAN and wine aromatics [
33,
34,
35]. In particular, foliar N was more effective in increasing juice YAN compared with early season soil applied N [
36] These results confirm significant improvements in the aromatic profile and intensity of wines made from Tempranillo grapes treated with foliar N. Lower aromatic intensity and pronounced herbaceous flavors were observed in wines not treated with foliar N. In addition, foliar N increased grape amino acid concentrations, which improved must N composition and enhanced fermentation kinetics. The above studies demonstrate that the use of 1–2% (
v/v) urea foliar applied at veraison shows considerable promise compared to traditional soil applied N.
In soil and plant nutrient surveys of numerous NC vineyards Havlin et al. [
17] reported over nearly 70% of full-bloom petiole samples were below critical N levels, while approximately 20% and 30% were below critical P and K levels, respectively. The low N status of
V. vinifera grown in NC is related to the minimal or no N applied by growers, a common practice used to reduce vine vigor and associated disease pressure. It is common to observe N deficiency symptoms on
V. vinifera grapevines, especially from pre-veraison through harvest growth stages.
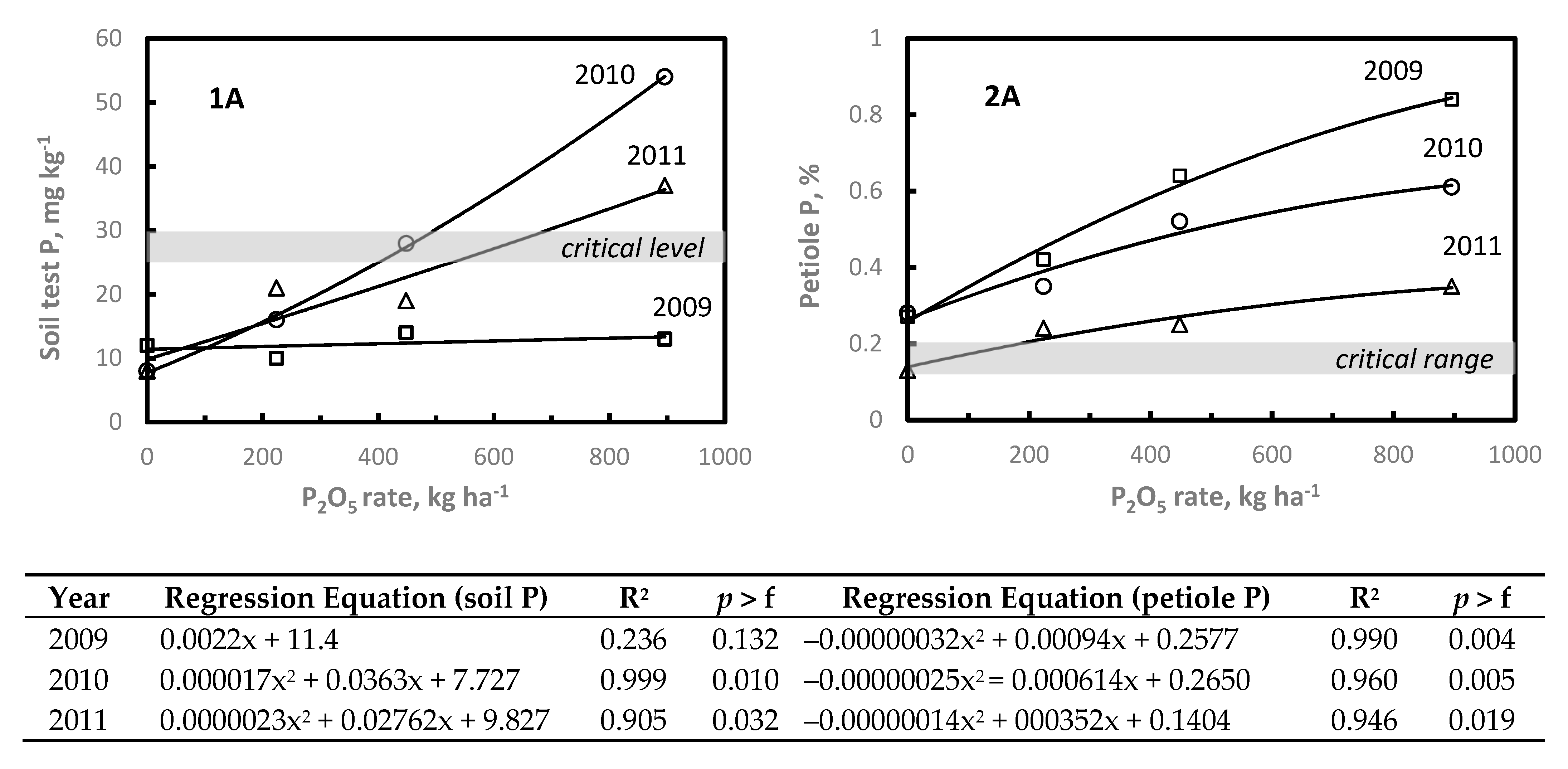
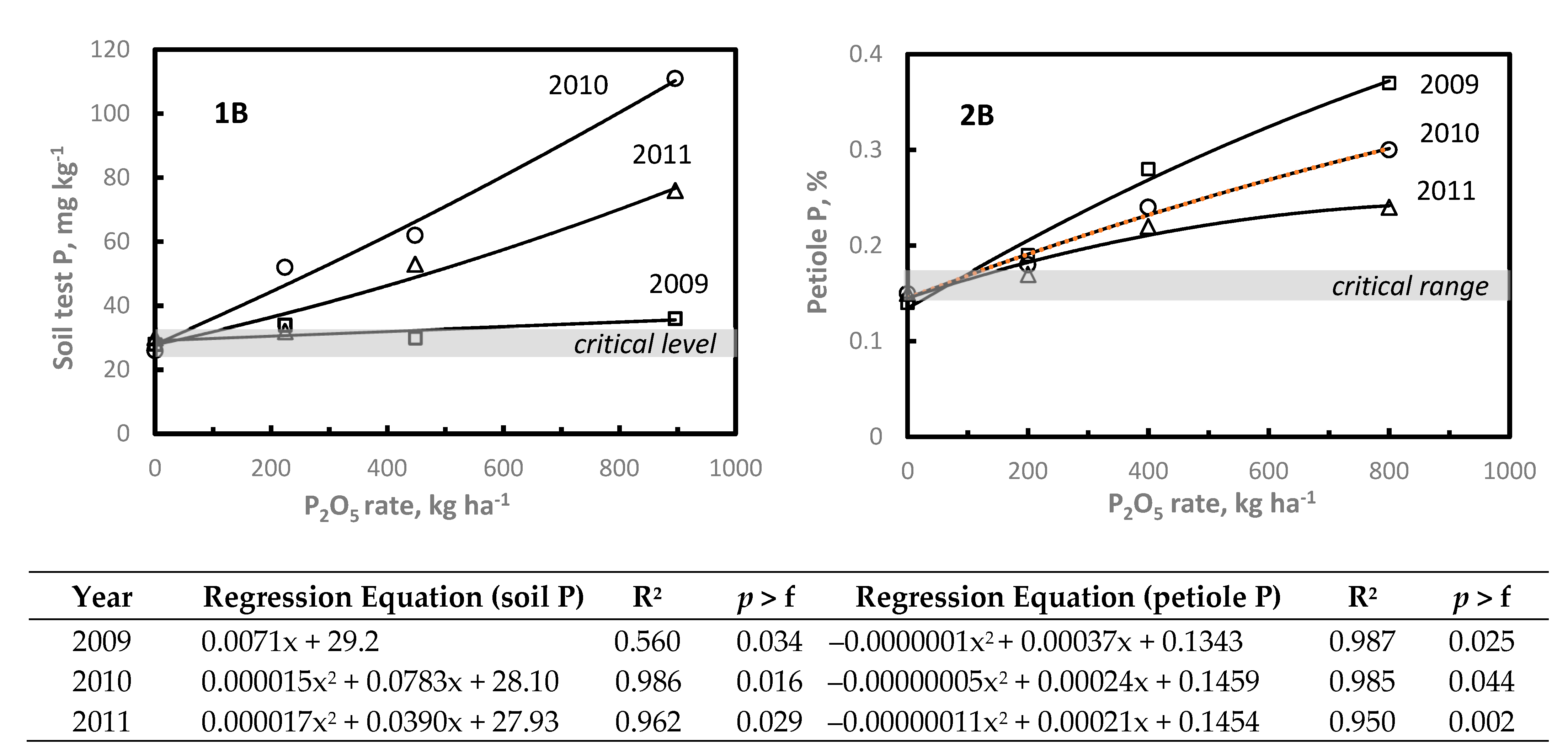
While only 30% or less of NC vineyards are P and/or K deficient, P and K fertilizer management decisions should be based on local research supporting interpretation of soil and plant analyses data [
7]. When soil test and/or plant analysis levels are above established critical levels, no additional P or K fertilizers are needed. Unfortunately, few data are available to assess the relative plant tissue P/K response to P/K applications. With P, Janat et al. [
37] reported two-year average petiole P increased from 0.21% to 0.30% with 100 kg P
2O
5 ha
−1 applied annually. Using two
V. vinifera varieties grown on P deficient soils, initial soil application of 300 kg P
2O
5 ha
−1 increased full bloom petiole P from 0.8% to 1.2% in the first year, increasing to 1.8% in the second year, and decreasing to 1.5% in the third year after P application [
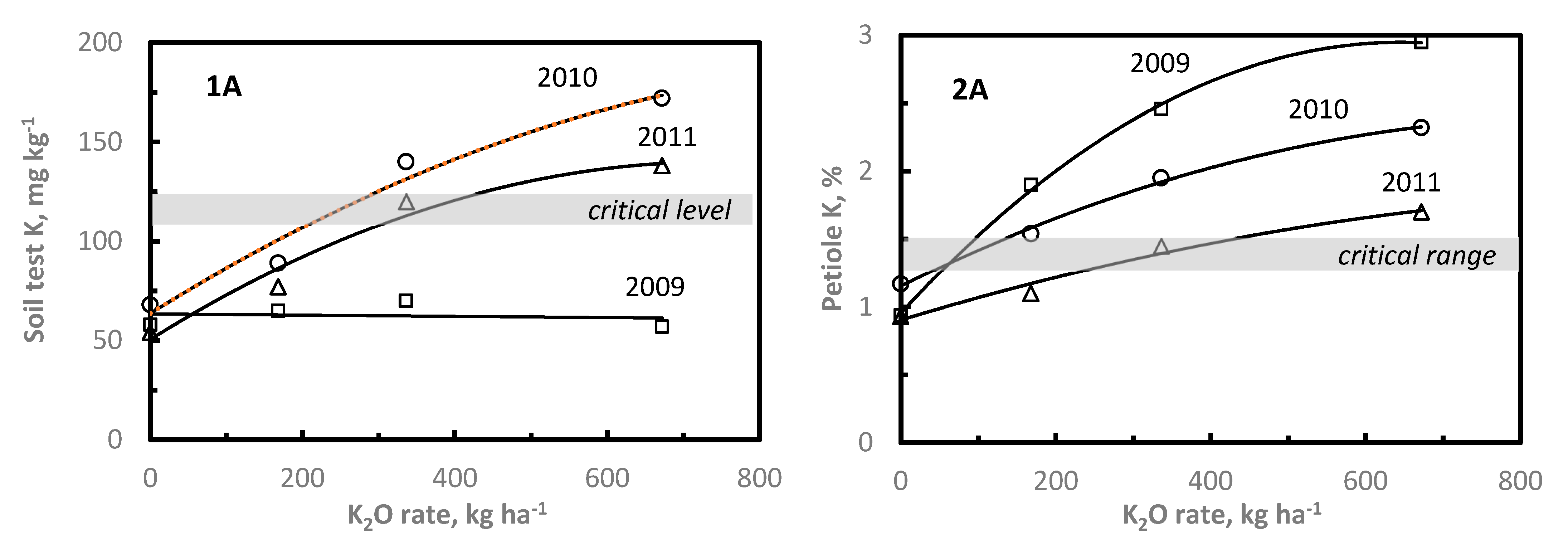
38]. In contrast, higher K rates are generally used compared to P, although excessive K applications can reduce wine quality by increasing must pH or decreasing titratable acidity [
39,
40]. For example, concern for excessive K effects on wine quality in Virginia resulted in a reduction in soil test critical K levels and subsequent fertilizer K recommendations [
41]. Using a French hybrid (cv. Foch) grown medium soil test K, sandy soil, full bloom petiole K increased from 2.8% to 3.2% K (average over five years) with annual applications of 600 kg K
2O ha
−1 [
42]. In a survey of 60 vineyard growers in central India, Naraboli et al. [
43] reported 2.50–2.75% petiole K at full bloom was associated with optimum yield and was achieved with an average 1000 kg K
2O ha
−1. After three years of annual applications of up to 200 kg K
2O ha
−1 to cv. Cabernet sauvignon in Brazil, Mehlich 1 K increased nearly 10-fold (50–464 mg kg
−1 and full bloom petiole K from 2.0% to 3.6% [
44].
Due to limited research resources available to establish critical N, P, and K plant tissue levels under NC conditions, critical nutrient levels (NC) were adopted from those established in other regions [
4,
5]. Despite this limitation, research information can be provided to assess relative plant nutrient response to N, P, and K application. Therefore, using established plant nutrient critical levels, vineyard managers can decide if additional nutrients are needed and should expect applied nutrients to increase plant nutrient levels to or above the critical levels. Therefore, the objectives of these studies were to: (1) evaluate late season foliar N application on YAN in the fruit, and (2) quantify soil and plant nutrient response to soil applied P and K.
4. Conclusions
The purpose of these studies was to quantify the effect of foliar applied N on selected N parameters in wine grapes related to wine quality, and to evaluate petiole P and K response to soil applied fertilizer P and K.
Petiole N content was below the critical level (1.2–1.6%) at each site used in the foliar N studies, which suggest that foliar N applied pre- and post-veraison could significantly improve grape N content and other parameters critical to enhancing flavor compound concentrations in wine grapes. Increasing foliar N rates generally increased YAN, while split N applications generally increased wine grape quality parameters to a greater extent than single foliar applied N rates. Pre-bud break soil applied N had little or no effect on wine grape quality unless applied at elevated rates. These preliminary data also demonstrate the potential use of UAV-based remote sensing in assessing N status in the vineyard. Although UAV’s provide a unique opportunity to capture images at resolutions needed to detect plant N status, sensitivity of the measurements can be affected early in the season when there is little growth. As this analysis suggests, if this technology is to detect early-season plant N in V. vinifera, timing of flights should correspond with adequate canopy development to providing robust, reliable measurements. Therefore, identifying N deficient grape plants at full bloom by either plant sampling/analysis or through remote sensing can direct the vineyard manager to initiate late-season foliar N management to improve wine grape quality.
The P and K response studies were located on soils testing at or below critical soil test P or K levels. At each site, increasing P or K rate increased soil test and petiole P and K levels. In the two- or three-years after application, petiole P and K declined to at or below critical levels; however, at the highest P or K rates petiole P or K levels remained at or above the critical nutrient range. Since plants accumulate nutrients throughout the rooting depth, it is difficult to base P or K management decisions on soil test P or K. Therefore, these data illustrate the importance of annual petiole analysis to determine when petiole P or K levels decline to or below established critical levels warranting application of P or K. Although additional P and K response studies representing a wider range in clay content and other soil properties are needed to establish specific P and K rates required to increase petiole P or K above established critical levels. Based on these limited data, growers should regularly monitor petiole P/K, and if below the critical levels soil applied 200–300 kg P2O5 ha−1 and 300–400 kg K2O ha−1. Subsequent P or K rates should be adjusted depending in petiole P/K the year of or following application.